Abstract
Purpose
Chk1 inhibition increases cell sensitivity to both chemotherapy and radiotherapy in several tumour types and is, therefore, a promising anti-cancer approach. Although several Chk1 inhibitors have been developed, their clinical progress has been hampered by low bioavailability and off-target toxicities.
Materials and Methods
We characterized the radiosensitizing activity of CCT244747, the first orally bioavailable Chk1 inhibitor. We used a panel of bladder and head and neck cancer cell lines and monitored the effect of combining CCT244747 with radiation both in in vitro and in vivo models.
Results
CCT244747 sensitized cancer cell lines to radiation in vitro and resulted in a growth delay in cancer xenograft models associated with a survival benefit. Radiosensitization was elicited by abrogation of the radiation-induced G2 arrest and premature entry into mitosis.
Conclusions
CCT244747 is a potent and specific Chk1 inhibitor that can be administered orally. It radiosensitizes tumour cell lines and represents a new therapy for clinical application in combination with radiotherapy
Keywords: Radiosensitization, Bladder cancer, Head and Neck cancer, Radiation, Chk1 inhibition
Introduction
Despite significant advances in treatments for locally-advanced bladder and head and neck squamous cell cancers (HNSCC), the 5-year disease-specific survival rates are poor [1, 2]. Most patients receive radiotherapy (RT), either as primary RT/chemoradiotherapy (CRT) in an organ-sparing approach, or post-operatively, as adjuvant RT/CRT. Unfortunately, failure to achieve loco-regional disease control and significant radiation-induced treatment toxicities mean that outcomes are suboptimal in many patients.
One strategy to improve the efficacy of RT is to target signaling pathways activated by radiation. Chk1 is one of the main regulators of the G2/M checkpoint and an essential signal transducer in the cellular response to DNA damage and replication stress [3, 4]. Therefore, this kinase represents an attractive target for sensitizing cancer cells to both RT and chemotherapy. Previous studies have shown that selectively targeting the G2/M cell cycle checkpoint can improve cancer cell sensitivity to RT [5, 6]. A number of Chk1 inhibitors have demonstrated tumour-specific radiosensitization in preclinical studies [7–9]. However, their translation into clinical trials has been limited by difficulties with pharmaceutical formulation and toxicity. Here, we present data on CCT244747, a potent, non-toxic, orally bioavailable Chk1 inhibitor [10] that mediates in vitro and in vivo radiosensitization by modulating G2/M checkpoint control. We show that death occurs through mitotic catastrophe and/or apoptosis in cells cycling with unrepaired DNA damage.
Materials and Methods
Cell lines, drug treatment and irradiation
RT112, T24 (bladder) and Cal27 (HNSCC) were obtained from ATCC. hTertRPE1 epithelial cells were obtained from Dr C. Bakal(ICR), London. All cancer cell lines were typed using short tandem repeat analysis (Bio-Synthesis Inc., Texas, USA). Cells were cultured in 10% Foetal Bovine serum (FBS) (Gibco® by life technologies), 1% glutamine and 0.5% penicillin/streptomycin, in Dulbecco’s modified Eagle’s medium (DMEM) (ICR, London, UK) and routinely tested for mycoplasma. CCT244747 was synthesized by the ICR, Sutton and dissolved in dimethyl sulfoxide (DMSO) (Fisher Scientific) for in vitro experiments and in 10% DMSO, 5% Tween-80, 20% PEG400 (Sigma-Aldrich) and 65% H2O for in vivo experiments. Irradiation was carried out as previously described [7].
Clonogenic assays
Cells were seeded into 6-well plates and, 16-24 hours later, treated with CCT244747 (0.25 µM, 0.5 µM or 2 µM). Plates were irradiated (2, 4 or 6 Gy) 6 hours post-treatment with CCT244727. Medium was replaced 48 hours after treatment with CCT244747. Cells were fixed and stained using 5% glutaraldehyde and 0.05% crystal violet (Sigma-Aldrich) 10-20 days after treatment. Colonies (≥50 cells) were counted manually. Surviving fractions were calculated as a ratio of the untreated control cells after normalizing for plating efficiency. Survival curves were generated using Prism 6, (GraphPad Software, San Diego, California, USA). Statistical differences were analysed with a 2-way ANOVA test.
Cell cycle distribution
Cells were seeded in 10 cm dishes and, 48 hours later, treated with 4 µM CCT244747. Plates were irradiated with 8 Gy in a single fraction 6 hours after exposure to CCT244747. Cells were fixed with 70% ethanol at indicated time points with CCT244747 and stained with pS10 histone H3 conjugated Alexa (R)647 antibody (Cell Signalling) and propidium iodide. Flow cytometry analysis was performed using a LSRII flow cytometer (BD Biosciences, Oxford, UK).
Western blotting analysis
Cells were seeded and treated with CCT244747 and radiation as described above. Whole-cell lysates were collected using radioimmunoprecipitation assay (RIPA) buffer at selected time-points after treatment with CCT244747. The following antibodies were used for western blotting: pS345 Chk1, total Chk1, Caspase-3, pS139 Histone H2A.X, β-Actin and GAPDH (Cell Signalling), PARP-1 (Santa Cruz Biotechnology) and pS10 Histone H3 (Merck Millipore).
Immunofluorescence analysis
Cells were plated in 35 mm glass-bottomed, collagen-coated dishes (MatTek, Massachusetts, USA) and irradiated with 4 Gy 6 hours after treatment with 4 µM CCT244747. Cells were fixed with 4% formaldehyde at the indicated time points and immunofluorescence was performed as previously described [7]. Cells were stained with pS139 Histone H2A.X (γ-H2AX; Cell signaling) and α-Tubulin (Sigma Aldrich) and visualized using Alexafluor-488-conjugated goat anti-rabbit and Alexfluor-546-conjugated goat anti-mouse antibodies (Invitrogen™, Life technologies) along with 4’,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Invitrogen, Molecular Probes™) nuclear stain. A minimum of 100 nuclei were examined. Nuclei were quantified as positive for foci when ≥5 foci were present within the nucleus, positive for pan-nuclear staining when ≥80% of the nucleus was stained positive for γ-H2AX. Micronucleated or multinucleated cells were scored as abnormal.
In vivo studies
Female 5- to 6-week-old athymic nude mice (CD-1® Nude Mouse Crl:CD1-Foxn1nu, Charles River) were used. All experiments were approved by the institutional review board in compliance with NCRI guidelines. 3×106 Cal27 cells were injected subcutaneously in the right flank. Once tumours had reached approximately 5 mm diameter, animals were randomized into 4 groups (n=8): control, RT, single-agent CCT244747 and CCT244747 plus RT. Radiotherapy consisted of a total dose of 10 Gy in 5 fractions on alternate days. CCT244747 (100 mg/kg) was administered by gavage 1 hr before each radiation fraction [10]. Tumour volume was calculated as Volume = (Width2 x Length)/2). Body weights were taken twice weekly. The time taken to reach the experimental endpoint (tumour diameter >15 mm) in each group was compared by log-rank test.
Results
CCT244747 radiosensitizes bladder and head and neck cancer cell lines
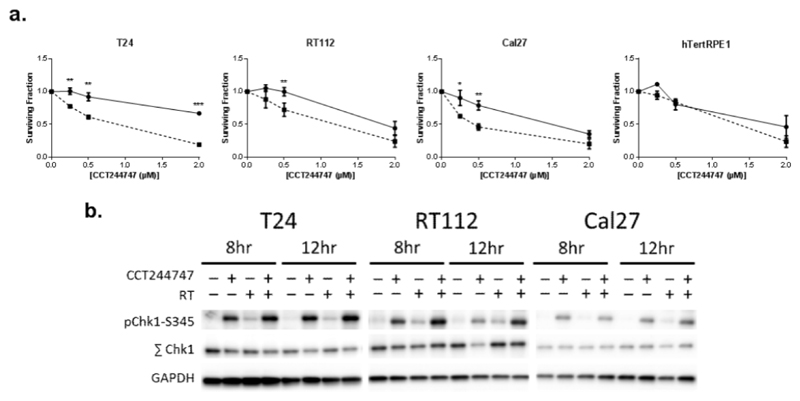
There was a significant difference between 0 and 2 Gy radiation when T24, RT112 and Cal27 cancer cells were treated with 0.5 µM CCT244747. This effect was not evident in hTertRPE1 epithelial cells (Fig 1a). The dose modifying factor (DMF) was calculated to quantify the degree of sensitisation (Fig S1 a,b). This showed a marked dose-modifying effect in T24, RT112 and Cal27 cancer cells that was not seen in hTertRPE1 epithelial cells, indicating cancer cell-specific radiosensitisation by CCT244747.
Fig. 1. CCT244747 increases the sensitivity of T24, RT112 and Cal27 cancer cell lines to radiation.
(a) Clonogenic survival of T24, RT112 and Cal27 cancer cells and hTert RPE1 epithelial cell lines following CCT244747 treatment (solid line), or CCT244747 combined with radiation treatment (dashed line). Data normalised to untreated control or CCT244747 only results and presented as mean±SEM of at least 3 individual experiments. * = P<0.05, ** = P<0.01, *** = P<0.001. (b) Western blots of lysates from T24, RT112 and Cal27 cells treated with CCT244747, RT, or a combination of both (CCT244747 and RT) demonstrating drug-on-target effect for each cell line used. Cells were collected 8 and 12 hours after CCT244747 exposure.
The on-target effect of CCT244747 was evaluated using western blot analysis at 8 and 12 hours after treatment with 4 µM CCT244747. CCT244747 induced phosphorylation at the Ser345 (pS345) site on Chk1, indicating increased DNA damage and protein phosphatase PP2A inhibition [11]. Radiation-induced pS345-Chk1 was evident in T24 and RT112 cell lines, but not to the same extent as for single-agent CCT244747 treatment. Combining CCT244747 with radiation increased pS345-Chk1 above levels seen with CCT244747 alone in T24, RT112 and Cal27 (Fig. 1c).
CCT244747 abrogates RT-induced G2 arrest
To determine whether CCT244747 would modify radiation-mediated cell cycle changes, cells were treated with CCT244747 6 hours before irradiation. To calculate the percentage of cells in mitosis, cells were stained for pS10-Histone H3 (p-HH3) and analyzed by flow cytometry. Treatment with CCT244747 significantly abrogated radiation-mediated G2 arrest at 12 hours (Fig. 2a). This continued cell cycling following irradiation, due to the presence of CCT244747, led to a significant increase in the mitotic and G1 populations in RT112 and Cal27 cells at 12 hours and the S phase population in all cells lines at 24 hours (Fig. S2).
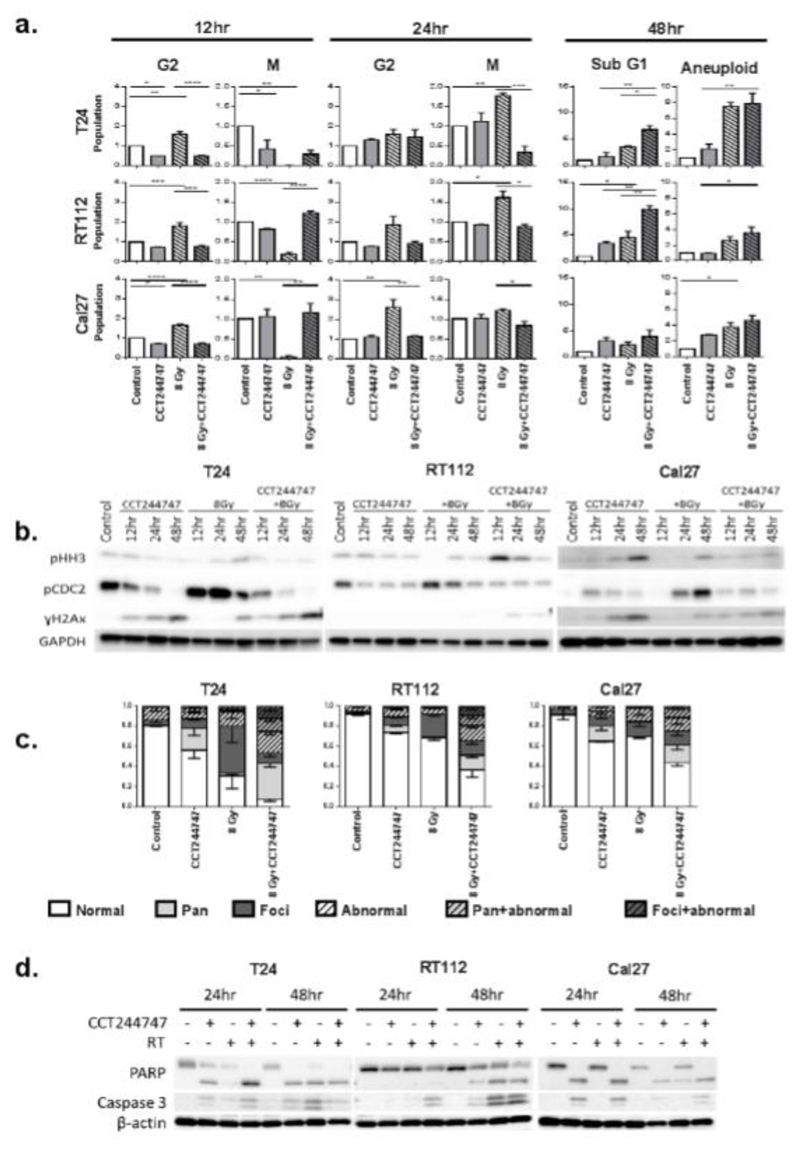
Fig. 2. CCT244747 abrogates the RT-induced G2 arrest and drives premature mitotic entry.
T24, RT112 and Cal27 were treated with radiation 6 hours after administration of CCT244747. (a)Relative change to various phases of the cell cycle at 12, 24 and 48 hour time-points following exposure to CCT244747. DNA content and Mitotic index were assessed by flow cytometry using propidium iodide and pS10-histone H3. Data are normalised to the untreated ‘control’ samples and presented as the mean ± SEM of 3 independent experiments. * = P<0.05, ** = P<0.01, *** = P<0.001. (b) Western blot showing cell cycle progression markers (pHH3) and DNA damage marker (γ-H2AX). (c) Quantification of immunofluorescent images from cells fixed 24 hours after treatment with CCT244747; positive for foci when ≥5 foci; positive for pan-nuclear staining when ≥80% of the nucleus was stained positive for (γ-H2AX) and cells with micronuclei and multinucleated cells were considered as abnormal nuclei. (d) Western blot showing apoptotic markers PARP-1 and caspase-3 at 24 and 48 hours.
All cell lines exhibited a significant increase in the G2 phase of the cell cycle and a decrease in the mitotic population at 6 hours post-irradiation (12 hour time point), indicating a DNA damage-induced G2 arrest (Fig 2a). Irradiation of cells with 8 Gy caused a decrease in the G1 population and a small increase in the S phase population at 12 hours after treatment. Both G1 and S phase populations were decreased in all cell lines at 24 hours post-irradiation (Fig S2).
CCT244747 alone reduced the G2 population in T24 and Cal27 cells and the mitotic population in T24 cells at 12 hours. This effect was not seen in RT112 cells and had recovered in other cells by 24 hours (Fig. 2a). There was also an observable increase in the S phase population in all cells 12 and 24 hours post-treatment with CCT244747 (Fig S2). This increase in the S phase may represent Chk1-dependent stalled replication [3, 12].
The flow cytometry results were confirmed by Western blotting for p-HH3, where a decrease in the expression of p-HH3 indicated G2 arrest. A dramatic reduction in the expression of p-HH3 was seen 6 hours after irradiation in all cell lines (12 hour time point) (Fig 2b). Treatment with CCT244747 abrogated radiation-mediated G2 arrest resulting in an increase in the expression of p-HH3 at 12-48 hours after treatment (compared to radiation alone).
To examine whether cells were dying by apoptosis or becoming aneuploid 48 hours after treatment, the sub-G1 and aneuploid (>4N) populations were analyzed by flow cytometry. Following CCT244747 and RT double therapy, there was a significant increase in the sub-G1 population in T24 and RT112 cells (Fig 2a). Although aneuploidy was increased following RT, addition of CCT244747 did not further increase the percentage of aneuploid cells present 48 hours after therapy (Fig 2a).
CCT244747 and RT combination therapy induces the formation of abnormal nuclei
To investigate changes in nuclear and cell morphology following RT and CCT244727, cells were stained with γ-H2AX and α-tubulin and analyzed by confocal microscopy. Untreated control cells had mainly normal nuclei (normal size and without micronuclei (Fig S3a, b)) containing few γ-H2AX foci (Fig. 2c). Cells treated with CCT244747 alone had mostly normal nuclear morphology, but there was an increase in the percentage of nuclei containing γ-H2AX foci (Fig S3b) or exhibiting pan-H2AX (Fig S3b) staining. As expected, RT increased the number of γ-H2AX foci, and significantly increased the percentage of cells with abnormal nuclear morphology (Fig S3b). CCT244747 and RT combination caused a further increase in the number of cells with abnormal nuclear morphology, either due to an increase in multinucleation or the presence of micronuclei (Fig 2c, S3a), suggesting cells were undergoing mitotic catastrophe. The percentage of cells with γ-H2AX nuclear staining (foci, pan, foci + abnormal, pan + abnormal) was significantly increased for all cell lines.
To further assess whether cells were undergoing apoptosis, cell lysates were collected 24 and 48 hours after treatment and analyzed for Caspase-3 and PARP cleavage by Western blotting. Single-agent CCT244747 increased cleaved PARP levels in T24 and Cal 27 at 24 hours and in all three cell lines at 48 hours. This was accompanied by an increase in cleaved Caspase-3 levels in T24 and Cal 27 at 48 hours. Radiotherapy increased PARP and Caspase-3 cleavage in T24 and RT112 but not Cal27 at 48 hours. In T24 and Cal27, combination therapy did not result in a further increase in Caspase-3 or PARP cleavage (Fig 2d). RT112 cells, showed increased PARP and Caspase-3 cleavage with combination therapy.
In vivo radiosensitization by CCT244747
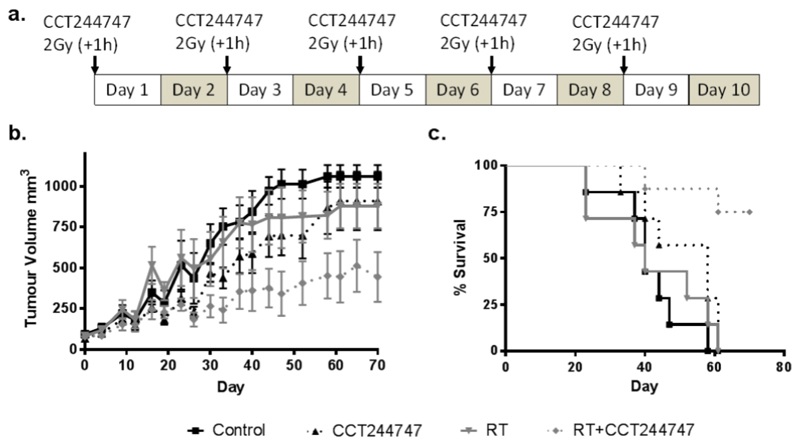
We tested the effect of CCT244747, either alone or in combination with RT, in Cal27 xenografts in vivo (Fig. 3a). We did not observe any clinical signs of toxicity (including a less than 5% drop in body weight) in all 4 treatment groups. The average tumour volume, which was between 70 and 90 mm3 at day 0, reached 1061 mm3 in the control group after 70 days (Fig. 3b). The increase in volume was less evident in the drug alone and RT alone groups compared to the controls (910 vs 879 vs 1061 mm). The mean tumour volume in the combination therapy group at 70 days was significantly lower than either drug or radiotherapy alone groups (445 mm3; P<0.05 for both). It took 35.4±9.6 days for the tumours in the combination treatment group to reach a minimum of 3 times their original volume. In contrast, it took 19.9±3.5 days in the radiation alone group, 24.7±8.3 days in the CCT244747 alone group and 20±3 days in the control group to reach this size.
Fig. 3. CCT244747 is an in vivo radiosensitizer.
Athymic nude mice were injected subcutaneously with 3x106 Cal27 cells and divided in 4 groups. Mice were treated with CCT244747 (100 mg/kg) by gavage 1h prior to RT (2 Gy x 5). (a) Schematic representation of the schedule used to treat the mice. (b) Tumour growth delay (mean ± SD) and (c) survival.
The median survival data reflected the lower tumour burden in the combination group compared with the others (Fig. 3c). Median survival values increased from 40 days (control and RT alone), to 58 days (drug only) and Not Reached (RT plus CCT244747) (P<0.01 vs all other treatment groups). Indeed, the combination group recorded 75% survival at 70 days, having achieved a stable average tumour volume of 450 mm3. The combination of RT and CCT244747 was significantly different from the single treatments (P=0.0042) (log-rank Mantel-Cox test).
Discussion
Cytotoxic chemotherapy is combined with radiotherapy to treat muscle invasive bladder cancers and locally advanced HNSCC, allowing organ preservation [13–15]. Due to the associated side-effects and poor outcomes, there is a need for less toxic and more effective treatments. Pre-clinical studies have evaluated several novel radiosensitisers in bladder cancer models [16–19], but no agent has translated into clinical trials reporting improved outcomes.
The current study confirms previously published work regarding the role of Chk1 in mediating tumour radioresistance [20]. This study demonstrated that the orally active Chk1 inhibitor CCT244747 radiosensitised tumour cells both in vitro and in vivo, and the combination demonstrated growth delay in a cancer xenograft model with a survival benefit. Radiosensitization was elicited through abrogation of RT-induced G2 cell cycle arrest, forcing cells into premature mitosis with unrepaired DNA-DSBs. Although previously described in pre-clinical head and neck cancer models [7], to our knowledge, this is the first study showing Chk1-mediated radiosensitization in bladder cancer cells.
Most cancer cells are more dependent than normal cells on the G2 checkpoint to repair DNA damage, because of a deficient G1 checkpoint [21]. Targeting the G2 checkpoint as an anticancer strategy has been explored as monotherapy [22] or in combination with DNA damaging agents [23]. Following DNA damage, Chk1 inhibition prevents G2 arrest forcing cells into premature M phase and cell death by mitotic catastrophe and/or apoptosis [7].
In addition to cellular signaling, the efficacy of the combination treatment is dependent on cell cycle perturbation. As demonstrated previously, Chk1 inhibition was able to overcome RT-mediated G2 arrest, causing cells to prematurely enter mitosis. We also observed a decrease in the S phase of the cell cycle 24 hours after RT, which was abrogated in cells treated with CCT244747/RT combination therapy. Western analysis confirmed these changes in the cell cycle and also indicated that, despite unrepaired DNA DSBs remaining at 24 and 48 hours after CCT244747/RT therapy, cells were still entering mitosis (evidenced by the expression of p-HH3) and, therefore, were likely to undergo mitotic catastrophe.
It has previously been shown that Chk1 inhibition during DNA replication can lead to disruption of the normal repair and restart pathways following stalled replication forks [3, 12, 24]. This can manifest as pan-nuclear H2AX staining, which has been linked to replication stress, S phase arrest [25] and apoptosis [12]. Indeed, our analysis revealed that cells treated with CCT244747/RT combination therapy exhibited increased pan-nuclear H2AX staining and abnormal nuclei, which resulted in apoptosis. Cells with pan-nuclear H2AX staining following RT alone may represent cells with foci alone, as this population was reduced after the combination of CCT24474 and RT.
PARP facilitates cellular disassembly and, following cleavage by Caspase-3, serves as a marker of cells undergoing apoptosis at later time points. CCT244747 increased cleaved PARP levels at 24 and 48 hours, whilst radiotherapy increased cleaved PARP levels at 48 hours. CCT244747 appeared to induce apoptotic cell death 24 hours earlier than radiotherapy. The mode of cell death following irradiation is dependent on several factors including the cell type and we note Cal 27 cells did not undergo apoptosis following radiotherapy alone.
Our in vivo model confirmed the in vitro findings and showed that CCT244747 radiosensitizes tumour cells. CCT244747 and RT monotherapies had modest effects on tumour growth, whereas the combination treatment demonstrated a significant tumour growth delay that translated into improved survival of the CCT244747 plus RT group. Based on these data, it will be important to investigate the combination of CCT244747 and radiation in early phase clinical trials in an attempt to develop efficacious treatments with less toxicity than conventional CRT.
Supplementary Material
Acknowledgments
KJH received support from the ICR/RM NIHR Biomedical Research Centre, the Oracle Cancer Trust, The Rosetree Trust and The Anthony Long Trust. KJH, HB, MTD acknowledge research funding from CRUK (C7224/A13407). RP received funding from the Department of Urology, the Royal Marsden Hospital, London and a generous research donation from Lord and Lady Colin Marshall of Knightsbridge. We would like to thank Ian Collins, Michelle Garrett and Tom Matthews for providing CCT244747.
Footnotes
Disclosure of Potential Conflicts of Interest: Intellectual property from the research collaboration with Sareum Ltd. on Chk1 inhibitors was licensed from The Institute of Cancer Research to Sareum Ltd. The Institute of Cancer Research has benefited from this and requires its employees to declare this potential conflict of interest.
References
- 1.Economopoulou P, Bourhis J, Psyrri A. Research Progress in Head and Neck Squamous Cell Carcinoma: Best Abstracts of ICHNO 2015. Am Soc Clin Oncol Educ Book. 2015:e323–8. doi: 10.14694/EdBook_AM.2015.35.e323. [DOI] [PubMed] [Google Scholar]
- 2.Economopoulou P, et al. Head and Neck Cancer Highlights of ESMO Congress 2014. European Oncology & Haematology. 2014:96–97. [Google Scholar]
- 3.Elvers I, et al. CHK1 activity is required for continuous replication fork elongation but not stabilization of post-replicative gaps after UV irradiation. Nucleic Acids Res. 2012;40(17):8440–8. doi: 10.1093/nar/gks646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai Y, Grant S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin Cancer Res. 2010;16(2):376–83. doi: 10.1158/1078-0432.CCR-09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillon MT, Good JS, Harrington KJ. Selective targeting of the G2/M cell cycle checkpoint to improve the therapeutic index of radiotherapy. Clin Oncol (R Coll Radiol) 2014;26(5):257–65. doi: 10.1016/j.clon.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Dillon MT, Harrington KJ. Human Papillomavirus-Negative Pharyngeal Cancer. J Clin Oncol. 2015;33(29):3251–61. doi: 10.1200/JCO.2015.60.7804. [DOI] [PubMed] [Google Scholar]
- 7.Borst GR, et al. Targeted radiosensitization by the Chk1 inhibitor SAR-020106. Int J Radiat Oncol Biol Phys. 2013;85(4):1110–8. doi: 10.1016/j.ijrobp.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell JB, et al. In vitro and in vivo radiation sensitization of human tumor cells by a novel checkpoint kinase inhibitor, AZD7762. Clin Cancer Res. 2010;16(7):2076–84. doi: 10.1158/1078-0432.CCR-09-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelke CG, et al. Sensitization of pancreatic cancer to chemoradiation by the Chk1 inhibitor MK8776. Clin Cancer Res. 2013;19(16):4412–21. doi: 10.1158/1078-0432.CCR-12-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walton MI, et al. CCT244747 is a novel potent and selective CHK1 inhibitor with oral efficacy alone and in combination with genotoxic anticancer drugs. Clin Cancer Res. 2012;18(20):5650–61. doi: 10.1158/1078-0432.CCR-12-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsels LA, et al. Assessment of Chk1 Phosphorylation as a Pharmacodynamic Biomarker of Chk1 Inhibition. Clinical Cancer Research. 2011;17(11):3706–3715. doi: 10.1158/1078-0432.CCR-10-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagou ME, Zuazua-Villar P, Meuth M. Enhanced H2AX phosphorylation, DNA replication fork arrest, and cell death in the absence of Chk1. Mol Biol Cell. 2010;21(5):739–52. doi: 10.1091/mbc.E09-07-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James ND, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366(16):1477–88. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 14.Soo KC, et al. Surgery and adjuvant radiotherapy vs concurrent chemoradiotherapy in stage III/IV nonmetastatic squamous cell head and neck cancer: a randomised comparison. Br J Cancer. 2005;93(3):279–86. doi: 10.1038/sj.bjc.6602696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furness S, et al. Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. Cochrane Database of Systematic Reviews. 2011;(4) doi: 10.1002/14651858.CD006386.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Tsai YC, et al. Synergistic Blockade of EGFR and HER2 by New-Generation EGFR Tyrosine Kinase Inhibitor Enhances Radiation Effect in Bladder Cancer Cells. Mol Cancer Ther. 2015;14(3):810–20. doi: 10.1158/1535-7163.MCT-13-0951. [DOI] [PubMed] [Google Scholar]
- 17.Nassim R, et al. Combining mTOR inhibition with radiation improves antitumor activity in bladder cancer cells in vitro and in vivo: a novel strategy for treatment. PLoS One. 2013;8(6):e65257. doi: 10.1371/journal.pone.0065257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoskin PJ, Saunders MI, Dische S. Hypoxic radiosensitizers in radical radiotherapy for patients with bladder carcinoma: hyperbaric oxygen, misonidazole, and accelerated radiotherapy, carbogen, and nicotinamide. Cancer. 1999;86(7):1322–8. [PubMed] [Google Scholar]
- 19.Ortiz T, et al. Radiosensitizer effect of wortmannin in radioresistant bladder tumoral cell lines. International Journal of Oncology. 2004;24(1):169–175. [PubMed] [Google Scholar]
- 20.Wang WJ, et al. MYC Regulation of CHK1 and CHK2 Promotes Radioresistance in a Stem Cell-like Population of Nasopharyngeal Carcinoma Cells. Cancer Research. 2013;73(3):1219–1231. doi: 10.1158/0008-5472.CAN-12-1408. [DOI] [PubMed] [Google Scholar]
- 21.Ho A, Dowdy SF. Regulation of G(1) cell-cycle progression by oncogenes and tumor suppressor genes. Current Opinion in Genetics & Development. 2002;12(1):47–52. doi: 10.1016/s0959-437x(01)00263-5. [DOI] [PubMed] [Google Scholar]
- 22.Sausville EA, et al. Phase I trial of 72-hour continuous infusion UCN-01 in patients with refractory neoplasms. Journal of Clinical Oncology. 2001;19(8):2319–2333. doi: 10.1200/JCO.2001.19.8.2319. [DOI] [PubMed] [Google Scholar]
- 23.Lara PN, Jr, et al. The cyclin-dependent kinase inhibitor UCN-01 plus cisplatin in advanced solid tumors: a California cancer consortium phase I pharmacokinetic and molecular correlative trial. Clin Cancer Res. 2005;11(12):4444–50. doi: 10.1158/1078-0432.CCR-04-2602. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Sanchez Y. Chk1 in the DNA damage response: conserved roles from yeasts to mammals. DNA Repair (Amst) 2004;3(8–9):1025–32. doi: 10.1016/j.dnarep.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Ewald B, Sampath D, Plunkett W. H2AX phosphorylation marks gemcitabine-induced stalled replication forks and their collapse upon S-phase checkpoint abrogation. Mol Cancer Ther. 2007;6(4):1239–48. doi: 10.1158/1535-7163.MCT-06-0633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.