Abstract
Background:
Despite limited recommendations for using sodium bicarbonate (SB) during cardiopulmonary resuscitation (CPR), we hypothesized that SB continues to be used frequently during pediatric in-hospital cardiac arrest (IHCA) and that its use varies by hospital-specific, patient-specific, and event-specific characteristics.
Methods:
We analyzed 3719 pediatric (<18 years) index pulseless CPR events from the American Heart Association Get With The Guidelines-Resuscitation database from 1/2000 to 9/2010.
Results:
SB was used in 2536 (68%) of 3719 CPR events. Incidence of SB use between 2000 and 2005 vs. 2006 and 2010 was 71.1% vs. 66.2% (P = 0.002). The primary outcome was survival to discharge. Secondary outcomes included 24-h survival and neurologic outcome. Multivariable logistic regression analyzed the association between SB use and outcomes. SB had increased use an ICU location, metabolic/electrolyte disturbance, prolonged CPR, pVT/VF, and concurrently with other pharmacologic interventions. Adjusting for confounding factors, SB use was associated with decreased 24-h survival (aOR 0.83, 95% CI: 0.69, 0.99) and decreased survival to discharge (aOR 0.80; 95% CI: 0.65, 0.97). Inclusion of metabolic/electrolyte abnormalities, hyperkalemia, and toxicologic abnormalities only (n = 674), SB use was not associated with worse outcomes or unfavorable neurologic outcome.
Conclusions:
SB is used frequently during pediatric pulseless IHCA, yet there is a significant trend toward less routine use over the last decade. Because SB is more likely to be used in an ICU, with prolonged CPR, and concurrently with other pharmacologic interventions; its use during CPR may be associated with poor prognosis due to an association with “last ditch” efforts of resuscitation rather than causation.
Keywords: Cardiopulmonary resuscitation, Cardiac arrest, Pediatrics, Sodium bicarbonate, Survival
1. Introduction
The use of sodium bicarbonate (SB) during adult and pediatric cardiopulmonary resuscitation (CPR) is discouraged because of a lack of evidence of benefit, and concerns regarding possible harm. The rationale for its use in cardiopulmonary arrest (CPA) was based on the premise that acidemia impairs myocardial performance and attenuates heart rate, cardiac contractility and thus cardiac output in response to catecholamines. However, adult randomized controlled trials failed to demonstrate the benefit of buffer therapy in adult out-of-hospital cardiac arrest,1–3 and a recent adult study even failed to demonstrate the benefit of using SB during prolonged CPR.4 There is little data that supports the use of SB during adult cardiac arrest, and no substantive data to support its use in the resuscitation of children in cardiac arrest.
The 2005 American Heart Association’s (AHA) Advanced Cardiac Life Support (ACLS) and Pediatric Advanced Life Support (PALS) guidelines state that the routine administration of SB has not been shown to improve outcome of resuscitation (Class Indeterminate), but may be used in certain resuscitation situations, such as pre-existing metabolic acidosis, hyperkalemia, or tricyclic antidepressant overdose. SB may be considered for prolonged arrest after effective ventilations and chest compressions and epinephrine have been administered (Class III, Level of Evidence B).5,6 In the 2010 ACLS and PALS guidelines the routine administration of SB was not recommended in cardiac arrest (Class III, Level of Evidence B), but could be administered for treatment of some toxidromes or certain resuscitation situations such as hyperkalemic cardiac arrest. The administration of SB for prolonged arrest was no longer recommended.7
Specific patterns of SB use during pediatric in-hospital cardiac arrest (IHCA), and its effect on survival have not been reported since these guidelines were published. The American Heart Association’s Get With The Guidelines®-Resuscitation (GWTG-R) database is a large, multicenter registry that prospectively and rigorously documents adult and pediatric IHCA.8 We conducted this study using the GWTG-R registry to characterize SB use during pediatric pulseless IHCA. We hypothesized that SB continues to be used frequently during pediatric IHCA and that its use varies by hospital-specific, patient-specific, and event-specific characteristics.
2. Methods
Study patients were derived from the GWTG-R registry (formerly known as the National Registry of Cardiopulmonary Resuscitation or NRCPR). This is a large, hospital-based, clinical registry of in-hospital cardiac arrests that is sponsored by the AHA with voluntary, fee-based membership. Its design was previously described in detail.9,10 Hospitals voluntarily participate in the database for the primary purpose of quality improvement, and as such are not required to obtain institutional review board (IRB) approval or informed consent from patients or families. The present study was exempted from IRB oversight at Medical City Children’s Hospital
2.1. Study population
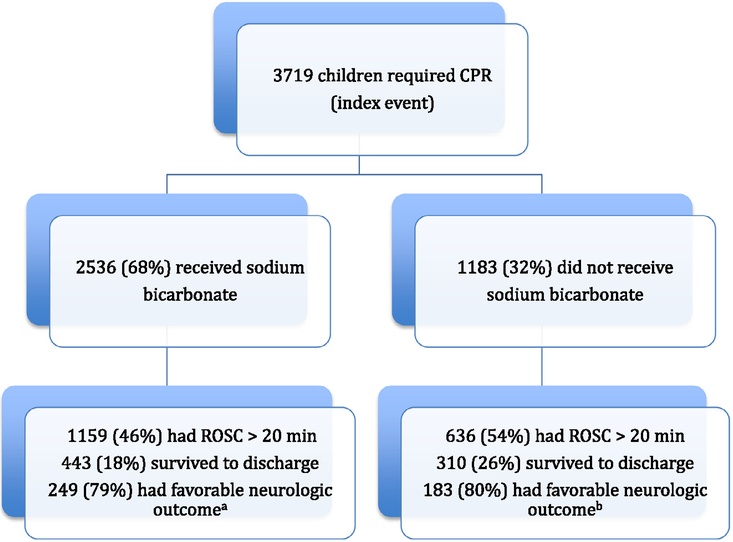
Between January 1, 2000, and September 14, 2010, we identified 3719 patients who were <18 years of age with an index in-hospital cardiac arrest from 289 participating hospitals (Fig. 1). All patients <18 years who experienced IHCA that required CPR at participating institutions were eligible for this study. An event was defined as an arrest that required chest compressions and/or defibrillation. An index event was defined as the patient’s first CPA that required CPR during hospitalization. Only index events were eligible for inclusion in the study. We excluded events in the delivery room (n = 665), newborn nursery (n = 72), or unknown location (n = 1), events in which epinephrine was not given (n = 1729), events in which a pulse was never lost (n = 1771), events with unknown CPR duration (n = 155), CPR duration <5 min (n = 468), CPR duration >120 min (n = 97), and events with no survival to hospital discharge data (n = 155). Some events met multiple exclusion criteria. Patients with very short duration of CPR and those patients with very, very prolonged duration of CPR were excluded as both of these groups are ‘contaminants’. Events with a CPR duration <5 min only very rarely require multiple medications (and rarely would receive SB), and are associated with very good outcome. Inclusion of this ‘patient population’ is a major contributor to better outcome in the non-bicarbonate group. Likewise, events that require prolonged CPR duration are more likely to include multiple doses of medications, including epinephrine, SB, calcium chloride, etc., and are associated with decreasing likelihood of ROSC the longer CPR is performed. Inclusion of this ‘patient population’ would be a major contributor to worse outcome in the sodium bicarbonate group.
Fig. 1.
Enrollment and outcomes. ROSC – restoration of spontaneous circulation. aData obtained from 602 patients. bData obtained from 1648 patients
After applying the exclusion criteria, there were 3719 events brought forward for analysis. Metabolic/electrolyte abnormality as defined by GWTG-R registry was any of the following within 4 h up to the time of the event: sodium <125 or >150 mEquiv./L, potassium <2.5 or >6 mEquiv./L, arterial pH <7.3 or >7.5, lactate >2.5 mmol/, blood glucose <60 mg/dL; and for (newborn/neonate) acidosis (pH <7.2 arterial, venous or capillary), ionized calcium <1 mmol/L or <4 mg/dL, glucose <40 mg/dL, sodium <125 mEquiv./L, magnesium >4 mEquiv./L, and potassium >6.5 mEquiv./L. Toxicologic abnormalities (adverse drug effect/reaction, drug overdose, poisoning) were defined according to GWTG-R registry as toxins, poisons, drug or medications taken by the patient or administered by staff.
2.2. Outcome measures
The prospectively selected primary outcome measure was survival to hospital discharge. The secondary outcome measures included survival of event (defined as restoration of spontaneous circulation for >20 min), 24-h survival, and neurologic outcome. The neurologic outcome was determined according to the pediatric cerebral performance category (PCPC) scale as follows: (1) a normal neurologic state, (2) mild disability, (3) moderate disability, (4) severe disability, (5) coma or vegetative state, and (6) death.11,12 Neurologic status before the arrest and at discharge was determined by chart review. A favorable neurologic outcome was defined by a discharge PCPC score of 1, 2, or 3.13
2.3. Statistical analysis
All statistical analyses were performed with a commercially available statistical package (SAS Institute Inc., Cary, NC, USA). Data were examined for normality assumption. Since all continuous variables are not normally distributed, median and inter quartile range were presented. Differences between groups were analyzed by the Wilcoxon rank-sum test for continuous variables and the Chi-squared test for dichotomous variables. Hospital, patient, and event variables associated with SB use by univariate analysis (P < .20) were included in stepwise multivariable logistic regression analysis. CPR duration was entered into the model as a continuous variable.14,15 Finally, all factors associated with primary and secondary outcomes on univariate analysis (P < .20) were included in stepwise multivariable logistic regression to describe the association of SB use with outcome measures adjusted for confounding factors. Odds ratios (ORs) with 95% confidence intervals (CIs) were reported. The sample size was not planned. A P-value of <0.05 was considered as statistically significant and all P-values are 2-sided.
3. Results
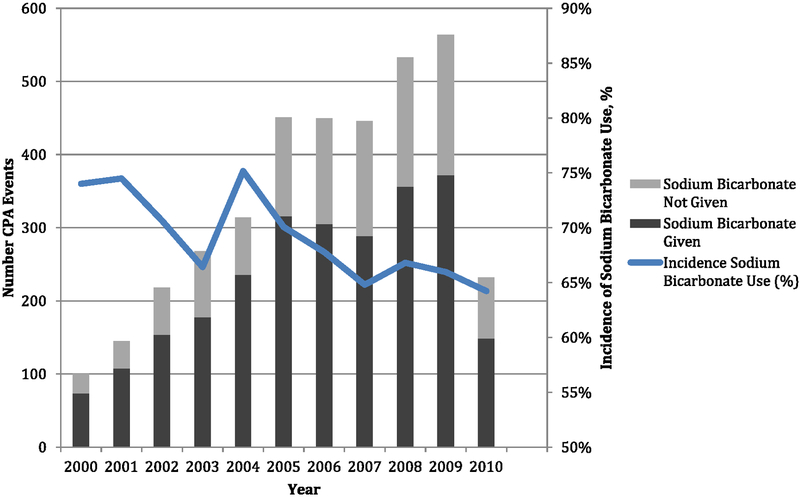
A total of 3719 index children received CPR for pediatric pulseless IHCA. Of these, 2536 (68%) received SB during CPR (Fig. 1). The incidence of SB use by age was 366 (59.4%) of 616 for infants <1 year of age, 822 (70.0%) of 1174 children age 1–7 years, and 1348 (69.9%) of 1929 children age 8–17 years. The incidence of SB use by years is depicted in Fig. 2. The incidence of SB use between 2000 and 2005 vs. 2006 and 2010 was 71.1% vs. 66.2% (P = 0.002). Pre-event and event characteristics of the patients that received SB during CPR were compared with those that did not receive SB during CPR (Tables 1 and 2). Cardiac illness category (both medical and surgical) was significantly associated with SB use during CPR, while the illness category of trauma and newborn were significantly less likely to be associated with SB use during CPR. In both groups, respiratory insufficiency and hypotension were the most common preexisting conditions. Hypotension, congestive heart failure, renal insufficiency, metabolic/electrolyte abnormalities, and septicemia were more common preexisting conditions among patients who received SB during CPR, compared with those who did not. A greater proportion of patients who subsequently received SB during CPR were on a vasoactive infusion at the time of the arrest, compared with those who did not receive SB (43% vs. 34%; P < .001). In both groups, acute respiratory insufficiency, hypotension, and arrhythmia were the most common immediate precipitating causes of the arrest; hypotension and metabolic/electrolyte abnormalities were more common immediate factors related to the arrest for patients who received SB during CPR compared with those who did not.
Fig. 2.
Year-wise sodium bicarbonate use during CPR. Arrow indicates publication of 2005 American Heart Association’s Guidelines for Pediatric Advanced Life Support.
Table 1.
Characteristics of the patients before cardiac arrest
| Characteristics | Sodium bicarbonate used (n = 2536) | Sodium bicarbonate not used (n = 1183) | P |
|---|---|---|---|
| Age, median (IQR), ya | 0.83(0.7) | 0.42(0.6) | <0.001 |
| Male gender, n (%) | 1448(57) | 657(55) | 0.371 |
| Weight, median (IQR), kga | 8.00(3.24) | 6.00(3.20) | <0.001 |
| White race, n (%)a | 1379(62) | 611(58) | 0.040 |
| Facility type | |||
| Pediatrica | 1054(42) | 446(38) | 0.025 |
| Adulta | 147(6) | 92(8) | 0.022 |
| Mixed | 1335(53) | 645(55) | 0.284 |
| Event location, n (%) | |||
| ICUa | 1823(72) | 763(65) | <0.001 |
| Operating room/PACUa | 81(3) | 69(6) | <0.001 |
| Emergency departmenta | 291(11) | 214(18) | <0.001 |
| Inpatient, monitoreda | 79(3) | 23(2) | 0.042 |
| Inpatient ward | 130(5) | 52(4) | 0.336 |
| Outpatient/ambulatory | 14(0.6) | 3(0.3) | 0.209 |
| Other | 118(5) | 59(5) | 0.656 |
| Illness category, n (%) | |||
| Medical, cardiaca | 399(16) | 139(12) | 0.001 |
| Medical, noncardiac | 957(38) | 433(37) | 0.500 |
| Surgical, cardiaca | 506(20) | 142(12) | <0.001 |
| Surgical, noncardiac | 180(7) | 81(7) | 0.778 |
| Newborna | 215(9) | 148(13) | <0.001 |
| Traumaa | 268(11) | 233(20) | <0.001 |
| Other/obstetric | 10(0.4) | 7(0.6) | 0.406 |
| Preexisting conditions, n (%) | |||
| Respiratory insufficiency | 1354(60) | 663(63) | 0.153 |
| Hypotensiona | 950(42) | 383(36) | <0.001 |
| Congestive heart failurea | 354(16) | 89(8) | <0.001 |
| Renal insufficiencya | 268(12) | 84(8) | <0.001 |
| Hepaticinsufficiency | 112(5) | 41(4) | 0.162 |
| Major traumaa | 208(9) | 131(12) | 0.005 |
| Metabolic or electrolyte abnormalitya | 486(22) | 164(16) | <0.001 |
| Septicemiaa | 421(19) | 146(14) | <0.001 |
| Pneumonia | 206(9) | 77(7) | 0.074 |
| Arrhythmia | 487(22) | 224(21) | 0.777 |
| Cardiac malformation acyanotic/cyanotic | 188(21) | 104(21) | 0.953 |
| Congenital malformation noncardiac | 107(12) | 66(13) | 0.431 |
| Mode of discovery of event, n (%) | |||
| Witnesseda | 2374(94) | 1072(91) | 0.001 |
| Monitored by ECG or pulse oximetiya | 2328(92) | 1022(86) | <0.001 |
| Interventions in place at time of event, n (%) | |||
| Vascular accessa | 1254(50) | 487(41) | <0.001 |
| Arterial cathetera | 740(29) | 293(25) | 0.005 |
| Assisted or mechanical ventilation | 1624(64) | 740(63) | 0.381 |
| Vasoactive infusiona | 1016(43) | 374(34) | <0.001 |
| Dialysis and extra corporeal therapies | 74(3) | 22(2) | 0.057 |
| Invasive airway | 1609(64) | 740(63) | 0.599 |
| Supplemental oxygen | 451(24) | 217(24) | 0.961 |
| Inhaled nitric oxide therapy | 89(5) | 37(4) | 0.455 |
| IV/IO infusion of antiarrhythmic | 85(4) | 29(3) | 0.135 |
IQR: interquartile range; ICU: intensive care unit; PACU: post-anesthesia care unit; ECG: electrocardiogram; IV/IO: intravenous/intraosseous.
P < 0.05.
Table 2.
Characteristics of cardiac arrest
| Characteristic | Sodium bicarbonate used (n = 2536) | Sodium bicarbonate not used (n = 1183) | P |
|---|---|---|---|
| Immediate factors related to event, n (%) | |||
| Acute respiratory insufficiency | 1141(49) | 556(50) | 0.323 |
| Hypotensiona | 1410(60) | 539(49) | <0.001 |
| Arrhythmia | 1125(48) | 494(45) | 0.086 |
| Metabolic or electrolyte disturbancea | 486(21) | 167(15) | <0.001 |
| Toxicologicproblem/overdose/poisoning | 24(1) | 7(0.8) | 0.236 |
| High potassium (neonate) | 20(13) | 9(11) | 0.805 |
| State of pulse during event, n (%) | |||
| Pulse absent throughout eventa | 1684(66) | 842(71) | 0.004 |
| Pulse initially present, subsequently absenta | 852(34) | 341(29) | 0.004 |
| First documented pulseless rhythm, n (%) | |||
| Asystoleb | 982(46) | 465(47) | 0.464 |
| Pulseless electrical activityb | 870(41) | 408(41) | 0.656 |
| Ventricular fibril la tionb | 173(8) | 62(6) | 0.080 |
| Pulseless ventricular tachycardiab | 119(6) | 50(5) | 0.586 |
| Unknown or not documented | 392(16) | 198(17) | 0.320 |
| Interval to initiation of CPR (min), (IQR)C | 0(0.0) | 0(0.0) | 0.359 |
| Duration of CPR (min), (IQR)a | 30(17.50) | 17(9.28) | <0.001 |
| Any VF/pulseless VT, n (%)a | 630(25) | 162(14) | <0.001 |
| Defibrillation attempted, n (%)a,d | 537(85) | 127(78) | 0.024 |
| Interval to first defibrillation (min), (IQR)e | 2(0.6) | 2(0.5) | 0.487 |
| Interval to invasive airway (min), (IQR)a,f | 5(2.10) | 6(3.10) | 0.033 |
| Interval VF to defibrillation (min), (IQR)g | 0(0.2) | 0(0.2) | 0.409 |
| Interval to first epinephrine dose (min), (IQR)a,h | 0(0.3) | 1 (0.4) | <0.001 |
| No. of epinephrine doses, (IQR)a,i | 4(3.7) | 3(2.4) | <0.001 |
| 1–2a | 480(21) | 505 (47) | <0.001 |
| 3–4 | 684(30) | 320(30) | 0.701 |
| 5–6a | 480(21) | 140(13) | <0.001 |
| 7–8a | 272(12) | 66(6) | <0.001 |
| 9–10a | 165(7) | 28(3) | <0.001 |
| >10a | 182(8) | 23(2) | <0.001 |
| Cardiac arrest at night (2300–0700)a | 758(30) | 392(33) | 0.046 |
| Cardiac arrest on weekend (2300 Friday-0700 Monday) | 761(30) | 389(33) | 0.077 |
| Pharmacologic interventions, n (%) | |||
| Fluida | 1230(49) | 420(36) | <0.001 |
| Dextrosea | 191(8) | 34(3) | <0.001 |
| Calciuma | 1641(65) | 249(21) | <0.001 |
| Vasopressina | 148(7) | 25(2) | <.0001 |
| Amiodaronea | 179(7) | 32(3) | <0.001 |
| Lidocainea | 292(12) | 70(6) | <0.001 |
| Magnesium sulfatea | 176(7) | 26(2) | <0.001 |
| Adenosine | 10(0.4) | 4(0.3) | 0.794 |
| THAM®j | 29(1) | 20(2) | 0.199 |
| Nonpharmacologic interventions, n (%) | |||
| Cardiopulmonary bypass/extracorporeal CPRa,k | 197(8) | 40(3) | <0.001 |
| Echo card iogra ma,l | 125(6) | 20(2) | <0.001 |
| Induced hypothermia initiated after ROSCm | 129(10) | 49(7) | 0.050 |
CPR: cardiopulmonary resuscitation; IQR: interquartile range; THAM: tromethamine; VF: ventricular fibrillation; VT: ventricular tachycardia
P < 0.05.
The data were obtained from 3129 patients.
The data were obtained from 3691 patients.
The data were obtained from 793 patients.
The data were obtained from 222 patients.
The data were obtained from 1150 patients.
The data were obtained from 615 patients.
The data were obtained from 3209 patients.
The data were obtained from 3345 patients.
The data were obtained from 3031 patients.
The data were obtained from 3682 patients.
The data were obtained from 3013 patients.
The data were obtained from 1928 patients.
Asystole and pulseless electrical activity (PEA) were the most common pulseless rhythm for both groups, with no differences seen in SB administration based on the etiology of the pulseless rhythm. The median duration of CPR was 30 min in the group that received SB, compared with 17 min in the group that did not receive SB (P < .001). Similarly, the median number of epinephrine doses administered was 4 in the group that received SB compared with 3 in the group that did not receive SB (P < .001). The types of CPR interventions were similar in both groups, although additional pharmacologic interventions (epinephrine, vasopressin, calcium, magnesium sulfate, amiodarone, lidocaine, dextrose, and fluids) were administered more often in the group that received SB during CPR.
The factors that were significantly associated with SB use during pediatric pulseless IHCA after controlling for duration of CPR by stepwise multivariable logistic regression are shown in Table 3. Sodium bicarbonate use during CPR was associated with arrests that occurred in an ICU location, events with vascular access already in place, and events with an immediate cause of metabolic/electrolyte disturbance. In addition, use of SB during CPR was independently associated with the duration of CPR, any pVT/VF during the event, and the concurrent use of other pharmacologic interventions (fluid, dextrose, calcium). Sodium bicarbonate was less likely to be used in pediatric only facilities, an illness category of newborn or trauma, and a pharmacologic intervention of only 1–2 doses of epinephrine.
Table 3.
Variables significantly associated with use of sodium bicarbonate.
| Variablea | Odds ratio (95% CI) | P |
|---|---|---|
| Hospital characteristics | ||
| Pediatric facility | 0.76(0.64,0.91) | 0.002 |
| Illness category | ||
| Trauma | 0.74 (0.57,0.96) | 0.025 |
| Newborn | 0.50 (0.39,0.64) | <0.001 |
| Event location | ||
| ICU | 1.34(1.12,1.61) | 0.001 |
| Immediate cause | ||
| Metabolic or electrolyte disturbance | 1.50(1.20,1.88) | <0.001 |
| Arrest characteristics | ||
| Interval to invasive airway (min) | 0.97 (0.96,0.99) | <0.001 |
| Interventions in place at time of event | ||
| Vascular access | 1.36(1.14,1.63) | 0.001 |
| Duration of CPR (min), (continuous) | 1.02(1.02,1.03) | <0.001 |
| Any pulseless VT/VF | 1.36(1.09,1.70) | 0.006 |
| Pharmacologic interventions | ||
| Dose of epinephrine 1–2 | 0.50(0.41,0.60) | <0.001 |
| Fluid | 1.39(1.18,1.64) | <0.001 |
| Dextrose | 2.02(1.33,3.06) | 0.001 |
| Calcium | 4.90(4.10,5.84) | <0.001 |
| THAM® | 0.34(0.17,0.66) | 0.001 |
CPR: cardiopulmonary resuscitation; ICU: intensive care unit; THAM: tromethamine; VF: ventricular fibrillation; VT: ventricular tachycardia.
After controlling for confounding factors (ethnicity, facility type, event location, illness category, preexisting conditions, interventions in place at the time of the event, immediate precipitating causes, arrest rhythm, concurrent advanced cardiac life support medications, and duration of CPR) SB administration during CPR was independently associated with worse survival to discharge (Table 4). Eighteen percent of patients survived to discharge when SB was used, compared with 26% who survived when SB was not used (aOR: 0.80; 95% CI: 0.65, 0.97). Survival at 24 h is also significant with an adjusted odds ratio of 0.83 (95% CI: 0.69, 0.99), suggesting a survival odds that is 17% lower in patients who received SB during CPR compared to those who did not receive SB. However, there was no difference seen in event survival (aOR:1.01; 95% CI: 0.85, 1.20) or neurologic outcome (aOR: 0.91; 95% CI:0.53, 1.58) between those who received SB and those who did not during CPR.
Table 4.
Sodium bicarbonate use and outcomes.
| Outcomes, n (%) | Sodium bicarbonate used | Sodium bicarbonate not used | Unadjusted OR (95% CI) | OR(95%CI)a |
|---|---|---|---|---|
| Primary outcome | ||||
| Survival to hospital discharge | 443(18) | 310(26) | 0.60(0.51,0.70) | 0.80 (0.65,0.97) |
| Secondary outcomes | ||||
| Survival of event (ROSC>20min) | 1159(46) | 636(54) | 0.72(0.63,0.83) | 1.01 (0.85,1.20) |
| 24-h survival | 745 (30) | 432(38) | 0.70(0.61.0.81) | 0.83 (0.69,0.99) |
| Favorable neurologic outcome (1/2/3 vs. 4/5/6) | 249(79)b | 183(80)c | 0.94(0.62,1.44) | 0.91 (0.53,1.58) |
The ORs were adjusted for factors associated with each outcome measure after multivariable logistic regression. The variables included in the model were ethnicity, facility type, event location, illness category, preexisting conditions, interventions in place at the time of the event, immediate precipitating causes, arrest rhythm, concurrent advanced cardiac life support medications, and duration of CPR as a continuous variable. ROSC indicates return of sustained circulation.
Data obtained from 314 patients.
Data obtained from 228 patients.
We also examined SB use during CPR in specific circumstances in which administration of SB might be indicated, such as pre-existing metabolic acidosis, hyperkalemia, or tricyclic antidepressant overdose. In the settings of metabolic/electrolyte abnormalities, hyperkalemia (neonate), and toxicologic abnormalities (n = 674), SB use during CPR was not associated with worse event survival (aOR: 1.54; 95% CI: 1.00, 2.37), survival at 24 h (aOR:1.54; 95% CI: 0.94, 2.53), survival to discharge (aOR: 1.52; 95% CI:0.84; 2.77), or unfavorable neurologic outcome (aOR: 0.61; 95% CI:0.09, 4.16) after adjustment for confounding factors as noted above. After excluding patients with metabolic/electrolyte abnormalities, hyperkalemia (neonates), and toxicologic abnormalities (n = 3045), SB use during CPR continued to be associated with worse survival to discharge (aOR: 0.73; 95% CI: 0.59, 0.90) and worse survival at 24 h (aOR 0.76; 95% CI: 0.62, 0.92) after adjustment for potentially confounding variables as noted above.
4. Discussion
This is the largest study analyzing the administration of SB during pediatric pulseless IHCA and its association with outcome. It documents that SB use during CPR is strongly influenced by hospital-specific, patient-specific, and arrest-specific characteristics. This report of 3719 consecutive children with pulseless IHCA documents frequent use of SB during the resuscitation of 2536 (68%) children, yet there is a significant trend toward less routine use over the last decade. The data from this large national registry suggest, although cannot confirm causation, that SB use during pediatric pulseless IHCA is associated with decreased survival at 24 h and decreased survival to hospital discharge when given outside current PALS recommendations. This association with poor prognosis, however, despite attempts at adjusting for confounding factors, may be due to differences between the two groups in the cohort (i.e., duration of CPR, vasoactive infusions in place, advanced pharmacologic interventions) rather than causation.
Despite ongoing clinical use of SB in pediatric cardiac arrest, there is very limited data to support the practice and differences of opinion exist among pediatric acute care physicians with respect to the timing and appropriateness of sodium bicarbonate administration during resuscitation.16 In a large retrospective study of 353 children with in-hospital CPR, Meert et al. found survivors were less likely to receive SB than non-survivors. In a regression model using both pre and post-arrest data, and adjusted for age, gender, and first documented rhythm, SB was associated with increased mortality (aOR 2.72; 95% CI: 1.66, 4.48).17 Within this cohort, children who received SB had longer duration of CPR, and were more likely to receive other pharmacologic interventions such as calcium, vasopressin, and a greater number of epinephrine doses.17 Despite our exclusion criteria of CPR events of <5 min or >120 min, and events in which a perfusing rhythm was never lost, there was a significant difference in CPR duration between the two groups. SB was given much more frequently during cardiac arrests of prolonged duration, with a median duration of 30 min in the SB group compared to only 17 min in the group that did not receive SB. This is a significant limitation to our findings as recent GWTG-R studies have demonstrated that CPR duration is independently associated with worse survival to hospital discharge and neurologic outcome.18,19
The frequent use of SB, and thus the poor outcome, may reflect failure of the patient to respond to initial BLS and ALS airway and ventilation interventions and thus a “last-ditch” effort to try all possible therapies during resuscitation, as suggested for why similar non-indicated therapies like calcium are administered during CPR.20 Further suggesting its use as part of an “epiphenomenon” of prolonged arrest and CPR in our cohort, SB use during CPR was significantly associated with administration of other advanced cardiac life support medications, particularly calcium with an adjusted OR of 4.90 (95% CI = 4.10,5.84).
Current indications for SB administration during CPR (preexisting severe metabolic acidosis, hyperkalemia, or tricyclic antidepressant overdose) are captured in the GWTG-R under the categories of metabolic/electrolyte abnormalities, hyperkalemia, and toxicologic abnormalities/overdose/poisoning. The combined incidence of metabolic/electrolyte abnormality, hyperkalemia and toxicologic abnormalities (as both pre-existing conditions and immediate precipitating causes) in the GWTG-R database is only ~23% of all pediatric cardiac arrests. Sodium bicarbonate was administered in 68% of the pulseless events, including 46% of the events with asystole as the presenting pulseless rhythm and 41% of those presenting with pulseless electrical activity. This suggests that SB is often used in circumstances other than those recommended by the current PALS guidelines. With the exception of the year 2004, the incidence of SB use during CPR has steadily declined each year. Since publication of the 2005 guidelines, use of SB during pulseless IHCA continued to decline from 70% in 2005 to 64% in 2010. This finding is encouraging and emphasizes that the published guidelines have impacted subsequent behavior and practice with regard to SB use in pediatric CPR.
Our findings should be interpreted in light of the following limitations. First, we do not have explicit documentation of specific indications for SB use or the details of SB dosing or timing during CPR as captured in the GWTG-R registry. A dose response effect, if available, could have provided stronger evidence for the effect of SB administration on outcome. Second, although data in the GWTG-R registry allowed us to adjust for a number of key variables, the possibility of residual confounding still remains; in particular we do not have detailed information on specific resuscitation ‘processes’ such as quantity or quality of chest compressions and interruptions in CPR. Another potential limitation of this study is lack of generalizability. The GWTG-R centers account for 10% of all hospitals in the United States and represent all geographic US Census regions. However, these volunteer centers pay a fee and thus may have more resources, as well as a greater interest in CPR outcomes, than other US hospitals. There are also potential limitations that pertain to the integrity and validity of the data and sampling bias. Uniform operational definitions, uniform data collection, rigorous abstractor training, detailed periodic reabstraction, and large sample size were instituted prospectively to address data integrity and validity. Sampling bias was minimized by the use of strict inclusion and exclusion criteria, comprehensive methods to verify all arrests as entered into the GWTG-R registry, a large sample, and the multicenter design. Finally, our study is limited by lack of long-term neurological follow-up and the use of only a global measure of neurological function, the PCPC, as a neurological outcome. However, previous studies suggest that neurological status at discharge is not substantially different from status at 6 months and 1 year after arrest, and the PCPC is the standard for Utstein reporting.12,21,22 Finally it is possible that the existing GWTG-R data elements fail to capture other, unmeasured confounders of outcomes, despite the use of suitable analytical techniques. Thus, it is likely that the observed association between SB use during pediatric pulseless IHCA and poor outcomes are not necessarily a causal relationship, but more likely represent an association of SB use in CPR events that are likely longer in duration, in a subset of patients more critical in nature, and perhaps just a “last-ditch” effort to try all resuscitative therapies
5. Conclusions
Sodium bicarbonate is administered during CPR in well over half of all pediatric pulseless IHCA events, yet data analysis from the GWTG-R registry confirms that the routine use of SB is steadily and significantly decreasing in prevalence, as the AHA guidelines recommend. The use of SB during CPR is strongly influenced by hospital-specific, patient-specific, and arrest-specific characteristics. The GWTG-R registry analysis suggests that the routine use of SB, even when controlling for appropriate confounding factors, is associated with worse survival outcomes, therefore reinforcing the current AHA guideline recommendations. This association however, is possibility related to the prolonged duration of CPR in some events, and cannot be confirmed to be the cause of worse prognosis. For special resuscitation circumstances where the AHA recommends consideration of SB use (e.g. hyperkalemia, severe academia, toxicologic overdose), there is no association of the use of SB with either harm or benefit. The GWTG-R registry analysis confirms the 2010 AHA guideline recommendations, but this is based upon strong associations and not causation. Thus, prospective trials of SB use are warranted, particularly in special circumstances where neither benefit nor harm has been clearly demonstrated. Giving SB routinely during cardiac arrest and CPR or after restoration of spontaneous circulation is not recommended.
Acknowledgements
We gratefully acknowledge Qilong Yi, PhD, MSc, and Science-Docs, Inc. for statistical analysis and all of that data abstractors, staff, and investigators who work so hard to contribute data to the American Heart Association Get With The Guidelines-Resuscitation registry.
Funding
The Chloe Duyck Memorial Fund at Medical City Children’s Hospital provided funding for the statistical analysis of this study but did not influence the design, conduct, management, analysis, or interpretation of the study.
Abbreviations:
- ACLS
Advanced Cardiac Life Support
- AHA
American Heart Association
- CPA
cardiopulmonary arrest
- CI
confidence interval
- CPR
cardiopulmonary resuscitation
- GWTG-R
Get With The Guidelines-Resuscitation
- IHCA
in-hospital cardiac arrest
- IRB
institutional review board
- OR
odds ratio
- PALS
Pediatric Advanced Life Support
- PCPC
pediatric cerebral performance category
- ROSC
restoration of spontaneous circulation
- SB
sodium bicarbonate
- pVT
pulseless ventricular tachycardia
- VF
ventricular fibrillation
Appendix A.
Get With The Guidelines-Resuscitation Investigators: Besides the author Tia Tortoriello Raymond, MD, and Arno L. Zaritsky, MD, members of the Get With The Guidelines-Resuscitation Pediatric Task Force include: Vinay Nadkarni, MD, The Children’s Hospital of Philadelphia; Melania Bembea, MD, The Johns Hopkins Hospital Ericka Fink, MD, Children’s Hospital of Pittsburgh of UPMC; Michael G. Gaies, M.D., M.P.H., C.S. Mott Children’s Hospital; Anne-Marie Guerguerian, MD, and Chris Parshuram, MD, The Hospital for Sick Children; Lynda Knight, RN, Lucile Packard Children’s Hospital; Monica Kleinman, MD, Boston Children’s Hospital; Peter C. Laussen, MB BS, Children’s Hospital Boston; Stephen M. Schexnayder, MD, Arkansas Children’s Hospital; Robert Sutton, MD, and Alexis A. Topjian, The Children’s Hospital of Philadelphia.
Footnotes
A Spanish translated version of the abstract of this article appears as Appendix in the final online version at http://dx.doi.org/10.1016/j.resuscitation.2015.01.007.
See Appendix A for Get With The Guidelines-Resuscitation Investigators
Conflict of interest statement
None.
References
- 1.Dybvik T, Strand T, Steen PA. Buffer therapy during out-of-hospital cardiopulmonary resuscitation. Resuscitation 1995;29:89–95. [DOI] [PubMed] [Google Scholar]
- 2.Vukmir RB, Katz L. Sodium bicarbonate improves outcome in prolonged prehospital cardiac arrest. Am J Emerg Med 2006;24:156–61. [DOI] [PubMed] [Google Scholar]
- 3.Aufderheide TP, Martin DR, Olson DW, et al. Prehospital bicarbonate use in cardiac arrest: a 3-year experience. Am J Emerg Med 1992;10:4–7. [DOI] [PubMed] [Google Scholar]
- 4.Weng Y-M, Wu S-H, Li W-C, et al. The effects of sodium bicarbonate during prolonged cardiopulmonary resuscitation. Am J Emerg Med 2013;31:562–5. [DOI] [PubMed] [Google Scholar]
- 5.Committee ECC, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005;13:IV1–203. [DOI] [PubMed] [Google Scholar]
- 6.Guidelines 2005 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 12: Pediatric Advanced Life Support. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation 2005;112:167–87. [PubMed] [Google Scholar]
- 7.Guidelines 2010 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 14: Pediatric Advanced Life Support. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation 2010;122:S876–908. [DOI] [PubMed] [Google Scholar]
- 8.Zaritsky A, Nadkarni V, Hazinski MF, et al. Recommended guidelines for uniform reporting of pediatric advanced life support: the pediatric Utstein Style—a statement for healthcare professionals from American Academy of Pediatrics, the American Heart Association, and the European Resuscitation Council. Circulation 1995;92:2006–20. [DOI] [PubMed] [Google Scholar]
- 9.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14,720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation 2003;58:297–308. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs I, Nadkarni V, Bahr J, et al. , International Liaison Committee on Resuscitation; American Heart Association; European Resuscitation Council; Australian Resuscitation Council; New Zealand Resuscitation Council; Heart and Stroke Foundation of Canada; InterAmerican Heart Foundation; Resuscitation Councils of Southern Africa; ILCOR Task Force on Cardiac Arrest and Cardiopulmonary Resuscitation Outcomes. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation 2004;110:3385–97. [DOI] [PubMed] [Google Scholar]
- 11.Fiser DH, Long N, Roberson PK, et al. Relation of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med 2000;28:2616–20. [DOI] [PubMed] [Google Scholar]
- 12.Reis AG, Nadkarni V, Perondi MP, Grisi S, Berg RA. A prospective investigation into the epidemiology of in-hospital pediatric cardiopulmonary resuscitation using the international Utstein reporting style. Pediatrics 2002;109:200–9. [DOI] [PubMed] [Google Scholar]
- 13.Samson RA, Nadkarni VM, Meaney PA, et al. Outcomes of in-hospital ventricular fibrillation in children. N Engl J Med 2006;354:2328–39. [DOI] [PubMed] [Google Scholar]
- 14.Slonim A, Patel K, Ruttimann U, et al. Cardiopulmonary resuscitation in pediatric intensive care units. Crit Care Med 1997;25:1951–5. [DOI] [PubMed] [Google Scholar]
- 15.Hajbaghery MA, Mousavi G, Akbari H. Factors influencing survival after inhospital cardiopulmonary resuscitation. Resuscitation 2005;66:317–21. [DOI] [PubMed] [Google Scholar]
- 16.Parker MJ, Parshuram CS. Sodium bicarbonate use in shock and cardiac arrest: attitudes of pediatric acute care physicians. Crit Care Med 2013;41: 2188–95. [DOI] [PubMed] [Google Scholar]
- 17.Meert K, Donaldson A, Nadkarni V, et al. Multicenter cohort study of in-hospital pediatric cardiac arrest. Peditr Crit Care Med 2009;10:544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Herce J, del Castillo J, Matamoros M, et al. Factors associated with mortality in pediatric in-hospital cardiac arrest: a prospective multicenter multinational observational study. Intensive Care Med 2013;39:309–18. [DOI] [PubMed] [Google Scholar]
- 19.Matos RI, Watson RS, Nadkarni VM, et al. , American Heart Association’s Get With The Guidelines-Resuscitation Investigators. Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation 2013;127:442–51. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan V, Morris M, Helfaer M, et al. Calcium use during in-hospital pediatric cardiopulmonary resuscitation: a report from the National Registry of Cardiopulmonary Resuscitation. Pediatrics 2008;121:e1144–51. [DOI] [PubMed] [Google Scholar]
- 21.López-Herce J, García C, Rodríguez-Núñez A, et al. , Spanish Study Group of Cardiopulmonary Arrest in Children. Long-term outcome of paediatric cardiorespiratory arrest in Spain. Resuscitation 2005;64:79–85. [DOI] [PubMed] [Google Scholar]
- 22.López-Herce J, García C, Domínguez P, et al. , Spanish Study Group of Cardiopulmonary Arrest in Children. Outcome of out-of-hospital cardiorespiratory arrest in children. Pediatr Emerg Care 2005;21:32. [DOI] [PubMed] [Google Scholar]