Abstract
Copeptin, a surrogate biomarker of vasopressin, has been associated with renal function decline and may serve as a useful early biomarker for preeclampsia. We measured serum copeptin using samples collected longitudinally during pregnancy among unaffected controls (n=136) and cases of preeclampsia (n=169), gestational diabetes (n=92), gestational hypertension (n=101) and preterm birth (n=86) in the Calcium for Preeclampsia Prevention trial (1992–1995). Preeclampsia and gestational hypertension were defined as having a diastolic blood pressure greater than or equal to 90 millimeters of mercury on two occasions with and without proteinuria, respectively. The risk of pregnancy complications associated with copeptin was estimated by logistic regression adjusting for maternal age, race, body-mass index, insurance status, marital status, current smoking, and clinical site. Baseline copeptin levels, at mean 16 weeks of gestation, were associated with increased preeclampsia risk (adjusted odds ratios and 95% confidence interval being 1.55 per log unit; 1.03–2.31) compared to controls (p=0.03). The association was stronger among cases diagnosed before 37 weeks (1.86; 1.08–3.20) than those diagnosed later (1.45; 0.91–2.32). Copeptin levels rose with increasing gestational age in both cases and controls but remained significantly higher among those who were diagnosed with preeclampsia. Differences in levels of copeptin between cases and controls became more apparent closer to time of diagnosis. No significant associations were found for gestational hypertension without proteinuria, gestational diabetes, or preterm birth without preeclampsia. Copeptin levels are elevated in pregnant women prior to diagnosis of preeclampsia with elevation specific to this pregnancy complication rather than hypertension alone.
Keywords: preeclampsia, gestational diabetes, gestational hypertension, copeptin, vassopressin
Summary
We investigated whether maternal serum copeptin, a biomarker for the antidiuretic hormone arginine vasopressin, differed in pregnancies complicated by preeclampsia, gestational hypertension, and gestational diabetes compared to uncomplicated pregnancies using stored samples from the Calcium for Preeclampsia Prevention trial (1992–1995). Baseline copeptin levels at mean 16 weeks of gestation were positively associated with increased risk of preeclampsia even after accounting for maternal age, race, body-mass index, insurance status, marital status, current smoking, and clinical site. Associations were stronger among cases diagnosed before 37 weeks of gestation and no associations were found for gestational diabetes or gestational hypertension. Our findings demonstrate that copeptin levels are elevated in pregnant women prior to diagnosis of preeclampsia with elevation specific to this pregnancy complication rather than hypertension alone.
Preeclampsia (PE), defined by new onset hypertension and proteinuria after 20 weeks of gestation, increases risks for both maternal and perinatal mortality and morbidity.1 Complicating 2–8% of pregnancies1, it remains a serious pregnancy complication with uncertain cause.2, 3 Therefore, the identification of potential biomarkers of PE prior to clinical diagnosis remain important.
Arginine vasopressin (also known as antidiuretic hormone) is a multi-functional regulatory hormone playing crucial roles in osmotic homeostasis and blood pressure regulation through three receptors.4, 5 Vasopressin activation of V1a receptors, expressed in vascular walls, leads to vasoconstriction, whereas V2 receptors triggers water reabsorption through aquaporin in the kidney.5 Arterial vasodilation systemically rises while osmolality declines from very early in pregnancy.6 This vasodilation triggers both increases in cardiac output and non-osmotic vasopressin release in normal pregnancy consequent to renal vasodilation.7 Vasopressin continues to rise in spite of the decrease in osmolality suggesting a resetting of homeostasis.8, 9 However in humans, vasopressinase, produced by the placental trophoblast, also rises dramatically to metabolize vasopressin, thus regulating the hormone’s effects.7
Multiple pathways linking vasopressin to the development of PE have been proposed. 5, 7, 10, 11 Directly, increased vasopressin may decrease kidney function and increase blood pressure through the V1a and V2 receptors.5, 10 Indirectly, abnormal endothelial function may lead to decreased vasodilation that alters vasopressin expression.7 Lastly, it has also been postulated that vasopressinase cleaved vasopressin byproducts may still have reactivity, particularly for increasing its pressor activity via the V1 receptor.11 However, empirical evidence suggests cleavage removes bioactivity.12
Given that vasopressin may play a role in PE, we measured copeptin, a biomarker of vasopressin, in maternal serum collected during pregnancy before the clinical diagnosis of PE. Copeptin is the cleavage product of the C-terminal part of pre-provasopressin and has no known function.5 Vasopressin, with a half-life of only 24 minutes,13 cannot serve as a reliable biomarker.14 In addition, during pregnancy, placentally produced vasopressinase breaks down vasopressin, adding to difficulties in its measurement15. Copeptin has the advantage of remaining in circulation longer and can serve as a potential biomarker of PE.16
We sought therefore to explore the association between copeptin and development of PE prospectively. In order to evaluate the potential of copeptin as a specific biomarker of PE, we also examined its relation to gestational hypertension, gestational diabetes (GDM) and preterm birth.
Methods
The Calcium for Preeclampsia Prevention (CPEP) trial was a randomized clinical trial (1992–1995), where 4,589 nulliparous women with singleton pregnancies were randomized to calcium supplements or placebo.17, 18 Women were excluded if lost to follow-up or had incomplete information on outcomes (n=283), had a pregnancy loss (n=49), had an infant with chromosomal abnormality (n=1), or had no serum collected at baseline or samples were misdated (n=589).19 We conducted a nested case control study selecting samples from the remaining 3,667 women with and without certain pregnancy complications of interest. Case definitions of PE and gestational hypertension have been previously published.18–20 See online supplement for details regarding outcomes and covariates.
Selection
All cases of preterm PE (n=72), GDM (n=88) and preterm birth (n=87 without PE or diabetes) were included. Additional randomly selected cases of term PE (n=89) and gestational hypertension (n=114) were also included. The control group consisted of 136 women who were normotensive, normoglycemic, had no elevated proteinuria and delivered a term infant who was not small for gestational age (SGA). Random selection was made while maintaining the distribution of participants from each site from among eligible participants.
Copeptin measurement
Women provided a non-fasting blood sample prior to randomization and twice during follow-up between gestational weeks 26–29 and at approximately week 36. Women were randomized before 21 weeks and 6 days of gestation. Serum copeptin was measured by BRAHMS Immunoluminometric Assay (Thermo Scientific, Berlin, Germany) using coated-tubes following the methods of Morgenthaler et al14. Additional details regarding copeptin measurement can be found online. Our current analysis with measured copeptin consisted of 136 controls, 71 preterm PE, 98 term PE, 78 gestational hypertension (without GDM), 88 GDM (with 10 cases also having term PE), and 86 preterm deliveries without PE. This study was exempted from institutional review board review and approval by the National Institutes of Health’s Office of Human Subjects Research due to use of existing anonymous samples.19
Statistical analysis
Crude and adjusted odds ratios (OR) and the 95% confidence intervals (CI) between copeptin and outcomes of interest were estimated by logistic regression. Measures were grouped into three gestational age periods of blood draw as originally designated by the trial.17 To ensure that associations remained prospective in nature at later gestational ages, we excluded values measured after the date of diagnosis of PE or gestational hypertension. We also included only one measure per woman for each time window. Please see Supplemental materials for more details.
The crude PE analysis was repeated dividing samples into seven finer categories of gestational age. Mean log values and their standard errors (SE) were plotted along with p-values estimated using Wilcoxon-rank sum test. Lastly, differences in copeptin measure by time until diagnosis was explored by pairing cases with control samples of similar gestational age at blood collection. For these analyses samples measured at time of diagnosis or past time of diagnosis were grouped as “0 weeks” from diagnosis. P-values between mean levels of copeptin at each of these time points were calculated using Wilcoxon-rank sum test.
Results
Women with PE were significantly more likely to have higher BMI and deliver lower birthweight babies (Table 1). Among women with preterm and term PE, 43 (61%) and 23 (24%) cases were severe, respectively. No significant differences in gestational age for the first and second blood draw by case status were found. In bivariate analyses, greater copeptin levels were significantly (p<0.05) associated with younger maternal age, African American race, having never married, not having private health insurance and clinical site (data not shown).
Table 1.
Characteristics of Participants by Preeclampsia and Gestational Hypertensive Status in the CPEP Study
| Characteristic | Controls | Preterm PE | Term PE | All PE | GH |
|---|---|---|---|---|---|
| N | 136 | 71 | 98 | 169 | 101 |
| Age (years) | 20.4 (3.7) | 21.0 (4.5) | 20.6 (4.5) | 20.7 (4.5) | 21.6 (5.6) |
| Weight (kg) | 66.6 (14.3) | 72.2 (19.1)* | 72.3 (17.1)† | 72.3 (17.9)† | 71.7 (17.2)* |
| BMI (kg/m2) | 25.5 (5.6) | 28.2 (7.2)† | 27.6 (5.7)† | 27.9 (6.4)‡ | 27.0 (5.7) |
| Current smoker, n(%) | 10 (7.4) | 6 (8.5) | 15 (15.3) | 21 (12.4) | 10 (9.9) |
| Race, n(%) | |||||
| White non-Hispanic | 47 (34.6) | 19 (26.8) | 32 (32.7) | 51 (30.2) | 38 (37.6) |
| Black | 63 (46.3) | 37 (52.1) | 53 (54.1) | 90 (53.3) | 47 (46.5) |
| Hispanic or other race | 26 (19.1) | 15 (21.1) | 13 (13.3) | 28 (16.6) | 16 (15.8) |
| Less than high school education, n(%) | 60 (44.1) | 29 (40.8) | 46 (46.9) | 75 (44.4) | 44 (43.6) |
| No private insurance, n(%) | 118 (86.8) | 64 (90.1) | 94 (95.9)* | 158 (93.5)* | 93 (92.1) |
| Married, n(%) | 30 (22.1) | 19 (26.8) | 17 (17.3) | 36 (21.3) | 26 (26.0) |
| Male infant, n(%) | 63 (46.3) | 38 (53.5) | 48 (49.0) | 86 (50.9) | 53 (52.5) |
| Birthweight (g) | 3314 (445) | 2126 (731)‡ | 3316 (494) | 2816 (843)‡ | 3359 (593) |
| Gestational age at delivery (weeks) | 39.7 (1.5) | 34.8 (2.9)‡ | 39.7 (1.2) | 37.6 (3.2)‡ | 39.7 (1.6) |
| Randomized to calcium, n(%) | 68 (50.0) | 40 (56.3) | 35 (35.7)* | 75 (44.4) | 52 (51.5) |
| Baseline copeptin (pmol/l) | 3.8 (2.3) | 5.4 (4.7)† | 4.9 (4.3)* | 5.1 (4.5)‡ | 4.1 (2.9) |
| Gestational age of 1st sample(weeks) | 16.0 (2.6) | 15.7 (2.9) | 16.0 (2.7) | 15.9 (2.8) | 16.2 (2.3) |
| Gestational age of 2nd sample (weeks) | 27.7 (1.5) | 27.6 (1.5) | 27.4 (1.6) | 27.2 (1.5) | 27.5 (1.7) |
| Gestational age of 3rd sample (weeks) | 36.5 (0.8) | 35.6 (0.9)† | 36.4 (0.6) | 36.3 (0.7)* | 36.5 (0.7) |
Mean (SD) unless specified.
Abbreviations: PE, preeclampisa, GH, gestational hypertension
p<0.05
p<0.01
p<0.001 for tests of difference between groups with pregnancy complications versus the control group.
Marital status was missing for one woman in the gestational hypertensive group.
The median (interquartile range) of baseline log copeptin levels among PE cases and controls measured on average at 16 weeks of gestation were 3.85 (2.57–6.30) and 3.15 (2.17–4.72) pmol/l, respectively. Baseline levels were significantly and positively associated with risk of PE before its diagnosis (Table 2). Each log unit increase in baseline copeptin was associated with increased risk of PE (OR 1.61), with similar associations for preterm and term cases. Adjustment for covariates slightly attenuated the associations and term PE risk was no longer significantly elevated while preterm PE remained associated with copeptin. Repeating analyses excluding HELLP syndrome cases or PE cases with concurrent GDM did not impact results (data not shown). Higher copeptin levels were associated with increased risk of preterm birth not affected by PE; however, the association did not persist after adjustment for race (Table 2). In addition, among those who had SGA without PE (n=22, some with other complications: 6 with GH, 3 GDM, 2 preterm) we found no difference in mean baseline levels of copeptin (geometric means of 2.91 for SGA versus 3.21 for non-SGA, p=0.46). Neither were differences observed in the risk of SGA (p>0.3) when compared to the controls at any time during pregnancy. Hence, differences observed for PE are not due to SGA or preterm birth. Copeptin was not associated with risks of gestational hypertension or GDM. The second and third copeptin measures were also associated with risk of PE.(Table 2)
Table 2.
Cross-sectional associations between log copeptin levels and risk of pregnancy complications, CPEP case-cohort
| Outcomes | N* | Crude OR (95% CI) | p-value | Adjusted OR (95% CI)† | p-value |
|---|---|---|---|---|---|
| Baseline copeptin (<22 weeks GA) | vs. 136 controls | ||||
| Preeclampsia (all cases) | 158 | 1.61 (1.11–2.33) | 0.01 | 1.55 (1.03–2.31) | 0.03 |
| Preterm PE (diagnosis <37 weeks) | 63 | 1.73 (1.06–2.83) | 0.03 | 1.86 (1.08–3.20) | 0.03 |
| Term PE (diagnosis ≥37 weeks) | 95 | 1.58 (1.03–2.44) | 0.04 | 1.45 (0.91–2.32) | 0.12 |
| Gestational Hypertension | 101 | 1.20 (0.78–1.86) | 0.41 | 1.33 (0.83–2.13) | 0.24 |
| Gestational diabetes | 92 | 0.87 (0.56–1.36) | 0.55 | 1.23 (0.72–2.12) | 0.45 |
| Preterm birth without PE | 86 | 1.83 (1.14–2.94) | 0.01 | 1.46 (0.87–2.46) | 0.15 |
| Second copeptin (22–32 weeks GA) | vs. 115 controls | ||||
| Preeclampsia (all cases) | 144 | 2.07 (1.33–3.21) | 0.001 | 2.06 (1.28–3.31) | 0.003 |
| Preterm PE (diagnosis <37 weeks) | 57 | 2.59 (1.47–4.57) | 0.001 | 2.76 (1.45–5.10) | 0.001 |
| Term PE (diagnosis ≥37 weeks) | 87 | 1.70 (1.05–2.76) | 0.03 | 1.68 (0.99–2.86) | 0.05 |
| Gestational hypertension | 92 | 1.32 (0.83–2.11) | 0.24 | 1.37 (0.83–2.28) | 0.22 |
| Gestational diabetes | 81 | 1.10 (0.69–1.75) | 0.69 | 1.25 (0.72–2.16) | 0.43 |
| Third copeptin (33–38 weeks GA) | vs. 110 controls | ||||
| Preeclampsia (all cases) | 99 | 2.64 (1.58–4.40) | 0.0002 | 2.52 (1.42–4.50) | 0.002 |
| Preterm PE (diagnosis <37 weeks) | 14 | 5.16 (1.87–14.27) | 0.002 | 8.56 (1.97–37.24) | 0.004 |
| Term PE (diagnosis ≥37 weeks) | 85 | 2.37 (1.38–4.07) | 0.002 | 2.40 (1.28–4.50) | 0.007 |
| Gestational hypertension | 77 | 1.23 (0.75–2.03) | 0.41 | 1.53 (0.85–2.73) | 0.15 |
Abbreviation: GA, gestational age; PE, preeclampsia; OR, odds ratio
Four term PE cases and 22 gestational hypertension cases also had GDM.
Adjusted for age, race, BMI, current smoking, married, private insurance, and clinical site
Because associations became progressively stronger closer to delivery, we investigated more finely divided categories of gestational age (Figure 1) and associations by time to diagnosis (Figure 2). As seen in Figure 1, copeptin levels increased with gestational age among both cases and controls. Copeptin levels were generally higher for cases at each cross-sectional comparison than controls although some of the time points with sparse data did not reach statistical significance and the difference in mean log copeptin levels was smaller for term than preterm cases. Mean copeptin levels increasingly differed by case, especially after 30 weeks of gestation. Mean levels did not differ by case until closer to time of diagnosis although differences were borderline significant (p=0.05) among preterm PE cases for samples taken from 9 to 14 weeks before diagnosis (Figure 2). Samples at or after diagnosis (time “0” in Figure 2) strongly differed among both term and preterm cases.
Figure 1.
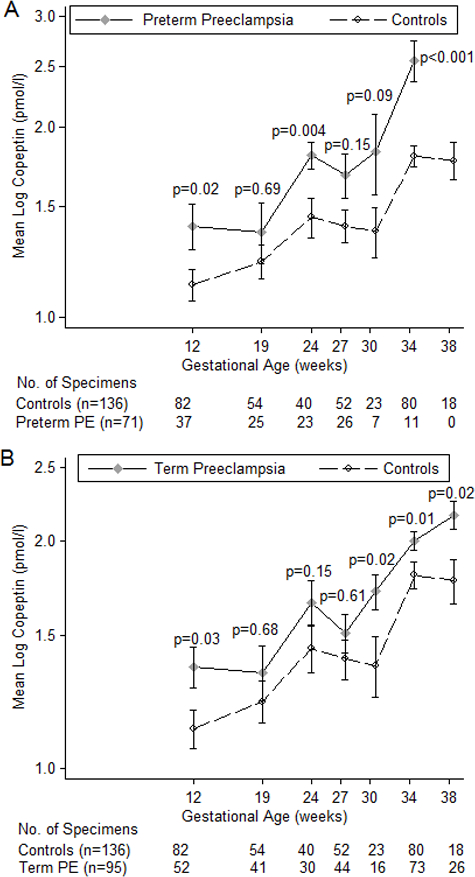
Mean log copeptin levels (± SE) over gestational age for PE cases diagnosed before 37 weeks (1a) and cases diagnosed after 37 weeks (1b) compared to controls in the Calcium for Prevention of Preeclampsia study
Figure 2.
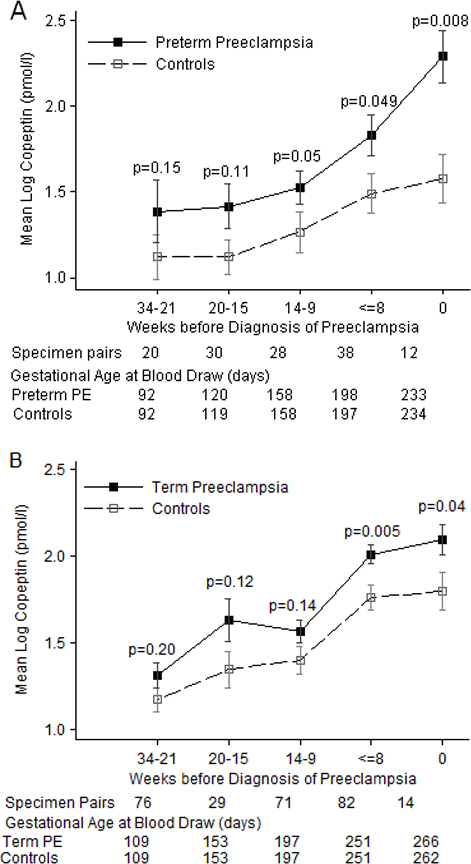
Mean log copeptin levels (± SE) by time before diagnosis of preeclampsia among cases diagnosed before (2a) or after (2b) 37 weeks gestation compared to controls in the Calcium for Prevention of Preeclampsia study
Copeptin levels had slightly stronger associations with severe than mild PE (Supplemental table S1) but confidence intervals overlapped. Reclassification of definitions for hypertension which include systolic blood pressure ≥140 mmHg as a diagnostic criterion also did not alter findings (Table S1). Lastly, among 56 women with measured sFlt-1 and PlGF before 22 weeks (42 of whom were PE cases), neither sFlt-1 (r=0.10, p=0.45) or PlGF (r=−0.08, p=0.54) were correlated with copeptin measures. Nor were significant correlations observed at later gestational age (data not shown).
Discussion
Copeptin is a biomarker of arginine vasopressin which acts on multiple systems to increase blood pressure and water retention. As a potential biomarker of PE, we prospectively evaluated copeptin levels among women who consequently developed PE and other pregnancy complications. Higher copeptin concentrations, measured before recognition of clinical disease, were associated with increased PE risk. Levels of copeptin increased across gestation regardless of PE case status but differed more markedly by case status closer to the time of diagnosis. Copeptin was not associated with GDM or gestational hypertension apart from PE, suggesting it was specific to development of PE.
Vasopressin secretion and metabolism are altered in normal pregnancy, with a rise in secretion very early in pregnancy.6 Vasopressinase produced by the placenta increases dramatically over pregnancy, leading to increased vasopressin clearance through cleavage of vasopressin.21 In fact, vasopressin is cleared 3–4 times faster after mid-gestation but plasma levels are maintained suggesting increased production rates.22 Our observation that copeptin increases over gestation supports copeptin as a good biomarker for vasopressin secretion.
Vasopressin function is regulated by three receptor subtypes.5 Although the V1a receptor function is suspected to play a role in hypertension, studies have not been able to find strong evidence.23 Rather, decline in kidney function has more recently been found to be associated with vasopressin via measurement of copeptin levels in large epidemiologic studies of non-pregnant individuals.23 Multiple animal studies suggest that vasopressin’s link with kidney function is causal23; for instance, blocking vasopressin V1a or V2 receptors protects the deterioration of kidney function in rat models for nephropathy.24, 25 Conversely, administration of desmopressin, a powerful V2-receptor agonist, in rats and humans increases GFR and urinary albumin excretion.26 In our pregnancy observations, it would seem women with gestational hypertension (i.e., those who avoid kidney damage despite elevated blood pressure) do not overly secrete vasopressin above and beyond that of normal pregnancy. This once again confirms the differences in pathophysiology of the two conditions.3 Among women who come to be diagnosed with PE, copeptin may serve as an early marker of the possible impact on renal function.
Studies measuring vasopressin directly in human pregnancy have not been conclusive. Risberg et al. found non-significantly higher levels of plasma vasopressin in weeks 12 and 24 of gestation and lower concentrations among mild PE cases at 36 weeks.27 Yet another study observed decreases in pregnancy, possibly due to vasopressinase metabolism.28 We believe our findings using copeptin as a more stable biomarker of vasopressin provide a more accurate picture of vasopressin in pregnancy and in association with PE.
Few studies have measured copeptin with regards to PE.29–32 The first study observed significant differences in copeptin levels between 64 PE cases and 32 normotensive controls but authors provided no information on time of PE diagnosis and cases may have been diagnosed prior to blood sampling.31 Three other studies have also observed increased maternal copeptin among PE cases.29, 30, 32 Santillan et al. prospectively observed a significant difference in copeptin levels beginning in the first trimester between 20 PE cases and 26 controls.32 They also observed using an animal model that infusion of vasopressin during pregnancy in mice leads to the clinical phenotypes observed in PE.32 Our findings remain novel with regards to investigating the prospective association in a larger sample of PE cases and including other pregnancy complications.
Vasopressin affects glucose homeostasis.33 Epidemiologic evidence have observed associations between elevated copeptin levels and increased risk of incident type 2 diabetes.16, 34 Despite these findings, our study showed no association between copeptin and risk of GDM. A lack of association has also been observed in another study conducted at delivery.35 We add to these findings that copeptin levels do not differ prior to diagnosis of GDM.
To our knowledge, our study is the largest study to date investigating copeptin levels during pregnancy with risk of multiple pregnancy complications. Prospective measurements allowed for investigations into the effect of gestational age and associations in relation to timing of diagnosis. The inclusion of only healthy primiparas may preclude generalizability of results for cases of recurrent PE. We also did not have sufficient numbers of cases of SGA without PE (n=22) to completely rule out an independent contribution of SGA. In addition, despite the prospective nature of the study, we cannot decipher whether elevation in vasopressin secretion was secondary to endothelial dysfunction caused by placental disease as angiogenic factors were measured at the same time as copeptin. Our limited observation that copeptin was not correlated with measured angiogenic factors suggests copeptin may provide additional information. A good biomarker for PE remains elusive and even angiogenic factors such as soluble endoglin and sFlt-1 both minimally impact prediction when examined against other clinical risk factors.36–39
Perspectives
Copeptin levels are elevated prior to diagnosis of PE. Levels progressively increase throughout pregnancy among normal pregnant women but significantly higher levels were observed only among women who develop PE and not for cases of gestational hypertension or GDM. Our observations add to the understanding of the role of vasopressin on the pathophysiology of PE.
Supplementary Material
Novelty and Significance.
What is new?
Maternal serum levels of copeptin, a biomarker for vasopressin, rise prior to clinical diagnosis of preeclampsia. Early pregnancy levels did not differ for other pregnancy complications such as gestational diabetes or gestational hypertension without proteinuria, or for preterm birth not complicated by PE suggesting that copeptin may serve as a specific biomarker for preeclampsia. Copeptin levels also rose as gestational age increased. This observation agrees with what has been shown for levels of vasopressin during pregnancy.
What is relevant?
As copeptin serves as a stable biomarker for vasopressin, our findings indicate that vasopressin may be dysregulated in cases of preeclampsia prior to its clinical diagnosis. Copeptin is associated with declining renal function in non-pregnant populations and this supports the notion that vasopressin is important in development of preeclampsia.
Acknowledgments
Sources of Funding
Supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health. The CPEP trial was supported by contracts (N01-HD-1-3121, −3122, −3123, −3124, −3125, and −3126; N01-HD-3154; and N01-HD-5-3246) with the National Institute of Child Health and Human Development, with cofunding from the National Heart, Lung, and Blood Institute. Recent biomarker assays including for copeptin was supported by contract (HHSN275201100002I-HHSN27500003) with the National Institute of Child Health and Human Development.
Footnotes
Disclosures
None to declare.
References
- (1).Steegers EA, von DP, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet 2010; 376:631–644. [DOI] [PubMed] [Google Scholar]
- (2).Duley L, Meher S, Abalos E. Management of pre-eclampsia. BMJ 2006; 332:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Naljayan MV, Karumanchi SA. New developments in the pathogenesis of preeclampsia. Adv Chronic Kidney Dis 2013; 20:265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Koshimizu TA, Nasa Y, Tanoue A, Oikawa R, Kawahara Y, Kiyono Y, Adachi T, Tanaka T, Kuwaki T, Mori T, Takeo S, Okamura H, Tsujimoto G. V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc Natl Acad Sci U S A 2006; 103:7807–7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Koshimizu TA, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, Tanoue A. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev 2012; 92:1813–1864. [DOI] [PubMed] [Google Scholar]
- (6).Schrier RW. Systemic arterial vasodilation, vasopressin, and vasopressinase in pregnancy. J Am Soc Nephrol 2010; 21:570–572. [DOI] [PubMed] [Google Scholar]
- (7).Tkachenko O, Shchekochikhin D, Schrier RW. Hormones and Hemodynamics in Pregnancy. Int J Endocrinol Metab 2014; 12:e14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Durr JA, Stamoutsos B, Lindheimer MD. Osmoregulation during pregnancy in the rat. Evidence for resetting of the threshold for vasopressin secretion during gestation. J Clin Invest 1981; 68:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Barron WM, Durr J, Stamoutsos BA, Lindheimer MD. Osmoregulation and vasopressin secretion during pregnancy in Brattleboro rats. Am J Physiol 1985; 248:R29–R37. [DOI] [PubMed] [Google Scholar]
- (10).Grazzini E, Breton C, Derick S, Andres M, Raufaste D, Rickwaert F, Boccara G, Colson P, Guerineau NC, Serradeil-le GC, Guillon G. Vasopressin receptors in human adrenal medulla and pheochromocytoma. J Clin Endocrinol Metab 1999; 84:2195–2203. [DOI] [PubMed] [Google Scholar]
- (11).Krege JH, Katz VL. A proposed relationship between vasopressinase altered vasopressin and preeclampsia. Med Hypotheses 1990; 31:283–287. [DOI] [PubMed] [Google Scholar]
- (12).Gordge MP, Williams DJ, Huggett NJ, Payne NN, Neild GH. Loss of biological activity of arginine vasopressin during its degradation by vasopressinase from pregnancy serum. Clin Endocrinol (Oxf) 1995; 42:51–58. [DOI] [PubMed] [Google Scholar]
- (13).Baumann G, Dingman JF. Distribution, blood transport, and degradation of antidiuretic hormone in man. J Clin Invest 1976; 57:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 2006; 52:112–119. [DOI] [PubMed] [Google Scholar]
- (15).Gordge MP, Robson SC, Williams DJ, Payne NN, Neild GH. Serum Vasopressinase and Platelet Responses to Arginine Vasopressin in Normal Pregnancy, Pregnancy-induced Hypertension and Pre-eclampsia. Platelets 1994; 5:90–95. [DOI] [PubMed] [Google Scholar]
- (16).Enhorning S, Wang TJ, Nilsson PM, Almgren P, Hedblad B, Berglund G, Struck J, Morgenthaler NG, Bergmann A, Lindholm E, Groop L, Lyssenko V, Orho-Melander M, Newton-Cheh C, Melander O. Plasma copeptin and the risk of diabetes mellitus. Circulation 2010; 121:2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Levine RJ, Esterlitz JR, Raymond EG, DerSimonian R, Hauth JC, Ben CL, Sibai BM, Catalano PM, Morris CD, Clemens JD, Ewell MG, Friedman SA, Goldenberg RL, Jacobson SL, Joffe GM, Klebanoff MA, Petrulis AS, Rigau-Perez JG. Trial of Calcium for Preeclampsia Prevention (CPEP): rationale, design, and methods. Control Clin Trials 1996; 17:442–469. [DOI] [PubMed] [Google Scholar]
- (18).Levine RJ, Hauth JC, Curet LB, Sibai BM, Catalano PM, Morris CD, DerSimonian R, Esterlitz JR, Raymond EG, Bild DE, Clemens JD, Cutler JA. Trial of calcium to prevent preeclampsia. N Engl J Med 1997; 337:69–76. [DOI] [PubMed] [Google Scholar]
- (19).Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006; 355:992–1005. [DOI] [PubMed] [Google Scholar]
- (20).Joffe GM, Esterlitz JR, Levine RJ, Clemens JD, Ewell MG, Sibai BM, Catalano PM. The relationship between abnormal glucose tolerance and hypertensive disorders of pregnancy in healthy nulliparous women. Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol 1998; 179:1032–1037. [DOI] [PubMed] [Google Scholar]
- (21).Braunstein G Endocrine Changes in Pregnancy. In: Melmed S, Polonsky KS, Larsen RP, Kronenberg HM, editors. Williams Textbook of Endocrinology 12th ed. Philadelphia: Saunders Elsevier; 2011. p. 819–32. [Google Scholar]
- (22).Davison JM, Sheills EA, Barron WM, Robinson AG, Lindheimer MD. Changes in the metabolic clearance of vasopressin and in plasma vasopressinase throughout human pregnancy. J Clin Invest 1989; 83:1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Bankir L, Bouby N, Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol 2013; 9:223–239. [DOI] [PubMed] [Google Scholar]
- (24).Okada H, Suzuki H, Kanno Y, Yamamura Y, Saruta T. Effects of vasopressin V1 and V2 receptor antagonists on progressive renal failure in rats. Clin Sci (Lond) 1994; 86:399–404. [DOI] [PubMed] [Google Scholar]
- (25).Okada H, Suzuki H, Kanno Y, Saruta T. Evidence for the involvement of vasopressin in the pathophysiology of adriamycin-induced nephropathy in rats. Nephron 1996; 72:667–672. [DOI] [PubMed] [Google Scholar]
- (26).Bardoux P, Bichet DG, Martin H, Gallois Y, Marre M, Arthus MF, Lonergan M, Ruel N, Bouby N, Bankir L. Vasopressin increases urinary albumin excretion in rats and humans: involvement of V2 receptors and the renin-angiotensin system. Nephrol Dial Transplant 2003; 18:497–506. [DOI] [PubMed] [Google Scholar]
- (27).Risberg A, Olsson K, Lyrenas S, Sjoquist M. Plasma vasopressin, oxytocin, estradiol, and progesterone related to water and sodium excretion in normal pregnancy and gestational hypertension. Acta Obstet Gynecol Scand 2009; 88:639–646. [DOI] [PubMed] [Google Scholar]
- (28).Eguchi K, Oguni N, Sawai T, Yonezawa M. Comparison of plasma concentrations of arginine vasopressin (AVP) and atrial natriuretic peptide (ANP) in normal and preeclamptic pregnancies. J Perinat Med 1996; 24:437–443. [DOI] [PubMed] [Google Scholar]
- (29).Foda AA, bdel Aal IA. Maternal and neonatal copeptin levels at cesarean section and vaginal delivery. Eur J Obstet Gynecol Reprod Biol 2012; 165:215–218. [DOI] [PubMed] [Google Scholar]
- (30).Wellmann S, Benzing J, Fleischlin S, Morgenthaler N, Fouzas S, Buhrer CA, Szinnai G, Burkhardt T, Lapaire O. Cardiovascular Biomarkers in Preeclampsia at Triage [published online ahead of print May 17, 2004] Fetal Diagn Ther doi: 10.1159/000361016. [DOI] [PubMed] [Google Scholar]
- (31).Zulfikaroglu E, Islimye M, Tonguc EA, Payasli A, Isman F, Var T, Danisman N. Circulating levels of copeptin, a novel biomarker in pre-eclampsia. J Obstet Gynaecol Res 2011; 37:1198–1202. [DOI] [PubMed] [Google Scholar]
- (32).Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, Leslie KK, Hunter SK, Zamba GK, Gibson-Corley KN, Grobe JL. Vasopressin in Preeclampsia: A Novel Very Early Human Pregnancy Biomarker and Clinically Relevant Mouse Model [published online ahead of print July 7, 2004]. Hypertension doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Fujiwara Y, Hiroyama M, Sanbe A, Aoyagi T, Birumachi J, Yamauchi J, Tsujimoto G, Tanoue A. Insulin hypersensitivity in mice lacking the V1b vasopressin receptor. J Physiol 2007; 584:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Abbasi A, Corpeleijn E, Meijer E, Postmus D, Gansevoort RT, Gans RO, Struck J, Hillege HL, Stolk RP, Navis G, Bakker SJ. Sex differences in the association between plasma copeptin and incident type 2 diabetes: the Prevention of Renal and Vascular Endstage Disease (PREVEND) study. Diabetologia 2012; 55:1963–1970. [DOI] [PubMed] [Google Scholar]
- (35).Oncul M, Tuten A, Kucur M, Imamoglu M, Ekmekci OB, Acikgoz AS, Madazli R. Copeptin concentrations are not elevated in gestational diabetes mellitus. Arch Gynecol Obstet 2013; 288:1045–1049. [DOI] [PubMed] [Google Scholar]
- (36).Boucoiran I, Thissier-Levy S, Wu Y, Wei SQ, Luo ZC, Delvin E, Fraser WD, Audibert F. Risks for preeclampsia and small for gestational age: predictive values of placental growth factor, soluble fms-like tyrosine kinase-1, and inhibin A in singleton and multiple-gestation pregnancies. Am J Perinatol 2013; 30:607–612. [DOI] [PubMed] [Google Scholar]
- (37).Kleinrouweler CE, Wiegerinck MM, Ris-Stalpers C, Bossuyt PM, van der Post JA, von DP, Mol BW, Pajkrt E. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG 2012; 119:778–787. [DOI] [PubMed] [Google Scholar]
- (38).Myatt L, Clifton RG, Roberts JM, Spong CY, Hauth JC, Varner MW, Thorp JM Jr., Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Iams JD, Sciscione A, Harper M, Tolosa JE, Saade G, Sorokin Y, Anderson GD. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet Gynecol 2012; 119:1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Myers JE, Kenny LC, McCowan LM, Chan EH, Dekker GA, Poston L, Simpson NA, North RA. Angiogenic factors combined with clinical risk factors to predict preterm pre-eclampsia in nulliparous women: a predictive test accuracy study. BJOG 2013; 120:1215–1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


