Abstract
Background
Risk factors for hepatocellular carcinoma (HCC) have not been well characterized among African immigrants with chronic hepatitis B virus (HBV) infection. We conducted a case-control study to identify demographic and clinical factors associated with HCC among a diverse cohort of patients with chronic HBV infection seen in a large academic health setting.
Methods
We identified a total of 278 patients with HCC and chronic HBV seen at two medical centers in a 14-year span from January 2002 to December 2015. These cases were age- and sex-matched in a 1:3 ratio with 823 non-cancer control subjects with chronic HBV. Conditional logistic regression was used to estimate the odds of HCC by race, with black race stratified by African-born status, after adjusting for diabetes, HIV or HCV coinfection, alcohol misuse and cirrhosis.
Results
Of the 278 HCC cases, 67% were 60 years of age or older, 78% were male, 87% had cirrhosis and 72% were Asian. HIV infection was present in 6% of cases. Only 7% (19 of 278) of HCC cases were black, of whom 14 were African immigrants. The median age at HCC diagnosis was 44 years in Africans. Notably, nearly all (93%) of the African-born patients with HCC were diagnosed at an age younger than 60 years compared with 52% of Asian cases (P<0.001). The main factors independently associated with greater odds of HCC overall were Asian race (adjusted odds ratio [aOR] 3.3, 95% confidence interval [CI] 1.9–5.5) and cirrhosis (aOR 19.7, 95% CI 12.2–31.8).
Conclusion
African immigrants accounted for a small proportion of HBV-associated HCC cases overall compared with Asians but appeared to have greater likelihood of early-onset HCC. Optimal strategies for HCC prevention in these key subroups with chronic HBV warrant further study.
Introduction
Liver cancer is a leading cause of cancer-related death worldwide, particularly in developing countries [1]. While the incidence of other leading cancers, particularly lung cancer, has declined in recent years due in large part to tobacco control measures, liver cancer rates have continued to increase in many parts of the world [2, 3]. In the United States (US), the incidence of liver cancer has more than doubled in the last three decades [4, 5]. Chronic viral hepatitis due to hepatitis B (HBV) and C (HCV) viruses is thought to account for at least 80% of hepatocellular carcinoma (HCC), the most common form of primary liver cancer [6]. Chronic HBV is estimated to increase the odds of HCC by 50- to 100-fold [7] and contributes overwhelmingly to the disproportionate burden of HCC in Asia and Africa, the world regions with the highest HCC incidence and HBV endemicity [3, 8]. Among chronic HBV-infected patients, there can be significant variability in HCC presentation by race and by geographic region. For example, data from African countries suggest that HCC occurs at younger ages than in other regions of the world, possibly due to greater lifetime exposure to aflatoxin or to HBV genotype [9–11]. These data have lead to US guidelines recommending initiation of HCC surveillance in African-born patients with chronic HBV after the age of 20 [12].
Few studies, however, have examined the risk of HCC in HBV-infected African-born patients who have immigrated to more developed countries. The number of individuals with chronic HBV may be increasing in the US as a result of immigration. From 2004–2008, an estimated 53,800 persons with chronic HBV infection immigrated to the US annually; immigrants now account for 95% of new chronic HBV cases in the US [13]. One population-based study from Minnesota demonstrated that the proportion of HCC attributable to HBV infection rose from 4% to 21% from 2000–2014 [14]. It remains unclear whether HBV-infected patients who immigrate to areas without the environmental risk factors (e.g., dietary iron or aflatoxin) of their native countries continue to have excess risk for HCC and to present at younger ages. More generally, risk factors for HCC have not been well documented among US immigrants with chronic HBV infection, particularly African-born immigrants [15]. Much of the clinical and epidemiologic data on HCC in persons with chronic HBV infection derive from native or immigrant Asian cohorts [12].
We conducted a case-control study to characterize the relative burden of HCC in a diverse population of patients with chronic HBV infection, including African-born patients, seen in a large US urban academic medical setting. Our aim was to describe the spectrum of age at HCC diagnosis and identify demographic and clinical factors associated with HCC in this mostly immigrant population.
Methods
Data source and study population
Our data source was a de-identified clinical data repository of all laboratory and clinical diagnoses abstracted from the electronic medical record system of University of Washington Medical Center (UWMC), Harborview Medical Center (HMC) and affiliated outpatient clinics which form a consolidated care network. The UWMC/HMC system provides both primary and specialty care and is the main academic medical center in the greater Seattle region. HMC is the regional safety-net hospital and both centers serve a large community of immigrant patients in King County, Washington. UWMC is a leading referral center for HCC, in part due to its role as the region’s first liver transplantation center. Chronic hepatitis B is identified in both settings as either a known diagnosis on entry to care or through screening as part of routine clinical practice. We selected all patients seen at UWMC or HMC with a diagnosis of chronic hepatitis B and HCC from January 2002 to December 2015. Chronic HBV was defined by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) codes 070.3X, 070.2X and confirmed by chart review. HCC cases were defined by ICD-9 CM 155.0 for hepatocellular carcinoma and confirmed by radiology or clinician documentation on chart review. Non-cancer controls with chronic HBV were matched by age and sex in a 3:1 ratio to patients with HCC. Age in controls was matched to within 3 years of the case’s age to permit this ratio. This research was approved by the Human Subjects Division of the University of Washington.
Key covariates
We collected demographic data on age, sex and race. We identified immigrant or non-US-born status for black patients and Asians primarily by the presence of non-English (primary spoken) language. For those black patients who had missing or English language, we conducted chart reviews to ascertain African-born status and identified patients as African-born if chart notes indicated country of origin or language. We stratified black patients into African-born and US-born in the race variable for our main analysis because they were the main focus of our inquiry on immigrant status. We did not split Asians by US-born status in the main analysis but did reexamine Asian race stratified as non-US-born versus US-born, using English language as our surrogate for US-born status, as a secondary analysis. HIV coinfection was identified by ICD-9 CM diagnosis codes 042 and V08 and then confirmed by chart review and laboratory results. We defined HCV coinfection as the presence of hepatitis C virus antibody on laboratory testing. We identified diabetes mellitus by ICD-9 CM code 250.X and alcohol use disorder by ICD-9 CM codes 291.x, 303.x and 305.0. Patients were considered to have cirrhosis if they met any of the following criteria: (1) Fibrosis-4 (FIB-4) score greater than 3.6 based on serum aspartate aminotransferase (AST), platelet count, and alanine aminotransferase (ALT) closest to the time of diagnosis [16–18], (2) diagnosis of chronic liver disease and cirrhosis (ICD-9 CM 571.X) or (3) cirrhosis of liver without mention of alcohol (ICD-9 CM 571.5). For additional information on liver disease severity, we collected diagnoses of hepatic complications including ascites (ICD-9 CM 789.5), esophageal varices (ICD-9 CM 456.9, 456.1, 456.2), hepatic encephalopathy (ICD-9 CM 572.2) and spontaneous bacterial peritonitis (ICD-9 CM 567.23).
Statistical analysis
We compared distributions of categorical variables between cases and controls (or between other strata) by performing Pearson chi squared tests. We compared ages between cases and controls using a two-sample Wilcoxon rank-sum (Mann-Whitney) test. In our primary analysis, we estimated the crude odds ratio (OR) and 95% confidence intervals (CI) for the association between race and the HCC using conditional logistic regression composed of clusters of matched case-control strata. We then calculated adjusted OR, adjusting for several hypothesized risk factors for HCC including HIV coinfection, HCV coinfection, alcohol use disorder, cirrhosis, and diabetes. We also performed a sensitivity analysis without cirrhosis in the model, since HCC can occur without cirrhosis in HBV-infected individuals, and cirrhosis is not always identified in clinical practice [19].
Results
We identified 296 potential HCC cases who were seen at UWMC or HMC between January 2002 to December 2015. Eighteen cases were excluded after they were determined not to have HCC on chart review, leaving a total of 278 confirmed cases with chronic HBV infection and HCC. A total of 859 controls were identified. After chart review to confirm chronic HBV status, 36 controls who were determined not to have chronic HBV were excluded, leaving a total of 823 controls with chronic HBV but without HCC who were seen between January 2002 and December 2015.
The demographic and clinical characteristics of HCC cases and non-cancer controls are presented in Table 1. Over sixty percent of cases and controls were 60 years of age or older, with very few (1.4%) younger than 40. The majority of HCC cases and non-cancer controls were men (78.4% and 78.3%, respectively). As expected, 86.7% of the cases had cirrhosis, compared with only 29% of the non-cancer controls (P<0.001). Complications of advanced liver disease including spontaneous bacterial peritonitis, esophageal varices, ascites or hepatic encephalopathy were documented more frequently in cases than controls. There were no statistically significant differences between cases and controls in the percentage of patients with alcohol use diagnoses, diabetes, or HCV coinfection.
Table 1. Demographic and clinical characteristics of HCC cases and controls.
| HCC cases n = 278 |
Controls n = 823 |
P-value | |
|---|---|---|---|
| Age, median (range) | 65 (33–93) | 64 (30–93) | 0.12 |
| Male sex, no. (%) | 218 (78) | 644 (78) | 0.95 |
| Race, no. (%) | <0.001 | ||
| White | 52 (19) | 235 (29)* | |
| Black (overall) | 19 (7) | 166 (20) | |
| Black: African immigrant | 14 (5) | 110 (13) | |
| Black: non-immigrant | 5 (2) | 56 (7) | |
| Asian | 199 (72) | 394 (48) | |
| Other | 8 (3) | 26 (3) | |
| Alcohol misuse diagnosis, no. (%) | 27 (10) | 73 (9) | 0.67 |
| HIV coinfection‡, no. (%) | 16 (6) | 87 (11) | 0.02 |
| Hepatitis C coinfection‡, no. (%) | 38 (14) | 126 (15) | 0.51 |
| Diabetes mellitus‡, no. (%) | 62 (22) | 188 (23) | 0.85 |
| Cirrhosis‡, no. (%) | 241 (87) | 239 (29) | <0.001 |
| Liver complications‡, no. (%) | |||
| Any of the following | 103 (37) | 79 (10) | <0.001 |
| Ascites | 37 (13) | 47 (6) | <0.001 |
| Hepatic encephalopathy | 37 (13) | 26 (3) | <0.001 |
| Esophageal varices | 58 (21) | 24 (3) | <0.001 |
| Spontaneous bacterial peritonitis | 12 (4) | 10 (1) | <0.001 |
*Among 821 controls with non-missing race.
‡Definitions–Cirrhosis: FIB4 index >3.6 or ICD-9 CM code 571.x. HIV: ICD-9 CM codes 042, V08. Hepatitis C: positive hepatitis C virus antibody. Alcohol misuse: ICD-9 CM codes 291.x, 303.x or 305.0. Diabetes mellitus: ICD-9 CM code 250.x. Liver complications: respective ICD-9 CM codes for these diagnoses
Race, age and disease severity at HCC diagnosis
Asians were the predominant racial group among patients with HCC, comprising 71.6% (n = 199) of the 278 cases. Median age at HCC diagnosis among Asians was 59 years (range 27–88). Men comprised 75% of those with HCC and cirrhosis was present in 86% of these Asian cases. The countries most commonly represented among the non-English speaking Asian cases were Vietnam, China and Korea.
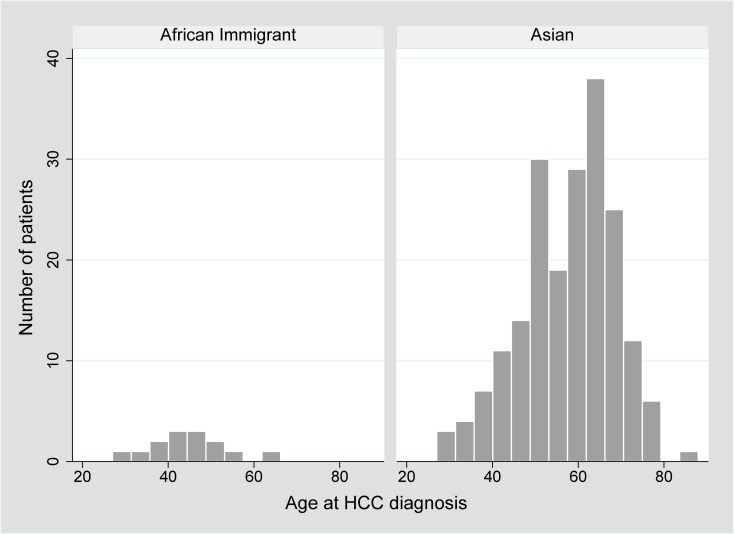
Black patients represented only 6.8% (n = 19) of all cases of HCC in the 14-year period of the study despite comprising 20% (n = 166) of the controls (P<0.001). Five percent (n = 14) of the HCC cases and 13% (n = 110) of controls were African immigrants. Most (86%, n = 12) of the HCC cases were of East African origin (mainly Ethiopia and Somalia), consistent with the demographics of African immigrants in King County, Washington [20]; the remaining two were from West African countries (Chad and Liberia). The median age at HCC diagnosis was 44 years (range 28–65), Fig 1. Notably, nearly all (93%) of the African patients were diagnosed with HCC at an age younger than 60 years compared with 52% of Asian cases (P<0.001). Most (79%, n = 11) African cases were men and 64% (n = 9) of patients met our critiera for cirrhosis. Of note, 89% (n = 8) of cirrhotic patients with HCC were diagnosed at an age younger than 50 years (median age 42). Among the African immigrant patients with HCC, 7% (n = 1) had HIV co-infection.
Fig 1. Demonstrates the distribution of age at HCC diagnosis across both African immigrants and Asians.
Seventy-one percent (n = 10) of African cases were diagnosed with HCC at an age younger than 50 years compared with 22% (n = 44) of Asian cases (P<0.001). Overall there were 71 patients who were diagnosed with HCC at an age younger than 50 years. The majority (62%) were Asian, 14% were African immigrant and 18% white. Eleven (15%) were HIV-coinfected and 77% had cirrhosis.
Factors associated with HCC
Asian race was the only race significantly associated with greater odds of HCC in univariate and multivariate analysis (Table 2) with an adjusted odds ratio (aOR) of 3.3 (95% CI 1.9–5.5) compared with white patients, after adjusting for diabetes, HIV or HCV coinfection, alcohol misuse and cirrhosis. The aOR for HCC in African immigrants was also elevated but to a lesser extent and did not meet statistical significance (aOR 1.3, 95% CI 0.6–2.9). Black non-immigrant status did not appear to be associated with HCC.
Table 2. Factors associated with hepatocellular carcinoma.
| Crude OR | 95% CI | P-value | Adjusted OR‡ | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| Race (reference: White) | ||||||
| African-immigrant | 0.6 | 0.3–1.1 | 0.12 | 1.3 | 0.6–2.9 | 0.58 |
| Black non-immigrant | 0.4 | 0.2–1.1 | 0.08 | 0.6 | 0.2–2.0 | 0.43 |
| Asian | 2.3 | 1.6–3.3 | <0.001 | 3.3 | 1.9–5.5 | <0.001 |
| Other | 1.4 | 0.6–3.2 | 0.44 | 1.9 | 0.6–5.7 | 0.27 |
| HIV coinfection | 0.5 | 0.3–0.9 | 0.02 | 0.8 | 0.4–1.8 | 0.66 |
| HCV coinfection | 0.9 | 0.6–1.3 | 0.56 | 0.9 | 0.6–1.6 | 0.80 |
| Diabetes | 1.0 | 0.7–1.3 | 0.77 | 0.6 | 0.4–0.9 | 0.03 |
| Alcohol | 1.1 | 0.7–1.8 | 0.63 | 0.9 | 0.5–1.7 | 0.71 |
| Cirrhosis | 17.3 | 11.1–27 | <0.001 | 19.7 | 12.2–31.8 | <0.001 |
OR, odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; HCV, chronic hepatitis C.
‡ Multivariable conditional logistic regression model that included all of the factors noted in the table. Cases and controls were matched for age and sex.
Cirrhosis had the strongest independent association with HCC overall, crude OR 17.3 (95% CI 11.1–27) and aOR 19.7 (95% CI 12.2–31.8). Diabetes was associated with decreased odds of HCC, aOR 0.6 (95% CI 0.4–0.9) in our primary model but not in our secondary analysis excluding cirrhosis (see below). HCV or HIV co-infections and alcohol use were not associated with increased odds of HCC in our main analysis.
In the secondary multivariable analysis that did not include cirrhosis (S1 Table, Asian race remained associated with HCC with aOR 2.6 (95% CI 1.7–3.9). In contrast to the primary analysis, diabetes was no longer associated with HCC, aOR 1.0 (95% CI 0.7–1.4). Alcohol use disorder was, however, associated with a greater odds of HCC in this model without cirrhosis, aOR 1.6 (95% CI 0.9–2.7, P = 0.08).
We also reexamined the multivariable model (including cirrhosis) with Asian race stratified as US-born versus not, using English language as our surrogate for US-born status. Among the 593 Asians, 171 (29%) were noted to be English speakers. Asians, either primary English or non-English speakers, had elevated risk for HCC (see S2 Table).
Discussion
We conducted a case-control study set in a large academic medical center and public safety-net hospital serving a heterogeneous population of patients with chronic hepatitis B including African immigrants. Among our mostly immigrant cohort, we identified 278 HCC cases over a 14-year span. Our cases were predominantly male, consistent with the prior observation that men carry a 4-fold higher risk of HCC than women [21, 22]. Asian race and cirrhosis were the independent factors most strongly associated with HCC after adjusting for key demographic and clinical factors, with Asians and cirrhotics comprising the majority of our cases. African-born patients comprised the majority of black patients with chronic hepatitis B in our cohort, consistent with the known epidemiology of this infection. They comprised 5% of all HCC cases (and 13% of controls) observed during this time and African immigrant status was not significantly associated with excess HCC risk compared with whites. Africans were however diagnosed, on average, at a younger age than HCC cases in Asians.
Studies have shown that African-born patients, particularly West Africans, are at increased risk for developing HCC at an earlier age than other populations [10, 11, 23–25]. However this finding has not been established in immigrant patients, specifically those with chronic hepatitis B, from these regions. Country of birth rather than race/ethnicity was an independent risk factor for early-onset HCC in one study based on the Surveillance, Epidemiology, and End Results (SEER) cancer registry, in which origin from West Africa or, to a lesser extent, Central/South or East Africa had a strong association with onset of HCC at ages younger than 40 years [9]. However, the SEER study did not capture data on liver disease severity or presence of viral hepatitis which are key determinants of HCC risk. Our study is the first to document this risk of early-onset HCC specifically in African-born immigrants with chronic HBV. The median age at HCC diagnosis in our study (i.e., 44 years) is comparable to those reported in native African cohorts [11]. The mechanism for this earlier presentation remains unclear. Screening for HCC at younger ages for African-born patients is unlikely to account fully for this given the lengthy period of case finding. Longer duration of chronic HBV infection may play a role but would not fully explain why African-born patients had younger median age at diagnosis than Asians, who frequently acquire the infection perinatally. Increased exposure to dietary iron or to aflatoxin (a mycotoxin produced in grains and peanuts that have been stored in warm, moist conditions and a known environmental risk factor for HCC), particularly among African immigrants, have been postulated to potentiate this early risk for HCC [23]. The role of genetic susceptibility to HCC has not been fully explored across multiple races, and most studies of candidate single nucleotide polymorphisms or genome wide association have been conducted primarily in Asians or Caucasians than Africans [26]. Regional differences in viral factors–HBV genotype, delta coinfection and specific genetic mutations–may also contribute to hepatocarcinogenesis [27–29] in ways not yet fully elucidated.
Our findings do underscore the greater odds of HCC in Asian patients overall. This increased risk may be attributable to HBV genotype C, prevalent in and specific to Asia, which has been linked with greater risk of HCC [30]. Greater time with higher HBV viremia may also be a contributing factor, as suggested by one study that compared Chinese and Senegalese patients with HBV infection [31] and other longitudinal cohort studies of patients with chronic HBV that have identified perinatal acquisition as a risk factor for significant fibrosis and HCC [32].
Cirrhosis had the strongest association for HCC in our study, and was present in the majority of chronic HBV patients with HCC regardless of their race/ethnicity. Unlike other causes of HCC where cirrhosis is nearly ubiquitous in HCC, HBV is an oncogenic virus that can, through epigenetic changes in the liver microenvironment, potentiate HCC independent of cirrhosis. However HCC incidence is several orders of magnitude higher in HBV-infected patients with cirrhosis than in those without [30, 33]. Five-year cumulative risk for HCC may be as high as 15% in untreated chronic HBV patients with cirrhosis [33, 34]. While older literature has suggested that African patients may be more likely to present with HCC without cirrhosis [35], our findings suggest that, even in younger African-born patients, cirrhosis remains a key contributor to HCC and that liver disease staging needs to occur even in these younger patients.
Diabetes was not associated with HCC in our univariate analysis but when we adjusted for cirrhosis, diabetes had a reduced odds for HCC. We believe this is likely an artifact of the unexpectedly low prevalence of diabetes in the small subgroup of patients with HCC and no cirrhosis (3 of 37, or 8%) compared with controls without HCC or cirrhosis (80 of 239, 33%) in our cohort. Diabetes and metabolic syndrome have both been shown to be associated with an increased risk of HCC in population-based studies [36, 37]. Additionally, alcohol use disorder was only associated with greater odds of HCC in our multivariable model without cirrhosis, suggesting that its contribution to excess HCC risk may be largely mediated through cirrhosis.
Limitations of this study include the retrospective nature and the inability of a cross-sectional, case-control design to establish causal association or provide an estimate of HCC incidence. The actual incidence rate ratio for HCC in these key subsets of patients with chronic HBV is uncertain and cannot be derived from the present study. We were not able to assess or adjust for variability in HCC screening practices, if they existed, between different subgroups of patients. While differences in screening intervals or practices may have accounted for some of our findings, studies suggest that screening guidelines are infrequently and inconsistently followed in clinical practice across multiple patient groups [38–41] and that the majority of HCC has historically been diagnosed outside of surveillance [14, 42]. Misclassification is possible when relying on clinical diagnostic codes but we were able to confirm the accuracy of diagnoses for chronic HBV infection and HCC by chart review. The use of non-English language as an indicator for non-US-born may only capture a portion of all such individuals, and we may underrepresent HBV-endemic countries like India with this approach. We did not have data on delta coinfection or HBV-specific data such as e antigen status or more importantly HBV DNA levels, which have been shown to be associated with increased risk of HCC [43, 44]. We also did not include HBV treatment, a potentially important modifier of HCC [45], because of our concern for missing data and inadequate capture in our system. We may not have identified all HCC diagnoses in our patient population since some patients could have been diagnosed or have received HCC care outside our system. Our sample of African-born patients with HCC was small, thus our finding no association with African-born status and HCC should be interpreted with caution. This study was conducted in an urban academic medical setting and therefore subject to potential referral and/or selection bias; our findings may also not be generalizable to other populations or settings. Despite these limitations, our study included a large diverse population of patients with chronic HBV infection, including a variety of non-US-born persons, seen over a 14-year timespan and offers a contemporary examination of race/ethnicity as a risk factor for HCC, independent of other key clinical factors.
In summary, Asian race and cirrhosis were the main risk factors and most strongly associated with HCC overall in our study sample. While African immigrants accounted for a small proportion of HBV-associated HCC cases in our cohort and did not appear to have excess risk of HCC overall, they were diagnosed with HCC at younger ages than other racial groups. Further work needs to be done to evaluate and overcome the structural, cultural and patient- and provider-specific barriers that impede the timely diagnosis and optimal care of patients with chronic HBV infection. Non-US-born patients may–due to language, stigma and socioeconomic factors–be particularly vulnerable to delayed entry to appropriate medical care and missed opportunities in the prevention of HCC [46].
Supporting information
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–48. Epub 2016/12/06. 10.1001/jamaoncol.2016.5688 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter SR, Thein H-H, Gidding HF, Amin J, Law MG, George J, et al. Risk factors for hepatocellular carcinoma in a cohort infected with hepatitis B or C. Journal of gastroenterology and hepatology. 2011;26:1757–64. 10.1111/j.1440-1746.2011.06785.x . [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. 10.3322/caac.21262 . [DOI] [PubMed] [Google Scholar]
- 4.Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–37. 10.1002/cncr.29936 ; PubMed Central PMCID: PMCPMC4840031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary KL, et al. SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017.
- 6.Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13(2):123–35. Epub 2013/01/25. 10.1038/nrc3449 . [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–73.e1. Epub 2012/04/28. 10.1053/j.gastro.2011.12.061 ; PubMed Central PMCID: PMCPMC3338949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organization WH. Global Hepatitis Report—2017. Geneva: 2017.
- 9.Yang JD, Altekruse SF, Nguyen MH, Gores GJ, Roberts LR. Impact of country of birth on age at the time of diagnosis of hepatocellular carcinoma in the United States. Cancer 2016. p. 81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JD, Gyedu A, Afihene MY, Duduyemi BM, Micah E, Kingham TP, et al. Hepatocellular Carcinoma Occurs at an Earlier Age in Africans, Particularly in Association With Chronic Hepatitis B. Am J Gastroenterol. 2015;110(11):1629–31. 10.1038/ajg.2015.289 . [DOI] [PubMed] [Google Scholar]
- 11.Kew MC. Epidemiology of hepatocellular carcinoma in sub-Saharan Africa. Annals of Hepatology. 2013;12:173–82. [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M. AASLD PRACTICE GUIDELINE Management of Hepatocellular Carcinoma: An Update. Hepatology (Baltimore, Md). 2010;42:1–35. 10.1002/hep.20933 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The increasing burden of imported chronic hepatitis B—United States, 1974–2008. PLoS One. 2011;6(12):e27717 Epub 2011/12/14. 10.1371/journal.pone.0027717 ; PubMed Central PMCID: PMCPMC3233539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang JD, Ahmed Mohammed H, Harmsen WS, Enders F, Gores GJ, Roberts LR. Recent Trends in the Epidemiology of Hepatocellular Carcinoma in Olmsted County, Minnesota: A US Population-based Study. J Clin Gastroenterol. 2017;51(8):742–8. Epub 2017/04/27. 10.1097/MCG.0000000000000810 ; PubMed Central PMCID: PMCPMC5552490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppola N, Alessio L, Pisaturo M, Macera M, Sagnelli C, Zampino R, et al. Hepatitis B virus infection in immigrant populations. World Journal of Hepatology. 2015;7:2955–61. 10.4254/wjh.v7.i30.2955 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. 10.1002/hep.21178 . [DOI] [PubMed] [Google Scholar]
- 17.Kim BK, Kim DY, Park JY, Ahn SH, Chon CY, Kim JK, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546–53. Epub 2010/01/16. 10.1111/j.1478-3231.2009.02192.x . [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Chen Y, Zhao Y. The diagnostic value of the FIB-4 index for staging hepatitis B-related fibrosis: a meta-analysis. PLoS One. 2014;9(8):e105728 Epub 2014/08/29. 10.1371/journal.pone.0105728 ; PubMed Central PMCID: PMCPMC4148327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker M, El-Serag HB, Sada Y, Mittal S, Ying J, Duan Z, et al. Cirrhosis is under-recognised in patients subsequently diagnosed with hepatocellular cancer. Aliment Pharmacol Ther. 2016;43(5):621–30. Epub 2016/01/20. 10.1111/apt.13505 ; PubMed Central PMCID: PMCPMC4742403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Affairs OoIR.
- 21.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–66. Epub 2015/03/11. 10.1111/liv.12818 ; PubMed Central PMCID: PMCPMC4691343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 Suppl 4:5–13. Epub 2010/12/09. 10.1634/theoncologist.2010-S4-05 . [DOI] [PubMed] [Google Scholar]
- 23.Ladep NG, Lesi OA, Mark P, Lemoine M, Onyekwere C, Afihene M, et al. Problem of hepatocellular carcinoma in West Africa. World Journal of Hepatology. 2014;6:783–92. 10.4254/wjh.v6.i11.783 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirk GD, Lesi OA, Mendy M, Akano AO, Sam O, Goedert JJ, et al. The Gambia Liver Cancer Study: Infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology. 2004;39(1):211–9. 10.1002/hep.20027 . [DOI] [PubMed] [Google Scholar]
- 25.Bandoh S, Chb MB, Duguru MJ, Okeke EN, Bch BM. Hepatocellular Carcinoma Occurs at an Earlier Age in Africans, Particularly in Association With Chronic Hepatitis B.—PubMed—NCBI. 2015;42:1629–31. 10.1038/ajg.2015.289 [DOI] [PubMed] [Google Scholar]
- 26.Ezzikouri S, Benjelloun S, Pineau P. Human genetic variation and the risk of hepatocellular carcinoma development. Hepatol Int. 2013;7(3):820–31. 10.1007/s12072-013-9463-y . [DOI] [PubMed] [Google Scholar]
- 27.Kramvis A, Kew MC. Epidemiology of hepatitis B virus in Africa, its genotypes and clinical associations of genotypes. Hepatology Research. 2007:9–19. 10.1111/j.1872-034X.2007.00098.x [DOI] [PubMed] [Google Scholar]
- 28.Lamarca A, Mendiola M, Barriuso J. Hepatocellular carcinoma: Exploring the impact of ethnicity on molecular biology. Crit Rev Oncol Hematol. 2016;105:65–72. Epub 2016/07/04. 10.1016/j.critrevonc.2016.06.007 . [DOI] [PubMed] [Google Scholar]
- 29.Cao GW. Clinical relevance and public health significance of hepatitis B virus genomic variations. World J Gastroenterol. 2009;15(46):5761–9. 10.3748/wjg.15.5761 ; PubMed Central PMCID: PMCPMC2791267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raffetti E, Fattovich G, Donato F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: a systematic review and meta-analysis. Liver Int. 2016;36(9):1239–51. Epub 2016/04/12. 10.1111/liv.13142 . [DOI] [PubMed] [Google Scholar]
- 31.Evans AA, O'Connell AP, Pugh JC, Mason WS, Shen FM, Chen GC, et al. Geographic variation in viral load among hepatitis B carriers with differing risks of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 1998;7(7):559–65. Epub 1998/07/29. . [PubMed] [Google Scholar]
- 32.Shimakawa Y, Lemoine M, Njai HF, Bottomley C, Ndow G, Goldin RD, et al. Natural history of chronic HBV infection in West Africa: a longitudinal population-based study from The Gambia. Gut. 2016;65(12):2007–16. Epub 2015/07/18. 10.1136/gutjnl-2015-309892 . [DOI] [PubMed] [Google Scholar]
- 33.Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H, van Boemmel F, et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology. 2017;66(5):1444–53. Epub 2017/06/18. 10.1002/hep.29320 . [DOI] [PubMed] [Google Scholar]
- 34.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50. Epub 2004/10/28. . [DOI] [PubMed] [Google Scholar]
- 35.Kew MC. Hepatocellular carcinoma with and without cirrhosis. A comparison in southern African blacks. Gastroenterology. 1989;97(1):136–9. Epub 1989/07/01. . [DOI] [PubMed] [Google Scholar]
- 36.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–9. 10.1136/gut.2004.052167 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54(2):463–71. Epub 2011/05/04. 10.1002/hep.24397 ; PubMed Central PMCID: PMCPMC4141525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hearn B, Chasan R, Bichoupan K, Suprun M, Bagiella E, Dieterich DT, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis. 2015;61(11):1742–8. Epub 2015/08/05. 10.1093/cid/civ654 ; PubMed Central PMCID: PMCPMC4809983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannini EG, Cucchetti A, Erroi V, Garuti F, Odaldi F, Trevisani F. Surveillance for early diagnosis of hepatocellular carcinoma: how best to do it? World J Gastroenterol. 2013;19(47):8808–21. Epub 2014/01/01. 10.3748/wjg.v19.i47.8808 ; PubMed Central PMCID: PMCPMC3870532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85–93. Epub 2011/01/19. 10.7326/0003-4819-154-2-201101180-00006 . [DOI] [PubMed] [Google Scholar]
- 41.Sarkar M, Stewart S, Yu A, Chen MS, Nguyen TT, Khalili M. Hepatocellular carcinoma screening practices and impact on survival among hepatitis B-infected Asian Americans. Journal of Viral Hepatitis. 2012;19:594–600. 10.1111/j.1365-2893.2011.01577.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santi V, Buccione D, Di Micoli A, Fatti G, Frigerio M, Farinati F, et al. The changing scenario of hepatocellular carcinoma over the last two decades in Italy. J Hepatol. 2012;56(2):397–405. Epub 2011/07/16. 10.1016/j.jhep.2011.05.026 . [DOI] [PubMed] [Google Scholar]
- 43.Mendy ME, Welzel T, Lesi OA, Hainaut P, Hall AJ, Kuniholm MH, et al. Hepatitis B viral load and risk for liver cirrhosis and hepatocellular carcinoma in The Gambia, West Africa. J Viral Hepat. 2010;17(2):115–22. Epub 2009/10/31. 10.1111/j.1365-2893.2009.01168.x ; PubMed Central PMCID: PMCPMC2817443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen CJ, Iloeje UH, Yang HI. Long-Term Outcomes in Hepatitis B: The REVEAL-HBV Study. Clinics in Liver Disease. 2007;11:797–816. 10.1016/j.cld.2007.08.005 . [DOI] [PubMed] [Google Scholar]
- 45.Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62(4):956–67. 10.1016/j.jhep.2015.01.002 . [DOI] [PubMed] [Google Scholar]
- 46.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51(3):729–33. 10.1002/hep.23561 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.