INTRODUCTION
HIV-infected individuals show significantly shorter leukocyte telomere length compared with uninfected controls.1–4 This may translate to accelerated cellular aging and immunosenescence in the HIV population. HIV-related factors, such as duration of HIV infection or severity of HIV disease progression, fail to explain the variance in telomere length associated with HIV infection. Although nucleoside reverse transcriptase inhibitors can inhibit human telomerase in vitro,5,6 no associations have been reported between telomere length and exposure to nucleoside reverse transcriptase inhibitors in vivo.7,8 In fact, exposure to combination antiretroviral therapy has been associated with longer telomere length in individuals living with HIV.9,10
In observational studies, smoking, alcohol, higher body mass index, and low income have been previously associated with shorter telomeres in the general population. However, these factors were not significantly associated with telomere length among the HIV-infected participants,4 suggesting that HIV infection masked their negative effects on telomere length. Among HIV-infected individuals, high HIV viral load set point has been independently associated with shorter telomeres in 2 independent studies.4,11 This is consistent with the reported association between shorter telomeres and poorer immunological recovery in persons living with HIV and achieving virological control on combination antiretroviral therapy.12 In addition, a history of hepatitis C virus (HCV) infection has been associated with shorter telomeres in both HIV-infected and uninfected individuals.4,13 A recent study reported that although telomere length in HIV-infected individuals was shorter than that in uninfected persons, the slope of telomere length vs. age was no different, suggesting an acute shortening early on during the course of infection.11 Our aim was to measure telomere length before and immediately after HIV and/or HCV seroconversion and explore whether apparent telomere shortening occurs immediately after HIV/HCV seroconversion, or rather over time throughout the chronic infection period.
METHODS
Study Sample Selection
The study sample was a subset of the Vancouver Injection Drug User Study (VIDUS), a longitudinal cohort study (1996-present) of injection drug users who provide a blood sample every 3 months.14 Among the 1584 VIDUS participants enrolled, 133 were HIV seroconverters and 38 HCV seroconverters. For this retrospective substudy, all participants who had at least 2 available stored peripheral blood mononuclear cell (PBMC) pellets collected at 2 time points, within a year before and after seroconverting for HIV or HCV, were included. In addition, among the 142 VIDUS participants who did not seroconvert for either virus, all those with 2 PBMC pellets collected approximately 1 year apart were included as controls. This study was approved by the Providence Health Care and the University of British Columbia Clinical Research Ethics Boards.
Monochrome Multiplex Quantitative Polymerase Chain Reaction
DNA was extracted from non-live PBMC pellets using a NucliSENS easyMAG automated nucleic acid extractor (BioMérieux Canada, Saint-Laurent, QC, Canada) as per the manufacturer’s instructions, and stored at −20°C until used. DNA samples were masked and randomized before telomere length quantification using monochrome multiplex quantitative polymerase chain reaction assay.15 Relative telomere length was expressed as the ratio of telomere (T) to single-copy nuclear gene (S) (albumin) copy number, yielding the T/S ratio. The intra-assay coefficient of variation for T/S ratio, T-Ct, and S-Ct were 4.2%, 0.5%, and 0.3%, whereas the inter-assay coefficient of variation were 4.7%, 2.2%, and 0.6%, respectively.
Statistical Analyses
Within-individual telomere length change between the 2 time points was compared using the Wilcoxon signed-rank test. Characteristics at seroconversion, as well as telomere length for the HIV, HCV, and control groups were compared using the Mann–Whitney or analysis of covariance tests. The proportion of individuals with shorter telomere length in each group was compared by the Fisher’s exact test.
RESULTS
Patient Characteristics
A total of 95 VIDUS participants were studied; 51 acquired HIV, 16 acquired HCV, and 1 person acquired both viruses. The remaining 29 were controls who did not seroconvert for either virus. At the time of seroconversion, participants in the HIV group were significantly older than controls {median [interquartile range (IQR)] age 35 [26–42] vs. 25 [22–32] years, P = 0.006}, but there were no differences between HIV and HCV [35 (26–42) vs. 30 (25–36) years, P = 0.1] or HCV and controls [30 (25–36) vs. 25 (22–32) years, P = 0.18]. The median time between preseroconversion (T1) sampling and seroconversion for HIV and HCV were 3 (IQR 3–8) and 4 (IQR 3–7) months, respectively, whereas the median time between seroconversion and postseroconversion (T2) sampling for HIV and HCV were 9 (IQR 8–14) and 9 (IQR 9–14) months, respectively. For controls, the 2 samples were a median 12 (IQR 12–14) months apart. There were no differences between groups with respect to sex or ethnicity. Among HCV seroconverters, 31% (5/16) were already HIV-infected, whereas 90% (46/51) of the HIV seroconverters were already HIV-infected.
Telomere Length Before and After Seroconversion
After adjusting for age, there were no differences in telomere length at T1 between participants in the 3 groups [HCV: 8.5 (IQR 6.9–10.0) vs. HIV: 9.1 (IQR 7.8–11.1) vs. Controls: 9.6 (IQR 8.7–11)] (Fig. 1A). At T2, telomere length was significantly shorter in both seroconverter groups compared with controls [HCV: 8.4 (IQR 7.2–9.9) and HIV: 8.2 (IQR 6.9–10.0) vs. Controls: 9.6 (IQR 8.8–11.2), P = 0.02 and P = 0.01, respectively] after adjusting for age. There was no difference between HIV and HCV seroconverters (Fig. 1B).
Figure 1.
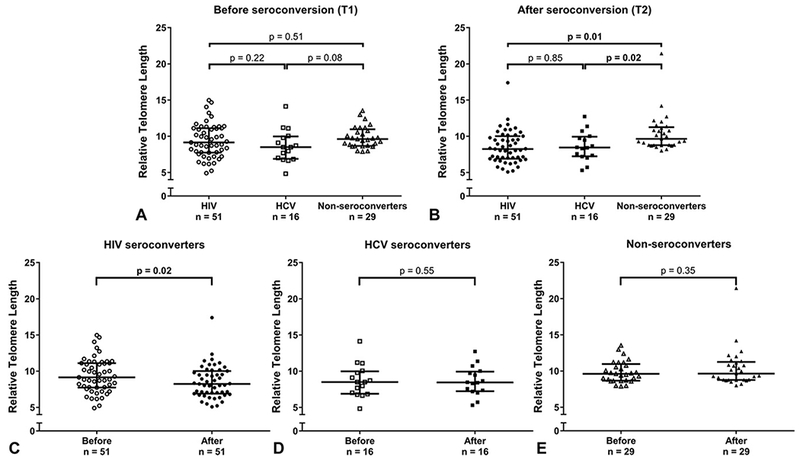
A, Age-adjusted comparisons of telomere length at T1 (before seroconversion) among participants in the 3 groups (HIV, HCV, and nonseroconverters). There were no differences in telomere length at T1 between participants in the 3 groups. P values shown are adjusted for age. B, Age-adjusted comparisons of telomere length at T2 (after seroconversion) among participants in the 3 groups (HIV, HCV, and nonseroconverters). At T2, telomere length was significantly shorter in both seroconverter groups compared with controls. There was no difference in telomere length between HIV and HCV seroconverters. P values shown are adjusted for age. C, Telomere length of participants before and after HIV seroconversion. Telomere length was significantly shorter postseroconversion compared with preseroconversion among those who acquired HIV. D, Telomere length of participants before and after HCV seroconversion. Telomere length was not significantly different postseroconversion compared with preseroconversion among those who acquired HCV. E, Telomere length of participants who did not seroconvert for either virus. Telomere length was not significantly different in the 2 PBMC samples obtained from each nonseroconverter.
Compared with preseroconversion, telomere length was significantly shorter (−13%) postseroconversion in those who acquired HIV [8.2 (IQR 6.9–10.0) vs. 9.1 (IQR 7.8–11.1), P = 0.02], but not among HCV seroconverters [8.4 (IQR 7.2–9.9) vs. 8.5 (IQR 6.9–10.0), P = 0.55]. Nonseroconverters were also similar at both samplings [9.6 (IQR 8.8–11.2) vs. 9.6 (IQR 8.7–11), P = 0.35] (Figs. 1C-E). Among HIV seroconverters, 67% experienced a decline (median 20%) in telomere length while this was true for 41% of controls (median 12%) and 44% of HCV seroconverters (median 9%). A significantly greater proportion of HIV seroconverters showed telomere shortening compared with controls (P = 0.036).
DISCUSSION
Our results show that PBMC telomere shortening occurs shortly after HIV seroconversion, an observation not detected after HCV seroconversion. This suggests that the reported association between HCV and shorter telomere length may be primarily driven by chronic infection and that telomere length changes after HIV infection are more rapid.
The increased susceptibility of persons living with HIV to acquire age-associated comorbidities earlier in life suggests that accelerated cellular senescence may disproportionately affect this population. Telomere length is a well-established biomarker of cellular aging and reflects the replicative potential of cells. Consequently, quantifying telomere length may help identify persons living with HIV who are at increased risk of developing diseases associated with cellular senescence. In addition, measuring intraindividual changes in telomere length can provide information on the rapidity of disease progression. An acute telomere shortening early on in the HIV infection course has been previously reported.13 Telomere shortening during HIV seroconversion has also been reported in a smaller sample (n = 31), using a different telomere length assay and an extended time gap (2 years) between preseroconversion and postseroconversion sampling.16 Our study confirms this and demonstrates that rapid telomere shortening occurs quickly after HIV seroconversion, and faster than after HCV seroconversion.
Several theoretical mechanisms could explain the fast telomere shortening observed in HIV seroconverters, irrespective of their disease/treatment status. HIV-induced death of a subset of leukocytes with long telomeres could result in a residual population of cells with shorter mean telomere length. Alternatively, certain HIV proteins can inhibit telomerase activity17,18 and could shorten telomeres within stem or progenitor cells, resulting in cell populations with irreversibly shorter telomeres. One small study reported reduced telomerase activity in peripheral blood lymphocytes from HIV-infected individuals.19 Short telomeres could also be a result of excessive immune cell replication during chronic immune activation, but our results suggest that this is virus dependent and more pronounced with HIV than HCV. Our study was not designed to investigate telomere length in cell subtypes, but changes in cell subsets could lead to shorter PBMC telomere length. For example, as the proportion of memory CD8+ cells increases and CD4+ cells decreases during HIV infection, this could modulate telomere length of the PBMC compartment. In support of this, a recent study observed that HIV-infected individuals had higher percentages of senescent, activated, and exhausted CD8 T cells compared with their uninfected peers, suggesting that HIV viremia may cause rapid differentiation of naive cells and therefore greater cell turnover.10 This could subsequently lead to an accumulation of senescent cell subtypes with short telomeres.
A major strength of our study is the availability of longitudinal samples from participants before and after seroconversion to enable paired telomere length comparisons. However, because of the rarity of such unique samples, our sample size is restricted. We lacked information on cytomegalovirus serostatus, another virus associated with shorter telomeres, but can assume that most participants were cytomegalovirus positive. Our study is also limited by the absence of other biomarkers of aging.
In conclusion, our results demonstrate that average telomere length measured in PBMCs undergoes significant shortening shortly after HIV seroconversion, and this is not seen over the same time period after HCV seroconversion, suggesting that different mechanisms modulate telomere shortening and cellular aging in these 2 infections. These findings should be replicated in a larger sample.
ACKNOWLEDGMENTS
The authors thank all study participants, as well as the staff from the British Columbia Centre for Excellence in HIV/AIDS and the Centre for Blood Research for their assistance and commitment to maintaining a state-of-the-art database.
Supported by grants from Genome BC, Genome Canada, and the Canadian Institutes of Health Research (CIHR). The VIDUS study was supported by the US National Institutes of Health (U01DA038886). A.G.-S. was supported by Instituto de Salud Carlos III (CD14/00320). A.A. was supported in part by a Canadian Foundation for AIDS Research grant to HCFC as well as a Canadian Institutes of Health Research (CIHR) team grant (in Cellular aging and HIV comorbidities in women and children 2013–18, TCO-125269) to HCFC. K.H. is supported by a Canadian Institutes of Health Research New Investigator Award (MSH-141971). R.P.H. has also received limited unrestricted funding, paid to his institution, from Gilead Sciences, Janssen, Merck, and ViiV Healthcare.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Wolthers KC, Bea G, Wisman A, et al. T cell telomere length in HIV-1 infection: no evidence for increased CD4+ T cell turnover. Science. 1996;274:1543–1547. [DOI] [PubMed] [Google Scholar]
- 2.Pathai S, Lawn SD, Gilbert CE, et al. Accelerated biological ageing in HIV-infected individuals in South Africa: a case-control study. AIDS. 2013;27:2375–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Effros RB, Allsopp R, Chiu CP, et al. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10:F17–F22. [DOI] [PubMed] [Google Scholar]
- 4.Zanet DAL, Thorne A, Singer J, et al. Association between short leukocyte telomere length and HIV infection in a cohort study: No evidence of a relationship with antiretroviral therapy. Clin Infect Dis 2014;58:1322–1332. [DOI] [PubMed] [Google Scholar]
- 5.Hukezalie KR, Thumati NR, Côté HC, et al. In vitro and ex vivo inhibition of human telomerase by anti-HIV nucleoside reverse transcriptase inhibitors (NRTIs) but not by non-NRTIs. PLoS One. 2012;7:e47505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leeansyah E, Cameron PU, Solomon A, et al. Inhibition of telomerase activity by human immunodeficiency virus (HIV) nucleos(t)ide reverse transcriptase inhibitors: a potential factor contributing to HIV-associated accelerated aging. J Infect Dis 2013;207:1157–1165. [DOI] [PubMed] [Google Scholar]
- 7.Solomon A, Tennakoon S, Leeansyah E, et al. No difference in the rate of change in telomere length or telomerase activity in HIV-Infected patients after three years of darunavir/Ritonavir with and without nucleoside analogues in the monet trial. PLoS One. 2014:9:e109718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montejano R, Stella-Ascariz N, Monge S, et al. Impact of antiretroviral treatment containing tenofovir difumarate on the telomere length of aviremic HIV-infected patients. J Acquir Immune Defic Syndr. 2017. September 1;76(1):102–109.: [DOI] [PubMed] [Google Scholar]
- 9.Côté HCF, Soudeyns H, Thorne A, et al. Leukocyte telomere length in HIV-infected and HIV-Exposed uninfected children: shorter telomeres with uncontrolled HIV viremia. PLoS One. 2012;7:e39266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianesin K, Noguera-Julian A, Zanchetta M, et al. Premature aging and immune senescence in HIV-infected children. AIDS. 2016;30:1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu JCY, Leung JM, Ngan DA, et al. Absolute leukocyte telomere length in HIV-infected and uninfected individuals: evidence of accelerated cell senescence in HIV-associated chronic obstructive pulmonary disease. PLoS One. 2015;10:e0124426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco J-R, Jarrin I, Martinez A, et al. Shorter telomere length predicts poorer immunological recovery in virologically suppressed HIV-1-infected patients treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr 2015;68:21–29. [DOI] [PubMed] [Google Scholar]
- 13.Grady BPX, Nanlohy NM, van Baarle D. HCV monoinfection and HIV/HCV coinfection enhance T-cell immune senescence in injecting drug users early during infection. Immun Ageing. 2016;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strathdee Sa, Patrick DM,Currie SL, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS. 1997;11:F59–F65. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh AYY, Saberi S, Ajaykumar A, et al. Optimization of a relative telomere length assay by monochromatic multiplex real-time quantitative PCR on the LightCycler 480: sources of variability and quality control considerations. J Mol Diagn. 2016;18:425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung JM, Fishbane N, Jones M, et al. Longitudinal study of surrogate aging measures during human immunodeficiency virus seroconversion. Aging (Albany NY). 2017;9:687–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Singh S, Jung HY, et al. HIV-1 Vpr protein inhibits telomerase activity via the EDD-DDB1-VPRBP E3 ligase complex. J Biol Chem 2013;288:15474–15480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comandini A, Naro C, Adamo R, et al. Molecular mechanisms involved in HIV-1-Tat mediated inhibition of telomerase activity in human CD4+ T lymphocytes. Mol Immunol. 2013;54:181–192. [DOI] [PubMed] [Google Scholar]
- 19.Ballon G, Ometto L, Righetti E, et al. Human immunodeficiency virus type 1 modulates telomerase activity in peripheral blood lymphocytes. J Infect Dis. 2001;183:417–424. [DOI] [PubMed] [Google Scholar]


