Abstract
Purpose:
Granulomas are a potentially severe condition that can last for several years in persons with primary immunodeficiency disorders (PIDD). We assessed the prevalence of granulomas in patients with PIDD.
Methods:
We used the Truven Health MarketScan® 2005–2015 Commercial Claims and Encounters and 2006–2015 Medicaid databases, and The U.S. Immunodeficiency Network (USIDNET) PIDD registry (a program of the Immune Deficiency Foundation). Our study population consisted of persons age <65 years with PIDD, defined as persons with ≥2 claims with a diagnostic code for PIDD in MarketScan databases, or patients enrolled in USIDNET. Granulomas were identified using diagnostic codes in MarketScan or provider report in USIDNET. We calculated annual prevalence of PIDD and of granulomas among PIDD patients.
Results:
We identified 247,474 and 40,395 persons with PIDD among commercially- and Medicaid-insured persons, respectively. PIDD prevalence was 6.0/10,000 in 2005 and 11.7/10,000 in 2015 among commercially-insured persons; 5.5/10,000 in 2006 and 9.6/10,000 in 2015 among Medicaid-insured persons. The prevalence of granulomas among PIDD patients was 1.2% and 1.5% among commercially- and Medicaid-insured persons, respectively. In USIDNET, prevalence of granulomas was 4.4% (177/4,021). The proportion with granulomas was similar across age groups in MarketScan, but varied from 2% to 9% in USIDNET. The reported prevalence of granulomas differed depending on PIDD condition: 1–2% in the MarketScan data, and 0–13% in USIDNET.
Conclusion:
Granuloma prevalence in PIDD patients was 1–4%. Our study provides an estimate of the proportion of PIDD patients and suggests that granulomas are an uncommon occurrence among patients with PIDD.
Keywords: primary immunodeficiency disorders, PIDD, granulomas, MarketScan, USIDNET Registry, United States
Introduction
Primary immunodeficiency disorders (PIDDs) represent a heterogeneous class of over 350 disorders, all involving an inherited defect in immune response.[1–3] Common PIDD disorders include antibody deficiencies, combined cellular and antibody deficiencies such as severe combined immunodeficiency (SCID), and disorders of the innate immune response.[4] Persons with PIDD are at an increased risk of infections and can have aberrant responses to infections.[1] Average life expectancy in PIDD patients ranges from 1 to 49 years (median), and morbidity can be considerable.[5]
Limited data are available on the national prevalence of PIDD in the United States. SCID was added to the recommended newborn screening panel in 2010, but no national surveillance system or screening program exists to identify patients with all forms of PIDD. One phone survey of 10,000 households conducted in 2005 estimated the PIDD prevalence to be 8.33 per 10,000 or 1:1,200, leading to an estimate of 150,000–360,000 PIDD cases in the United States.[1] Another study that used national administrative data found the PIDD prevalence to be 3.80–5.05 per 10,000 among privately insured and 2.91–4.11 per 10,000 among Medicaid-insured individuals during 2001–2007.[6] A regional study examining common variable immunodeficiency (CVID), the most common treatable PIDD condition, found a frequency of 1 per 10,000.[7] Lower estimates found in disease registries have raised concern about discordance between registries and insurance data and about PIDD misdiagnosis.[6, 8] Two studies have found that the prevalence of PIDD may be increasing with time, as diagnostics, treatment, and provider awareness improve.[6, 9] The lack of comprehensive data on PIDD in the United States has hampered outreach efforts, understanding of the prevalence of complications, and the development of diagnostic guidelines and testing algorithms.
Cutaneous manifestations are common in patients with PIDD and are some of the early concerning signals for diagnosis of an immunodeficiency in early childhood.[10, 11] Both autoimmune cutaneous conditions and frequent infections have been identified as potential signs that an individual may have PIDD. Granulomas are inflammatory infiltrates, which can occur in different tissues as a self-limited or severe condition that can persist for years. Granulomas can be associated with infectious or inflammatory diseases, and it is hypothesized that they occur as a result of antigen persistence or immune dysregulation.[12, 13] Granulomas have been described more commonly in adults than children with PIDD[14] including patients with chronic granulomatous disease (CGD), ataxia telangiectasia (AT), CVID, and SCID.[11, 12, 14–18] Patients with AT are more likely to develop cutaneous granulomas compared to other PIDD patients.[19, 20] Granulomas are thought to be a rare occurrence in PIDD patients [12]; however, information on the prevalence of granulomas in persons with PIDD is limited.[3, 11, 12, 21]
The prevalence of patients at risk and the frequency of granulomas is not known. Therefore, we assessed the prevalence of granulomas in patients with PIDD in the United States during 2005–2015 using a large national healthcare claims database, and in the United States Immunodeficiency Network (USIDNET) registry.
Methods
Data Sources
We used healthcare claims data from the Truven Health MarketScan® 2005– 2015 Commercial Claims and Encounters and 2006– 2015 Medicaid databases (Truven Health MarketScan Databases, Truven Health Analytics, Inc., Ann Arbor, MI).[22, 23] The commercial databases include data for approximately 30–40 million persons (employees and their beneficiaries) covered by employer-sponsored insurance for each year from all U.S. states; these data are a representative sample of the approximately 50% of persons with employer-sponsored insurance in the United States.[22] The Medicaid databases include data for approximately 6–13 million persons for each year from 8–12 unidentified states. The Medicaid population includes children in low-income families, children born to mothers on Medicaid (these children are automatically covered for their first year of life), and individuals with disabilities who qualify for the Supplemental Security Income program; states have flexibility in their Medicaid programs to include additional groups.[24] Both commercial and Medicaid databases include information on enrollment, inpatient and outpatient services, and outpatient pharmaceutical claims data for a subset of enrollees, including information on age and sex, clinical characteristics, and medical procedures and pharmaceutical treatment. Enrollees are assigned a de-identified unique number for both commercial and Medicaid databases, which allows linkage of claims over time. Only the Medicaid databases include information on race/ethnicity. This study of de-identified data was determined not to require institutional review board (IRB) review.
We also used data from the USIDNET registry, a research program of the Immune Deficiency Foundation that is funded by the National Institutes of Health. It is a voluntary registry that collects longitudinal data on patients with PIDD in the United States and Canada.[4, 25, 26] Forty-five institutions contribute patient data to the registry, who have either IRB-approved protocols or use the USIDNET IRB consent protocol.[4, 26, 27] The registry collects information on patients’ PIDD disorder or diagnosis, demographics (age, gender, race, ethnicity), and various laboratory and clinical data fields. At the time of analysis (September 2017), the USIDNET registry included a total of 6,407 patients, of whom 4,021 had sufficient demographic and clinical information for our analysis.
Case and Study Definitions
We only included persons who were aged <65 years of age in our analysis of both the MarketScan and USIDNET data. Age in the MarketScan data was defined as the age when the first claim with PIDD diagnostic claim was captured during the study period, whereas age in the USIDNET data was the age at the last visit.
In the MarketScan administrative claims data, persons with PIDD were identified by ICD-9 diagnostic codes for PIDD diagnoses [Supplemental Table 1], including a diagnostic code for autoinflammatory diseases, combined immunodeficiencies (which includes the diagnostic code for AT), complement deficiencies, congenital defects of phagocyte number or function, defects in intrinsic and innate immunity, diseases of immune dysregulation, immunodeficiencies affecting cellular and humoral immunity, and predominantly antibody deficiencies. An individual was defined as having PIDD if he/she had 2 or more inpatient and/or outpatient claims for a PIDD condition. Granulomas were defined by ICD-9 diagnostic codes for various types of granulomas, including skin, foreign body, giant cell, muscle, lethal midline, conjunctival or eye, gingiva or pyogenic, neurologic, intracranial or intraspinal, or upper body [Supplemental Table 1]. We excluded persons with ≥2 claims for secondary immune deficiencies (HIV, solid organ tumors, lymphoid tumors, chronic leukemias, nutritional deficiencies, other metabolic disorders and immune disease, and organ/tissue transplant) [6] [Supplemental Table 1] prior to their diagnostic code for PIDD. We also excluded persons who were aged ≥65 years, as their medical claims data may not be fully captured in the MarketScan data. Lastly, since ICD-10 codes were officially implemented on October 1, 2015 [28] and our study relied on data using ICD-9 diagnostic codes, we excluded data from October-December 2015.
In the USIDNET registry, PIDD diagnosis is made by a physician in one of the enrolling institutions. There are >300 conditions included in the registry. Cases of PIDD diagnosed by a provider in one of the enrolling institutions are further categorized into different PIDD diagnoses using specified diagnostic criteria (AT, CGD, CVID, DiGeorge syndrome, Bruton’s tyrosine kinase (BTK) deficiency, Wiskott-Aldrich syndrome (WAS), SCID, Hypogammaglobulinemia of unknown cause or unlisted gene defect, Hyper IgM due to uncertain or unlisted cause, other condition). The presence of granulomas is defined by the enrolling clinician; the provider may check a checkbox to indicate whether the granuloma is considered “prominent.” There are no standardized criteria for a prominent granuloma. Since the USIDNET registry provided more detailed clinical data, we were able to examine the type of granulomas among a subset of PIDD patients with AT, CGD, and CVID. Laboratory data, including T-cell count, are also included in the USIDNET registry. We hypothesized that T-cell control of antigen persistence or T-cell immune dysregulation contributed to granuloma formation. Thus, we compared T-cell levels in PIDD patients with and without granulomas by using the first T-cell count available in patients age ≥12 years old, when T-cell counts are less affected by age.
Analysis
We performed all analyses using SAS version 9.3 (SAS Institute, Cary, North Carolina). Annual prevalence was calculated using the MarketScan data as persons with PIDD, divided by 10,000 persons enrolled in MarketScan in that calendar year. We assessed trends in prevalence of PIDD over time using the Cochrane Armitage trend test. We calculated the proportion with reported granulomas among persons with PIDD. We conducted univariate analyses to compare whether persons with PIDD differed between databases by selected characteristics in 2015, the most recent year of available data, among the commercially- and Medicaid-insured persons using Pearson Chi square or Fisher’s exact test. We considered results with a p-value <0.05 as statistically significant. In the USIDNET data, we also assessed whether T-cell counts differed by PIDD patients with and without granulomas using a t-test based on Satterthwaite methods.
Results
Prevalence of PIDD: MarketScan health care claims databases
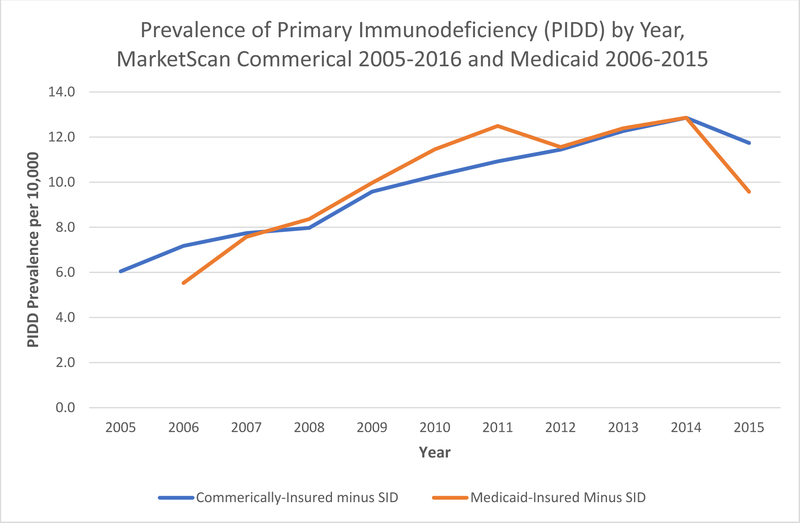
We first analyzed the reported prevalence of PIDD in the United States to understand the demographics and frequencies of different categories of PIDD. In the MarketScan databases, the reported prevalence of PIDD increased from 6.0/10,000 in 2005 to 11.7/10,000 in 2015 among commercially-insured persons aged <65 years (p<0.0001 for test of trend) and from 5.5/10,000 in 2006 to 9.6/10,000 in 2015 among Medicaid-insured persons aged <65 years (p<0.0001 for test of trend) [Figure 1]. In 2015, among commercially-insured persons, PIDD prevalence was highest among those aged 0–19 (15.0/10,000), followed by 55–64 years (14.0/10,000), and 45–54 years (11.9/10,000). PIDD prevalence was also higher in females compared to males (14.6 versus 8.7/10,000), and in those living in urban versus rural areas (12.1 versus 8.8/10,000). In 2015, among Medicaid-insured persons, the PIDD prevalence was highest among those aged 45–54 years (10.9/10,000), followed by 0–19 (10.7/10,000), and 55–64 years (10.6/10,000). The prevalence in females was similar to males (9.7 versus 9.4/10,000). The prevalence was higher in whites (11.1/10,000) and Hispanics (10.7/10,000) than in blacks (5.2/10,000).
Figure 1.
Prevalence of primary immunodeficiency (PIDD) among persons aged <65 years by year and insurance type (2005–2015 MarketScan Commercial, and 2006–2015 Medicaid data)
Characteristics and conditions of PIDD patients: MarketScan and USIDNET registry
We first examined whether there were differences among the PIDD populations included in the data sources. In the MarketScan databases, we identified 247,474 unique patients with PIDD among the commercially-insured during 2005–2015 and 40,395 among the Medicaid-insured during 2006–2015. In the USIDNET registry, 4,021 with PIDD were included in the analysis; among 1,076 with data on enrollment, they were enrolled for a median of 1.6 years (0.1–8.6 years) in the registry.
Demographic characteristics of patients identified with PIDD from all data sources are shown in Table 1. In the MarketScan databases, the age distribution (based on age of the first PIDD claim during the study period) was: 35% age 0–19 and 65% age 20–64 years among commercially-insured, and 71% age 0–19 and 29% aged 20–64 years among Medicaid-insured PIDD patients. In the USIDNET registry, the age distribution (based on age of last visit) was as follows: 57% aged 0–19 and 43% aged 20–64 years. The proportion of females among PIDD patients was 64% in the MarketScan commercially-insured population, 56% in the Medicaid-insured, and 37% in the USINDET registry.
Table 1.
Demographic characteristics of patients with primary immune deficiency (PIDD) and proportion with granulomas, MarketScan and USIDNET Registry
| MarketScan Commercial Data (N=247,474) |
MarketScan Medicaid Data (N=40,395) |
USIDNET Registry (N=4,021) |
||||
|---|---|---|---|---|---|---|
| Selected Variables | # | % | # | % | # | % |
| Agea | ||||||
| 0–19 | 86,783 | 35% | 28,530 | 71% | 2295 | 57% |
| 20–34 | 41,006 | 17% | 3,950 | 10% | 727 | 18% |
| 35–44 | 33,574 | 14% | 2,631 | 7% | 356 | 9% |
| 45–54 | 45,807 | 19% | 2,967 | 7% | 321 | 8% |
| 55–64 | 40,304 | 16% | 2,317 | 6% | 322 | 8% |
| Gender | ||||||
| Male | 88,303 | 36% | 17926 | 44% | 2273 | 57% |
| Female | 159,171 | 64% | 22464 | 56% | 1476 | 37% |
| Unknown | 272 | 7% | ||||
| Race | ||||||
| White | NA | 21,928 | 54% | 1700 | 76% | |
| Black | NA | 8,047 | 20% | 146 | 7% | |
| Hispanic | NA | 2,986 | 7% | 298 | 13% | |
| Other/Unknown | NA | 7,434 | 18% | 101 | 4% | |
| Region | ||||||
| Northeast | 46,354 | 19% | NA | NA | ||
| North Central | 57,463 | 23% | NA | NA | ||
| South | 95,341 | 39% | NA | NA | ||
| West | 43,403 | 18% | NA | NA | ||
| Unknown | 4,846 | 2% | NA | NA | ||
| Metropolitan Statistical Area | ||||||
| No | 32,392 | 13% | NA | NA | ||
| Yes | 215,082 | 87% | NA | NA | ||
| Granuloma | ||||||
| Any granuloma | 2,954 | 1.2% | 624 | 1.5% | 177 | 4.4% |
Age based on first PIDD claim during study period for MarketScan data, and age based on age at last visit for USIDNET registry data.
In the MarketScan data, the most common type of PIDD disorders among commercially-insured and Medicaid-insured PIDD patients were combined immunodeficiencies with associated syndromic features (50% and 51%, respectively) with 3% having a specific code for combined immunodeficiency, SCID or WAS; and immunodeficiencies affecting cellular and humoral immunity (31% and 34%, respectively) [Table 2];. In the USIDNET registry, the most common PIDD disorders were CVID (31%), CGD (10%), and DiGeorge syndrome (10%) [Table 3].
Table 2.
Breakdown of type of primary immunodeficiency (PIDD) disorder and proportion with granulomas by type of primary immunodeficiency disorder, MarketScan
| MarketScan Commercial Data (N=247,474) |
MarketScan Medicaid Data (N=40,395) |
|||||
|---|---|---|---|---|---|---|
| PIDD Patients | Granulomas | PIDD Patients | Granulomas | |||
| Type of PIDD | No. of PIDD patients | Frequency of PIDD type among PIDD patients (column %) | No. (row %) with Granulomas among PIDD patients | No. of PIDD patients | Frequency of PIDD type among PIDD patients (column %) | No. (row %) with Granulomas among PIDD patients |
| Autoinflammatory Diseases | 32,342 | 13% | 422 (1%) | 4,755 | 12% | 95 (2%) |
| Combined immunodeficiencies with associated or syndromic features (includes ataxia-telangiectasia) | 124,649 | 50% | 1,635 (1%) | 20,781 | 51% | 325 (2%) |
| Combined immunodeficiency, SCID, WAS | 6978 | 3% | 107 (1%) | 1,056 | 3% | 20 (2%) |
| Other Combined Immunodeficiency and Associated or syndromic Features (e.g.,Dyskeratosis Congenita, Myelodysplasia, Short Telomeres) | 117,671 | 48% | 1,528 (1%) | 19,725 | 49% | 305 (2%) |
| Complement deficiencies | 7,303 | 3% | 98 (1%) | 1,137 | 3% | 23 (2%) |
| Congenital defects of phagocyte number or function | 24,570 | 10% | 213 (1%) | 3,513 | 9% | 38 (1%) |
| Defects in Intrinsic and Innate Immunity | 22,287 | 9% | 329 (2%) | 3,948 | 10% | 80 (2%) |
| Diseases of Immune Dysregulation | 27,866 | 11% | 396 (2%) | 4,492 | 11% | 90 (2%) |
| Immunodeficiencies affecting cellular and humoral immunity | 77,607 | 31% | 1,003 (1%) | 13,936 | 34% | 241 (2%) |
| Predominantly Antibody Deficiencies | 46,484 | 19% | 589 (1%) | 5,726 | 14% | 91 (2%) |
Table 3.
Breakdown of type of primary immunodeficiency (PIDD) disorder and proportion with granulomas by type of primary immunodeficiency disorder, USIDNET Registry
| PIDD Patients | Granulomas | ||
|---|---|---|---|
| Type of PIDD | No. of PIDD patients | Frequency of PIDD type among PIDD case-patients (column %) | No. (row %) with Granulomas among PIDD patients |
| Ataxia telangiectasia | 15 | 0% | 2 (13%) |
| Chronic Granulomatous Disease (CGD)a | 471 | 12% | 54 (11%) |
| Common variable immunodeficiency disorders (CVID)b | 1408 | 35% | 96 (7%) |
| DiGeorge syndrome | 478 | 12% | 3 (1%) |
| BTK deficiency | 366 | 9% | 1 (0%) |
| Wiskott-Aldrich syndrome (WAS) | 193 | 5% | 0 (0%) |
| SCID unknown type | 98 | 2% | 0 (0%) |
| Hypogammaglobulinemia of unknown cause or unlisted gene defect | 139 | 3% | 1 (1%) |
| Hyper IgM due to uncertain or unlisted cause | 112 | 3% | 0 (0%) |
| Other conditions | 741 | 18% | 20 (3%) |
Chronic Granulomatous Disease (CGD) defined as diagnosis of “CGD, uncertain genetic cause”, “Autosomal recessive CGD - p22 phox deficiency (CYBA)”, “Autosomal recessive CGD - p47 phox deficiency (NCF1)”, “Autosomal recessive CGD - p67 phox deficiency (NCF2)”, “Autosomal recessive CGD – p22 phox deficiency (CYBA)”, “Autosomal recessive CGD – p47 phox deficiency (NCF1)”, “X-linked chronic granulomatous disease (CYBB)”
Common variable immunodeficiency disorders (CVID) defined as diagnosis of “Common variable immunodeficiency disorders with unknown genetic basis”
Granulomas and type of granulomas: MarketScan and USIDNET
In the MarketScan databases, the reported prevalence of granulomas among PIDD patients was 1.2% (2,954/247,474) among the commercially-insured and 1.5% (624/40,395) among Medicaid-insured persons. In the USIDNET registry, the prevalence of granulomas was 4.4% (177/4,021). The proportion with granulomas by age group was similar across age groups for the MarketScan commercially-insured population (1.1–1.5%), and MarketScan Medicaid-insured population (0.8–1.7%). In the USIDNET registry, the proportion who ever had granulomas ranged from 2% to 9%, with the lowest proportion in PIDD patients aged 0–19 years and highest proportion in those aged 35–44 years [Table 4].
Table 4.
Proportion with granulomas among PIDD patients by age group, MarketScan and USIDNET Registry
| MarketScan Commercial Data (N=247,474) |
MarketScan Medicaid Data (n=40,395) |
USIDNET Registry (N=4,021) |
||||
|---|---|---|---|---|---|---|
| No. of PIDD patients | No. (%) with granuloma among PIDD patients | No. of PIDD patients | No. (%) with granuloma among PIDD patients | No. of PIDD patients | No. (%) with granuloma among PIDD patients | |
| Agea | ||||||
| 0–19 | 86,783 | 1,051 (1%) | 28,530 | 493 (2%) | 2,295 | 55 (2%) |
| 20–34 | 41,006 | 410 (1%) | 3,950 | 52 (1%) | 727 | 56 (8%) |
| 35–44 | 33,574 | 417 (1%) | 2,631 | 27 (1%) | 356 | 31 (9%) |
| 45–54 | 45,807 | 574 (1%) | 2,967 | 33 (1%) | 321 | 22 (7%) |
| 55–64 | 40,304 | 502 (1%) | 2,317 | 19 (1%) | 322 | 13 (4%) |
Age based on first PIDD claim during study period for MarketScan data, and age based on age at last visit for USIDNET registry data.
Among commercially-insured PIDD patients, the proportion with a claim for granulomas during the study period ranged from 1.0% among those with congenital defects of phagocyte number or function to 1.6% among those with defects in intrinsic and innate immunity, or diseases of immune dysregulation. Among Medicaid-insured PIDD patients, the proportion with a claim for granulomas during the study period ranged from 1.1% among those with congenital defects of phagocyte number or function to 2.0% among those with autoinflammatory diseases, complement deficiencies, defects in intrinsic and innate immunity, or diseases of immune dysregulation [Table 2]. The proportions were statistically different by commercially- versus Medicaid-insured population for any type of granuloma, skin granulomas, and foreign-body granulomas (p<0.0001). In the USIDNET registry, the proportion who ever had granulomas varied by PIDD category: 0% among individuals with BTK deficiency (a humoral immunodeficiency), WAS, SCID unknown type, or Hyper IgM syndrome due to uncertain or unlisted cause; 11% among those with CGD, which would be coded with diagnostic code for congenital defects of phagocyte number or function in MarketScan; and 13% among those with AT, which would be coded with a diagnostic code for combined immunodeficiency with syndromic features in MarketScan [Table 3].
In the MarketScan databases, the most common types of granulomas were skin, foreign body, and muscle in both the commercially-insured and Medicaid-insured PIDD patients [Table 5]. Overall, among 177 individuals with granulomas in the USIDNET registry, most common types of granulomas included sino-pulmonary (50%), hematologic-lymphoid (33%), and skin (16%); 42% were considered to have prominent granulomas. Among 96 CVID patients who ever had granulomas, the most common types of granulomas were sino-pulmonary (58%), hematologic-lymphoid (41%), and skin (9%). Among 54 patients with CGD who ever had granulomas, the most common types of granulomas were sino-pulmonary (35%), hematologic-lymphoid (26%), skin (20%), and gastrointestinal (20%). There were 2 patients with AT with reported granulomas, both of whom had skin granulomas [Table 6].
Table 5.
Type of granuloma, MarketScan
| MarketScan Commercial Data (N=2,954)a |
MarketScan Medicaid Data (N=624)a |
|||
|---|---|---|---|---|
| Type of Granuloma | # | % | # | % |
| Skin | 1,713 | 58% | 475 | 76% |
| Foreign body | 1,025 | 35% | 119 | 19% |
| Orbital or conjunctival | 116 | 4% | 16 | 3% |
| Giant cell | 23 | 1% | 0 | 0% |
| Muscle | 155 | 5% | 27 | 4% |
| Lethal midline | 6 | 0% | 0 | 0% |
Proportion among the 1% (2,954/247,474) persons with granulomas among PIDD case-patients among commercially insured, and 2% (624/40,395) persons with granulomas among PIDD case-patients among Medicaid-insured persons.
Table 6.
Type of granuloma, USIDNET Registry
| Type of Granuloma | Persons with PIDD and granulomas N=177 |
Ataxia telangiectasia N=2 |
Chronic Granulomatous Disease (CGD)a N=54 |
Common variable immunodeficiency disorders (CVID)b N=96 |
||||
|---|---|---|---|---|---|---|---|---|
| # | % | # | % | # | % | # | % | |
| Autoimmunity | 7 | 4% | 0 | 0% | 1 | 2% | 6 | 6% |
| Cardiovascular | 1 | 1% | 0 | 0% | 0 | 0% | 1 | 1% |
| Constitutional | 1 | 1% | 0 | 0% | 0 | 0% | 1 | 1% |
| Oral/dental | 1 | 1% | 0 | 0% | 0 | 0% | 0 | 0% |
| Endocrine - Metabolic | 1 | 1% | 0 | 0% | 0 | 0% | 1 | 1% |
| Gastrointestinal | 17 | 10% | 0 | 0% | 11 | 20% | 5 | 5% |
| Genitourinary | 10 | 6% | 0 | 0% | 8 | 15% | 1 | 1% |
| Hematologic-Lymphoid | 58 | 33% | 0 | 0% | 14 | 26% | 39 | 41% |
| Musculoskeletal | 1 | 1% | 0 | 0% | 1 | 2% | 0 | 0% |
| Neoplastic | 3 | 2% | 0 | 0% | 0 | 0% | 1 | 1% |
| Neurologic | 1 | 1% | 0 | 0% | 0 | 0% | 1 | 1% |
| Sino-Pulmonary | 88 | 50% | 0 | 0% | 19 | 35% | 56 | 58% |
| Skin | 29 | 16% | 2 | 100% | 11 | 20% | 9 | 9% |
| Classified as prominent granuloma | 75 | 42% | 2 | 100% | 24 | 44% | 34 | 35% |
Chronic Granulomatous Disease (CGD) defined as diagnosis of “CGD, uncertain genetic cause”, “Autosomal recessive CGD - p22 phox deficiency (CYBA)”, “Autosomal recessive CGD - p47 phox deficiency (NCF1)”, “Autosomal recessive CGD - p67 phox deficiency (NCF2)”, “Autosomal recessive CGD – p22 phox deficiency (CYBA)”, “Autosomal recessive CGD – p47 phox deficiency (NCF1)”, “X-linked chronic granulomatous disease (CYBB)”
Common variable immunodeficiency disorders (CVID) defined as diagnosis of “Common variable immunodeficiency disorders with unknown genetic basis”
To test our hypothesis that T-cell control of antigen persistence or T-cell immune dysregulation contributed to granuloma formation, we compared the T-cell count in PIDD patients with and without granulomas. In the USIDNET registry, 968 PIDD patients had T-cell measurements at age ≥12 years. The average initial T-cell count was significantly lower in the 86 PIDD patients with granulomas (572.0, range: 479.1–664.9) compared to the 882 without granulomas (820.4, range: 777.3–863.5) (p<0.0001).
Discussion
Granulomas are one sign of potential primary immunodeficiency,[12, 29] although its pathogenesis is not well understood.[12] Our data demonstrate an overall frequency of granulomatous inflammation in 1–4% of PIDD patients, with skin granulomas being the most common. We also found that the prevalence of granulomas was higher in persons with specific diagnoses. The higher prevalence of granulomas in the USIDNET registry may be due to a number of factors, including the use of clinical diagnosis to capture information on granulomas. Additionally, we found that granulomas were associated with lower T-cell counts in PIDD patients in the USIDNET registry. Although relatively rare, granulomas may be the presenting manifestation among persons with PIDD.[16, 17] Identifying PIDD disorders that are more common and associated with granulomatous inflammation may be useful to inform algorithms for immunologic testing of patients with idiopathic granulomas. Early diagnosis and appropriate therapy may decrease morbidity and mortality among persons with PIDD disorders.
Recently, cutaneous granulomas due to vaccine-strain rubella have been identified in patients with diverse PIDD.[13, 14] More than 50 patients have now been identified internationally with this rare complication of rubella vaccination. Granulomatous lesions are composed of inflammatory cells, including macrophages.[14] Rubella virus was identified in Type 2 macrophages in a number of cases and some studies found long-term persistence of these viruses in macrophages (up to 15 years).[13, 30] So far, vaccine-derived rubella sequences have been detected in granulomas of PIDD patients with 1–2% sequence divergence from parental vaccine RA27/3.[13, 14, 30] The most common diagnosis in the patients with rubella virus–positive granulomas was AT.[30]
This study also provides pivotal information on the prevalence of PIDD. Using large national health care claims databases, we found that the annual reported prevalence of PIDD in the United States ranged from 6 to 12 per 10,000 persons in the United States and increased over the study period. Our findings were consistent with the prevalence observed in other studies. One study based on national telephone survey found a prevalence of 8.3/10,000.[1] Another study that looked at pediatric rates of PIDD in children aged 2–18 years, using the Healthcare Cost and Utilization Project Kids’ Inpatient Database, found a prevalence of 6.7 and 12.7 per 10,000 in 2003 and 2012, respectively.[31] However, we did find a higher prevalence than some other U.S. national estimates,[6, 9, 32] which might be explained by different methods for defining PIDD and different years included in the studies. Moreover, data from disease registries may underestimate the true prevalence of PIDD.[8, 33] The increasing prevalence of PIDD in the United States over time may be due to increasing awareness, better availability of diagnostic tests, changes in health-seeking behavior, and increasing longevity of patients. The age distribution is consistent with the most common diagnosis being CVID, an adult-onset disorder with a normal life expectancy in approximately half of the cases.[34, 35] People with CVID can have pulmonary granulomas as an isolated finding or in a setting of systemic granulomas with or without autoimmunity.[16, 18] In the setting of CVID, granulomas are associated with lower T-cell counts [16–18] and a significant decrease in decrease in T cells was observed with the appearance of cutaneous granulomas among case-patients with AT. [19]
There were differences in demographic characteristics of the PIDD patients captured in both the MarketScan commercial and Medicaid databases and in the USIDNET registry. The age distribution, however, was more similar between the MarketScan Medicaid database and USIDNET than with the MarketScan commercial database. The proportion of PIDD patients 20–64 years of age was 65% among the commercially-insured group, 30% in the Medicaid-insured and 40% in USIDNET group. The higher proportions of pediatric PIDD patients in Medicaid may be explained in part because data from Medicaid tend to include a higher proportion of children and pregnant women,[24] and in the USIDNET registry it is possible that there is a larger proportion of enrolling providers from pediatric institutions. In contrast, the MarketScan commercial databases may have a better capture of data from the adult population. Due to the differences in the composition of the MarketScan commercially-insured and Medicaid populations, we examined the PIDD prevalence by age group in 2015, and found similar patterns- with rates highest in the children aged 0–19 and adults ages 45–64 years.
Most PIDD cases were in females in both the MarketScan commercial and Medicaid databases, whereas most cases were in males in the USIDNET registry. The reason for these differences is unknown. Adult-onset CVID is more common in women while pediatric-onset PIDDs are influenced by the high rate of X-linked disorders. Disease registries have typically found a higher proportion of cases among males.[8, 26] Boyle found that the proportion of males among PIDD patients ranged from 42–56% with a telephone survey.[1] We hypothesize that the differences in characteristics of PIDD patients included in each of the databases may be partially due to the types and frequencies of PIDD conditions captured by each of the specific databases.
Our study has several limitations. MarketScan databases and the USIDNET registry are very different regarding the populations they represent and the data collected, thus, not directly comparable. It is possible that a patient may be included in both MarketScan databases and the USIDNET registry, and we were unable to determine the overlap among databases. MarketScan enrollees have employer-sponsored insurance or Medicaid insurance in the states with data managed by Truven; the data for PIDD patients may not be generalizable to all U.S. persons with PIDD. MarketScan is an administrative database and it is possible that not all health conditions and diagnoses were recorded or correctly identified, which was also noted in other studies that have used diagnostic codes.[6, 32, 35–37] We were unable to validate the ICD-9 diagnostic codes with medical record review and some of the available ICD-9 diagnostic codes may not be specific for primary immune deficiencies or secondary immune deficiencies, which may lead to misclassification of these persons in this study. For example, there is no specific diagnostic code for Dyskeratosis Congenita (DKC), Myelodysplasia, and Short Telomeres. In contrast, the USIDNET registry is based on voluntary reporting by providers. Many of the PIDD patients included in the registry are seen in tertiary care centers, and therefore may not reflect all PIDD patients. Despite having a physician-validated diagnosis of PIDD, case-definitions and enrollment criteria for the USIDNET may vary by provider. Granulomas may be caused by a number of possible etiologies, infectious or non-infectious; we did not attempt to identify the causes of the granulomas in this study.
Conclusions
In conclusion, we were able to estimate the prevalence of granulomas among PIDD patients in the United States using a convenience sample of persons with commercial or Medicaid insurance and a disease registry. Analysis of data from multiple databases is useful for improving our understanding of disease prevalence; neither database contained sufficient information to do so on its own. The prevalence of PIDD ranged from 6 to 12 per 10,000 in our study population. Given the shortage of physicians with expertise in immunology, this is a concern. Our prevalence of granulomas of 1–4% among PIDD patients suggests that granulomas are an uncommon occurrence among patients with PIDD. These data may be used as a baseline estimate for future studies. Specific groups of PIDD patients with higher rates of granulomas could be targeted for improved surveillance of infectious causes, including rubella.
Supplementary Material
Acknowledgements
We want to thank Manisha Patel, MD, MS for her thoughtful review of the manuscript. We thank Mary Ann Hall, MPH, for her editorial assistance.
The authors would also like to thank the patients and many physicians whose time and effort made the tools provided by USIDNET possible.
USIDNET is supported by a cooperative agreement, U24AI86837, from the National Institute of Allergy and Infectious Diseases (NIAID)/NIH,that has been awarded to the Immune Deficiency Foundation (IDF).
Funding Source: K.E.S.: Received support from NIH through the USIDNET grant for her work. No financial disclosures from other co-authors.
Abbreviations
- AT
ataxia telangiectasia
- CGD
chronic granulomatous disease
- CVID
common variable immunodeficiency
- IRB
institutional review board
- PIDD
primary immune deficiency disorder
- SCID
severe combined immunodeficiency
- USIDNET
United States Immunodeficiency Network registry
Footnotes
Conflicts of Interest: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, US Department of Health and Human Services.
References
- 1.Boyle JM, Buckley RH. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. Journal of clinical immunology. 2007;27(5):497–502. [DOI] [PubMed] [Google Scholar]
- 2.Notarangelo L, Casanova JL, Conley ME, Chapel H, Fischer A, Puck J, et al. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee Meeting in Budapest, 2005. The Journal of allergy and clinical immunology. 2006;117(4):883–96. [DOI] [PubMed] [Google Scholar]
- 3.Picard C, Bobby Gaspar H, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. Journal of clinical immunology. 2018;38(1):96–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephani Gray RS, Bialek Stephanie, Balajadia Ronald,. Varicella Epidemiology Among Children Aged 1, 2, and 6 Years, American Samoa, 2008–2009 44th National Immunization Conference Atlanta, GA2010.
- 5.Gathmann B, Grimbacher B, Beaute J, Dudoit Y, Mahlaoui N, Fischer A, et al. The European internet-based patient and research database for primary immunodeficiencies: results 2006–2008. Clinical and experimental immunology. 2009;157 Suppl 1:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobrynski L, Powell RW, Bowen S. Prevalence and morbidity of primary immunodeficiency diseases, United States 2001–2007. Journal of clinical immunology. 2014;34(8):954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapel H, Cunningham-Rundles C. Update in understanding Common Variable Immunodeficiency Disorders (CVIDs) and the management of patients with these conditions. British journal of haematology. 2009;145(6):709–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gathmann B, Binder N, Ehl S, Kindle G. The European internet-based patient and research database for primary immunodeficiencies: update 2011. Clinical and experimental immunology. 2012;167(3):479–91. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Joshi AY, Iyer VN, Hagan JB, St Sauver JL, Boyce TG. Incidence and temporal trends of primary immunodeficiency: a population-based cohort study. Mayo Clinic proceedings. 2009;84(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehman H Skin manifestations of primary immune deficiency. Clinical reviews in allergy & immunology. 2014;46(2):112–9. [DOI] [PubMed] [Google Scholar]
- 11.Relan M, Lehman HK. Common dermatologic manifestations of primary immune deficiencies. Current allergy and asthma reports. 2014;14(12):480. [DOI] [PubMed] [Google Scholar]
- 12.Harp J, Coggshall K, Ruben BS, Ramirez-Valle F, He SY, Berger TG. Cutaneous granulomas in the setting of primary immunodeficiency: a report of four cases and review of the literature. International journal of dermatology. 2015;54(6):617–25. [DOI] [PubMed] [Google Scholar]
- 13.Neven B, Perot P, Bruneau J, Pasquet M, Ramirez M, Diana JS, et al. Cutaneous and Visceral Chronic Granulomatous Disease Triggered by a Rubella Virus Vaccine Strain in Children With Primary Immunodeficiencies. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;64(1):83–6. [DOI] [PubMed] [Google Scholar]
- 14.Bodemer C, Sauvage V, Mahlaoui N, Cheval J, Couderc T, Leclerc-Mercier S, et al. Live rubella virus vaccine long-term persistence as an antigenic trigger of cutaneous granulomas in patients with primary immunodeficiency. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(10):O656–63. [DOI] [PubMed] [Google Scholar]
- 15.Fasano MB, Sullivan KE, Sarpong SB, Wood RA, Jones SM, Johns CJ, et al. Sarcoidosis and common variable immunodeficiency. Report of 8 cases and review of the literature. Medicine. 1996;75(5):251–61. [DOI] [PubMed] [Google Scholar]
- 16.Ardeniz O, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Clinical immunology (Orlando, Fla). 2009;133(2):198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sillevis Smitt JH, Kuijpers TW. Cutaneous manifestations of primary immunodeficiency. Current opinion in pediatrics. 2013;25(4):492–7. [DOI] [PubMed] [Google Scholar]
- 18.Boursiquot JN, Gerard L, Malphettes M, Fieschi C, Galicier L, Boutboul D, et al. Granulomatous disease in CVID: retrospective analysis of clinical characteristics and treatment efficacy in a cohort of 59 patients. Journal of clinical immunology. 2013;33(1):84–95. [DOI] [PubMed] [Google Scholar]
- 19.Chiam LY, Verhagen MM, Haraldsson A, Wulffraat N, Driessen GJ, Netea MG, et al. Cutaneous granulomas in ataxia telangiectasia and other primary immunodeficiencies: reflection of inappropriate immune regulation? Dermatology (Basel, Switzerland). 2011;223(1):13–9. [DOI] [PubMed] [Google Scholar]
- 20.Paller AS, Massey RB, Curtis MA, Pelachyk JM, Dombrowski HC, Leickly FE, et al. Cutaneous granulomatous lesions in patients with ataxia-telangiectasia. The Journal of pediatrics. 1991;119(6):917–22. [DOI] [PubMed] [Google Scholar]
- 21.Rose CD, Neven B, Wouters C. Granulomatous inflammation: The overlap of immune deficiency and inflammation. Best practice & research Clinical rheumatology. 2014;28(2):191–212. [DOI] [PubMed] [Google Scholar]
- 22.Quint JB.Health Research Data for the Real World: The MarketScan Databases. Truven Health Analytics White Paper. https://marketscan.truvenhealth.com/marketscanuniversity/publications/2015%20MarketScan%20white%20paper.pdf. Accessed Feb 7, 2017.
- 23.Shoffstall AJ, Gaebler JA, Kreher NC, Niecko T, Douglas D, Strong TV, et al. The high direct medical costs of Prader-Willi syndrome. The Journal of pediatrics. 2016;175:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CMS Medicaid.List of Medicaid eligibility groups. https://www.medicaid.gov/medicaid-chip-program-information/by-topics/waivers/1115/downloads/list-of-eligibility-groups.pdf. Accessed Feb 6, 2017.
- 25.Griffith LM, Cowan MJ, Notarangelo LD, Kohn DB, Puck JM, Pai SY, et al. Primary Immune Deficiency Treatment Consortium (PIDTC) report. The Journal of allergy and clinical immunology. 2014;133(2):335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan KE, Puck JM, Notarangelo LD, Fuleihan R, Caulder T, Wang C, et al. USIDNET: a strategy to build a community of clinical immunologists. Journal of clinical immunology. 2014;34(4):428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayor PC, Eng KH, Singel KL, Abrams SI, Odunsi K, Moysich KB, et al. Cancer in primary immunodeficiency diseases: Cancer incidence in the United States Immune Deficiency Network Registry. The Journal of allergy and clinical immunology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CMS Medicaid.ICD-10. https://www.cms.gov/Medicare/Coding/ICD10/index.html. Accessed June, 18 2018.
- 29.Mitra A, Pollock B, Gooi J, Darling JC, Boon A, Newton-Bishop JA. Cutaneous granulomas associated with primary immunodeficiency disorders. The British journal of dermatology. 2005;153(1):194–9. [DOI] [PubMed] [Google Scholar]
- 30.Perelygina L, Plotkin S, Russo P, Hautala T, Bonilla F, Ochs HD, et al. Rubella persistence in epidermal keratinocytes and granuloma M2 macrophages in patients with primary immunodeficiencies. The Journal of allergy and clinical immunology. 2016;138(5):1436–9.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin Z, Pappalardo A, Schwartz A, Antoon JW. Prevalence and Outcomes of Primary Immunodeficiency in Hospitalized Children in the United States. The journal of allergy and clinical immunology In practice. 2018. [DOI] [PubMed] [Google Scholar]
- 32.Resnick ES, Bhatt P, Sidi P, Cunningham-Rundles C. Examining the use of ICD-9 diagnosis codes for primary immune deficiency diseases in New York State. Journal of clinical immunology. 2013;33(1):40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bousfiha AA, Jeddane L, Ailal F, Benhsaien I, Mahlaoui N, Casanova JL, et al. Primary immunodeficiency diseases worldwide: more common than generally thought. Journal of clinical immunology. 2013;33(1):1–7. [DOI] [PubMed] [Google Scholar]
- 34.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112(2):277–86. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham-Rundles C, Sidi P, Estrella L, Doucette J. Identifying undiagnosed primary immunodeficiency diseases in minority subjects by using computer sorting of diagnosis codes. The Journal of allergy and clinical immunology. 2004;113(4):747–55. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez-Trujillo H, Orange JS, Roy JA, Wang Y, Newcomb CN, Liu Q, et al. Validity of Primary Immunodeficiency Disease Diagnoses in United States Medicaid Data. Journal of clinical immunology. 2015;35(6):566–72. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan KE, Boyle M, Nauman E, Carton T. Health care utilization by patients with common variable immune deficiency defined by International Classification of Diseases, Ninth Revision code 279.06. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2015;115(3):248–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.