Abstract
Floral scent is an important part of volatile organic compounds (VOCs) emitted from plants, and is influenced by many environmental and endogenous factors. To investigate the influence of temperature on the emission of the floral scent of Osmanthus fragrans, the number of chemical compounds and their relative release amounts from four cultivars of O. fragrans under different temperature treatments, were identified using the solid-phase microextraction (SPME) technique and gas chromatography-mass spectrometry (GC-MS) in this study. Results revealed that the numbers and release amounts of floral scent components were significantly influenced by different temperatures, and depend on different cultivars and different types of compounds. Overall, most cultivars had the largest number of chemical compounds in 19 °C and the numbers of chemical compounds decreased with the increase or decrease in the temperature. Alcohols and ketones were the two main kinds of compounds responding to temperature change. The response of a specific chemical compound to temperature change was different in four cultivars. Generally, linalool, α-ionone, β-ionone, and γ-decalactone accounted for the highest proportion in the nine main compounds, and changes of these four chemical compounds to different temperatures had obvious contributions to the floral scent of O. fragrans. The results obtained provide evidence that temperatures can greatly influence the emission of floral scent.
Keywords: floral scent, Osmanthus fragrans, temperature, volatile organic compounds, SPME, GC-MS
1. Introduction
Plant organs, such as flowers, fruits, leaves and roots, can produce and emit a large variety of volatile organic compounds (VOCs) that are useful in their interactions with their immediate environment. At present, more than 1000 VOCs have been reported to be emitted from vegetative parts as well as flowers [1]. Most of these VOCs can be assigned to the following classes: terpenoids, phenylpropanoids, and benzenoids, fatty acid derivatives, and amino acid derivatives [2,3]. As an important part of VOCs emitted from plants, a floral scent plays a significant role in many eco-physiological processes [4]. Floral scents are not only responsible for attraction of butter flies, moths, and bats for pollination, but also act as semiochemicals for the communication with neighboring plants [5]. Moreover, some floral scent compounds are usually distillated to obtain essential oils for further applications such as aromatherapy, perfume, flavoring, and food additives [5].
Many studies have shown that the environmental factors such as temperature [6,7,8,9,10,11,12,13], light [8,14], atmospheric CO2 concentration [12,15,16,17], agricultural practices (e.g., irrigation and fertilization) [18], and endogenous factors including circadian rhythms [19,20,21], leaf age [16,22,23], floral developmental stages [24,25] and plant age [26], are responsible for the production and emission rates of floral scents. In the past, researchers have focused on the effect of temperature on the VOCs emitted from plant leaves and fruits. With the enhancement of temperature, the volatile concentrations of apples increase, although production rate is reduced above 32 °C [27]. The emission rate of five monoterpenes shows positive correlation with leaf temperature between 20 °C and 46 °C in Pinus elliottii [28]. Isoprene emission from plant leaves increases with temperature and reaches the highest release rate at 40 °C [29]. Terpenoids and indole emissions increase with temperature to an optimum between 22 and 27 °C and thereafter decrease in corn plants [30]. Analysis of volatiles from blended leaves in sweet basil by gas chromatography-mass spectrometry (GC-MS) showed that the plants grown at 25 °C contained significantly more volatile oils than those grown at 15 °C [31].
Since the floral scent plays a key role in plant pollination and plant defense response, more and more attention has been paid to the effect of temperature on floral scent emission. It has been found that the total release amount of floral scent are greatly affected by the different temperatures in Trifolium repens [32], Petunia axillaris [33], and Lilium ‘Siberia’ [8]. The total endogenous amount of scent components decreased as the temperature increased, the total emission showing a peak at 30 °C in Petunia axillaris [33]. The results in Lilium ‘Siberia’ revealed that the numbers and release amounts of floral scent components were significantly influenced by temperature, and 30 °C resulted in the highest numbers and release amounts of the floral scent components [8].
Osmanthus fragrans belongs to the Oleaceae family and is one of the ten most famous flowers in China. They are evergreen trees and shrubs, grown as ornamental plants for their attractive foliage and fragrant edible flowers. Their flowers are usually distillated to obtain essential oils for further applications such as aromatherapy, haute couture perfumes, flavoring, food additives, and consumer products, such as soaps, shampoos, and detergents just because their particular fragrance. Floral fragrance is a desirable character for ornamental plants [34]. Because of the unique and remarkable scent, much research has focused on the identification of the components of the floral scent of O. fragrans [35,36,37]. A wide variety of volatile compounds were identified in different cultivars of O. fragrans, including α-ionone, β-ionone, linalool, cis- and trans-linalool oxide, γ-decalactone, and so on. In previous studies, these volatile compounds were greatly influenced by different temperatures in other ornamental plants Petunia axillaries [33], Lilium ‘Siberia’ [8], and different cultivars of Petunia × hybrida [7]. Since environmental temperature could greatly change in nature during the floral opening of O. fragrans, the release of floral scent is supposed to be influenced, which motivates us to gain knowledge of the relationships between floral scents and the surrounding temperatures. In this study, the floral scent emitted from four cultivars, “Yu Linglong” (YL) from the Albus group, “Jin Qiugui” (JQ) from the Luteus group, and “Yanhong Gui” (YH) and “Yingye Dangui” (YD) from the Aurantiacus group (Figure 1), of O. fragrans was collected by dynamic headspace at different levels of temperatures and further analyzed by using the solid-phase microextraction (SPME) technique and GC-MS technique. The numbers of chemical compounds and relative release amounts were subsequently identified to investigate the influence of temperature on the emission of floral scent.
Figure 1.
The floral morphology of four cultivars “Jin Qiugui” (JQ), “Yu Linglong” (YL), “Yanhong Gui” (YH), and “Yingye Dangui” (YD) of Osmanthus fragrans in this study.
2. Results
2.1. Influence of Temperature on the Number of Chemical Compounds Emitted from Osmanthus fragrans
In total, 53 chemical compounds were identified (Table 1 and Table 2) in this study. As shown in Table 3, with the treatment of medium temperature (19 °C), 29 chemical compounds were identified in YL, which was the highest of the four cultivars, while only 23 chemical compounds were detected in YH. Many of these compounds belong to the class of alkanes, but just one is an alkene compound. High-temperature treatment (32 °C) reduced the number of compounds of alkanes and ketones in JQ, YL, and YD (Table 3) and the number of compounds of alcohols in JQ and YL; therefore, the number of total chemical compounds in these cultivars, was respectively reduced by 25.92%, 17.24%, and 33.33%, compared with the treatment of 19 °C. However, four additional alkane compounds (4,6-dimethyldodecane, 3-methyltetradecane, 2-methylheptadecane, and pentadecane) were found in YH with high-temperature treatment. Generally, the number of ketones was reduced in four cultivars with high-temperature treatment.
Table 1.
All identified volatile compounds in this study.
| No. | Volatile Category | Compound Name | Code |
|---|---|---|---|
| 1 | Alcohols | cis-Linalool oxide | A1 |
| 2 | trans-Linalool oxide | A2 | |
| 3 | Linalool | A3 | |
| 4 | 3,7-Dimethyl-1,5,7-octatrien-3-ol | A4 | |
| 5 | Epoxy linalool | A5 | |
| 6 | 4-(2,6,6-Trimethyl-cyclohex-1-enyl)-butan-2-ol | A6 | |
| 7 | Cedrol | A7 | |
| 8 | 2,6-Dimethyl-1,7-octadiene-2,6-diol | A8 | |
| 9 | 2-(4-Methoxyphenyl)ethanol | A9 | |
| 10 | Ketones | 6-Methyl-5-hepten-2-one | B1 |
| 11 | 2,2,6-Trimethyl-6-ethenyldihydro-2H-pyran-3(4H)-one | B2 | |
| 12 | 4-(2,6,6-Trimethyl-2-cyclohexen-1-ylidene)-2-butanone | B3 | |
| 13 | 2H-α-Ionone | B4 | |
| 14 | 2H-β-Ionone | B5 | |
| 15 | α-Ionone | B6 | |
| 16 | Geranyl acetone | B7 | |
| 17 | β-Ionone | B8 | |
| 18 | 4-(2,2,6-Trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)-3-buten-2-one | B9 | |
| 19 | Pseudo ionone | B10 | |
| 20 | 6-Pentyl-2H-pyran-2-one | B11 | |
| 21 | Alkenes | (E)-Ocimene | C1 |
| 22 | (Z)-Ocimene | C2 | |
| 23 | 2,2,4,6,6-Pentamethyl-3-heptene | C3 | |
| 24 | 2,2,4,10,12,12-Hexamethyl-7-(3,5,5-trimethylhexyl)-6-tridecene | C4 | |
| 25 | 7-Tetradecene | C5 | |
| 26 | α-farnesene | C6 | |
| 27 | Esters | (Z)-3-Hexenyl butanoic acid ester | D1 |
| 28 | (Z)-4-Hexen-1-yl butanoic acid ester | D2 | |
| 29 | cis-3-Hexenyl-α-methylbutyrate | D3 | |
| 30 | cis-3-Hexenyl n-valeric acid ester | D4 | |
| 31 | γ-Decalactone | D5 | |
| 32 | Alkanes | Tridecane | E1 |
| 33 | 3,7-Dimethyldecane | E2 | |
| 34 | Tetradecane | E3 | |
| 35 | 4,6-Dimethyldodecane | E4 | |
| 36 | 3-Methyltetradecane | E5 | |
| 37 | Pentadecane | E6 | |
| 38 | 2-Methylheptadecane | E7 | |
| 39 | 3-Methylpentadecane | E8 | |
| 40 | Hexadecane | E9 | |
| 41 | Nonadecane | E10 | |
| 42 | 2,6,10,14-Tetramethylpentadecane | E11 | |
| 43 | 8-Hexylpentadecane | E12 | |
| 44 | 2,6,10,14-tetramethylhexadecane | E13 | |
| 45 | Heptadecane | E14 | |
| 46 | Octadecane | E15 | |
| 47 | Pentacosane | E16 | |
| 48 | Heneicosane | E17 | |
| 49 | Eicosane | E18 | |
| 50 | Hexatriacontane | E19 | |
| 51 | Dotriacontane | E20 | |
| 52 | Tetrapentacontane | E21 | |
| 53 | Tetracontane | E22 |
Table 2.
Average relative content of each volatile compounds response to different temperatures. Code of each volatile compound is the same as that in Table 1.
| Volatile Category | Compound Code | Average Relative Content (%) of Each Volatile Compounds Identified at Different Temperatures (°C) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JQ | YL | YH | YD | ||||||||||||||
| 12 | 15 | 19 | 32 | 12 | 15 | 19 | 32 | 12 | 15 | 19 | 32 | 12 | 15 | 19 | 32 | ||
| Alcohols | A1 | 1.14 | 1.09 | 0.42 | 0.70 | 4.21 | 4.13 | 2.23 | 1.39 | 0.9 | 2.61 | 2.14 | - | 4.91 | 4.31 | 0.66 | 0.64 |
| A2 | 2.03 | 1.59 | 0.76 | 2.58 | 5.42 | 5.38 | 3.17 | 4.75 | 1.01 | 4.4 | 2.63 | - | 11.84 | 9.62 | 1.37 | 2.22 | |
| A3 | 26.38 | 24.39 | 12.21 | 10.33 | 28.69 | 28.88 | 31.23 | 8.33 | 62.55 | 56.25 | 57.63 | 14.04 | 15.95 | 16.85 | 15.61 | 28.15 | |
| A4 | - | - | - | - | - | - | - | - | - | - | - | 0.38 | - | - | - | - | |
| A5 | 0.48 | 0.36 | 0.25 | 0.56 | 1.28 | 1.3 | 0.79 | 5.7 | 0.2 | 1.06 | 0.62 | 0.49 | 3.3 | 1.92 | 0.52 | 0.54 | |
| A6 | 0.29 | 0.26 | 0.82 | 1.59 | 0.17 | 0.11 | 0.12 | 0.72 | - | 2.7 | - | 1.41 | 0.46 | 1.67 | 0.52 | 0.17 | |
| A7 | 0.14 | - | - | - | 0.42 | - | - | - | - | - | - | - | - | - | - | - | |
| A8 | - | - | - | - | 0.07 | - | 0.05 | - | - | - | - | - | - | 0.7 | - | - | |
| A9 | 1.68 | 0.29 | 2.33 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Ketones | B1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| B2 | - | 0.07 | - | - | - | - | - | - | - | - | - | - | 0.47 | - | - | - | |
| B3 | - | - | - | - | 0.05 | - | 0.07 | - | - | - | - | - | - | - | - | - | |
| B4 | 0.14 | 0.2 | 0.27 | - | - | - | 0.23 | 0.02 | - | - | - | - | - | - | - | - | |
| B5 | 3.55 | 1.4 | 2.02 | 2.70 | 4.86 | 3.62 | 4.81 | 36.43 | 0.22 | 2.87 | 1.13 | 15.24 | 1.16 | 2.78 | 2.46 | 3.39 | |
| B6 | 7.29 | 3.25 | 11.6 | 5.70 | 7.47 | 8.21 | 12.09 | 10.96 | 1.14 | 2.79 | 2.86 | 4.78 | 4.93 | 6.6 | 8.2 | 5.13 | |
| B7 | 0.08 | 0.04 | 0.07 | 0.91 | - | - | 0.06 | 0.16 | - | - | 0.15 | 0.41 | - | 0.19 | 0.31 | ||
| B8 | 22.37 | 25.8 | 39.28 | 10.42 | 17.59 | 22.77 | 25.59 | 10.49 | 2.45 | 7.16 | 9.68 | 4.26 | 32.11 | 30.31 | 36.27 | 32.55 | |
| B9 | - | - | 0.05 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| B10 | - | 0.03 | 0.11 | - | - | - | 0.08 | - | - | - | - | - | - | - | - | - | |
| B11 | - | 0.17 | - | - | - | - | - | - | - | 1.16 | 0.32 | - | - | - | - | - | |
| Alkenes | C1 | - | 0.57 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| C2 | 19.5 | 31.14 | 4.28 | 2.40 | 10.71 | 13.77 | 9.23 | 1.32 | - | - | 1.86 | - | 7.94 | 9.4 | 3.72 | 2.07 | |
| C3 | - | - | - | - | - | - | - | - | - | - | - | 0.29 | - | - | - | - | |
| C4 | - | - | - | - | - | - | - | - | 0.22 | 1.18 | - | 0.21 | - | - | - | - | |
| C5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| C6 | - | 0.03 | 0.34 | 2.14 | 0.14 | - | - | - | - | - | - | - | 0.17 | - | - | ||
| Esters | D1 | - | - | - | - | 0.09 | 0.45 | 0.14 | 0.32 | 0.09 | 0.39 | - | 0.46 | - | - | 0.29 | |
| D2 | 0.45 | 0.3 | 0.68 | 0.22 | - | - | - | 1.35 | 0.81 | 0.24 | 0.53 | 0.63 | - | - | - | - | |
| D3 | - | - | - | - | - | - | 0.13 | - | - | - | - | - | - | - | - | - | |
| D4 | - | - | - | - | - | - | - | - | - | - | 0.45 | - | - | - | - | - | |
| D5 | 6.74 | 4.24 | 13.63 | 3.78 | 1.67 | 1.35 | 3.67 | 2.11 | 12.69 | 6.01 | - | 1.02 | 3.93 | 3.58 | 12.36 | 5.6 | |
| Alkanes | E1 | - | - | - | 1.89 | - | - | - | - | - | - | - | - | - | - | - | - |
| E2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| E3 | 0.12 | 0.14 | 0.37 | - | 0.2 | 0.34 | 0.17 | 0.7 | 0.12 | 0.54 | 0.51 | 1.79 | 1.62 | 0.42 | 0.33 | 0.5 | |
| E4 | - | - | - | - | 0.15 | 0.21 | 0.11 | 0.35 | - | - | - | 0.51 | - | - | 0.26 | - | |
| E5 | - | - | - | - | - | - | - | - | - | - | - | 0.32 | - | - | 0.07 | - | |
| E6 | 0.34 | - | 0.66 | 5.52 | - | - | 0.27 | 0.52 | - | - | - | 4.53 | - | 0.24 | 0.77 | - | |
| E7 | 0.26 | - | 0.28 | - | 0.06 | 0.07 | 0.03 | 0.11 | 0.09 | 0.13 | - | 0.3 | - | - | - | - | |
| E8 | - | - | 0.08 | - | - | - | - | - | - | - | - | - | - | - | 0.37 | - | |
| E9 | - | - | - | - | - | - | - | - | - | - | - | - | 1.2 | 0.23 | 1.23 | 0.35 | |
| E10 | 0.62 | 0.2 | 0.66 | - | 0.37 | 0.35 | 0.38 | 0.83 | 0.41 | 0.68 | 0.41 | 1.29 | 0.94 | 0.37 | 0.96 | 1.46 | |
| E11 | 0.34 | 0.14 | 0.41 | 1.75 | - | - | 0.36 | 1.02 | 0.17 | 0.59 | 0.43 | 1.81 | 0.95 | 0.34 | 0.68 | 0.59 | |
| E12 | - | - | - | - | - | - | 0.05 | 0.17 | 0.16 | 0.09 | - | - | - | - | - | - | |
| E13 | 0.27 | 0.06 | 0.21 | 1.75 | 0.13 | 0.2 | 0.18 | 0.38 | 0.23 | 0.45 | 0.35 | 0.55 | 1.37 | 0.45 | 0.08 | 0.14 | |
| E14 | 0.05 | 0.18 | - | 2.76 | - | - | 0.18 | 0.93 | 0.14 | 0.11 | 0.76 | 1.19 | 0.97 | 0.14 | 0.59 | - | |
| E15 | 0.08 | 0.13 | 0.14 | 1.36 | - | - | - | - | 0.18 | 0.45 | 0.2 | 1.52 | - | - | 0.03 | - | |
| E16 | 0.25 | 0.19 | - | - | - | - | - | - | - | - | - | - | - | 0.25 | - | ||
| E17 | 0.36 | 0.11 | 0.31 | 2.56 | 1.91 | 0.49 | 0.29 | 0.36 | 0.46 | 0.85 | 0.76 | 4.14 | 0.59 | 0.24 | 0.48 | 0.49 | |
| E18 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.16 | 0.08 | - | |
| E19 | - | - | 1.29 | - | - | 1.21 | 0.36 | - | - | - | - | - | - | 0.33 | 1.34 | 0.7 | |
| E20 | - | - | - | 6.06 | 1.58 | 0.18 | 0.37 | - | - | - | 0.97 | 9.84 | - | - | - | - | |
| E21 | - | - | - | - | - | - | - | - | - | 0.08 | 0.14 | 0.32 | - | - | - | - | |
| E22 | 0.72 | 0.52 | 1.18 | 6.64 | - | - | - | - | 3.67 | 1.14 | 0.18 | 8.99 | - | 0.36 | 1.63 | - | |
Table 3.
Numbers of chemical compounds identified in floral scent emitted from four different cultivars of Osmanthus fragrans at different levels of temperature.
| Volatile Category | Number of Chemical Compounds Identified at Different Temperatures (°C) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JQ | YL | YH | YD | |||||||||||||
| 12 | 15 | 19 | 32 | 12 | 15 | 19 | 32 | 12 | 15 | 19 | 32 | 12 | 15 | 19 | 32 | |
| Alcohols | 7 | 6 | 6 | 5 | 7 | 5 | 6 | 5 | 4 | 5 | 4 | 4 | 5 | 6 | 5 | 5 |
| Ketones | 5 | 8 | 7 | 4 | 4 | 3 | 7 | 5 | 3 | 4 | 5 | 4 | 4 | 4 | 4 | 3 |
| Alkenes | 1 | 3 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 |
| Esters | 2 | 2 | 2 | 2 | 1 | 2 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 1 | 1 | 2 |
| Alkanes | 11 | 9 | 11 | 9 | 7 | 8 | 12 | 10 | 10 | 11 | 10 | 14 | 7 | 11 | 16 | 7 |
| Total | 26 | 28 | 27 | 22 | 21 | 20 | 29 | 24 | 21 | 24 | 23 | 26 | 19 | 24 | 27 | 18 |
Low temperature (15 °C) treatment increased the number of alkenes and decreased the number of alkanes in JQ, YL, and YD, but had no significant effect on those two kinds of chemical compounds in YH. Moreover, the treatment of 15 °C had no significant effect on the number of alcohols and esters in four cultivars. In YL, the number of ketones was reduced by more than half with the treatment of 15 °C, while the number of ketones was similar in the three other cultivars with the treatments of 15 °C and 19 °C. Under lower temperature treatment (12 °C), the number of total chemical compounds in YL and YD was much lower than that with treatments of 19 °C; however, for JQ and YH, the number of total chemical compounds was similar to that with treatments of 19 °C. Generally, the treatment of 12 °C had no significant effect on the number of alcohols and alkenes among the four cultivars. The treatment of 12 °C greatly decreased the number of ketones in JQ, YL, and YH, and the number of alkanes in YL and YD.
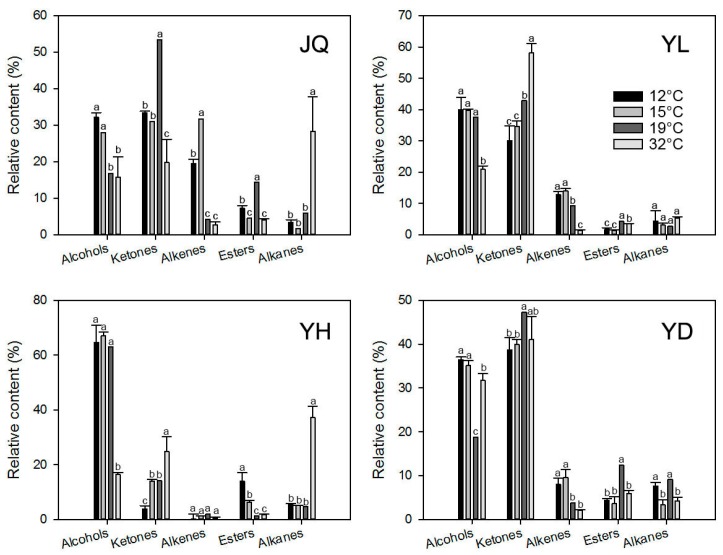
2.2. Influence of Temperature on the Relative Content of Chemical Compounds Emitted from Osmanthus fragrans
The relative release content of volatile compounds in the floral scent of O. fragrans at different temperature levels was also compared. Different temperatures significantly influenced the relative release content of different compound categories (p < 0.05; Figure 2). Compared to 19 °C, the relative release content of alcohols significantly increased in JQ and YD with the treatments of 12 and 15 °C; however, that in YL and YH was not changed under the treatments of 12 and 15 °C. The relative release content of alcohols with the treatment of 32 °C significantly decreased in YL and YH. Low temperatures of 12 and 15 °C decreased the relative release content of ketones in JQ and YD, but high temperatures of 32 °C reduced the relative release content of ketones in JQ. Similarly, in YL, the relative release content of ketones decreased when the temperature decreased to 12 and 15 °C, but increased when the temperature increased to 32 °C. In YH, the relative release content of ketones did not change with the treatments of 15 °C, but decreased with treatment of 12 °C and increased with treatment of 32 °C. The relative release content of alkenes significantly increased in most cultivars except in YH when the temperature decreased to 12 or 15 °C; however, it decreased in YL or did not change at all in the three other cultivars when the temperature increased to 32 °C. The relative release content of esters decreased whenever the temperature decreased to 12 and 15 °C or increased to 32 °C in JQ, YL, and YD. However, in YH, it increased when the temperature decreased to 12 and 15 °C and did not change at all when the temperature increased to 32 °C. Alkanes in all cultivars were not changed when the temperature decreased to 12 and 15 °C, but they significantly increased in JQ and YH when the temperature increased to 32 °C.
Figure 2.
Release amounts of different volatile categories in floral scent emitted from four cultivars of Osmanthus fragrans at different levels of temperature (12, 15, 19, and 32 °C). Error bars indicate SE (n = 3). Letters indicate significant differences (p < 0.05 by least significant difference) among the release amounts at different temperature treatments.
2.3. Influence of Temperature on The Relative Content of Main Chemical Compounds Emitted from Osmanthus fragrans
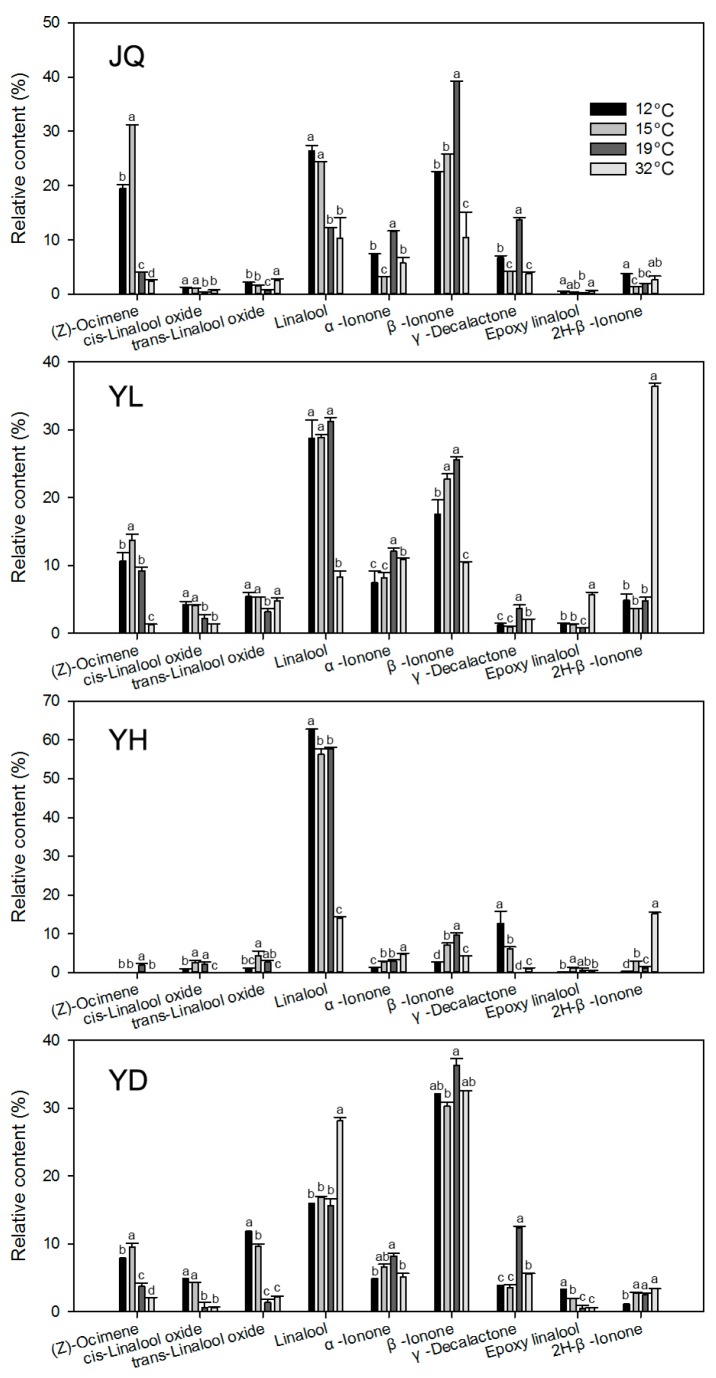
We also investigated the relative release amount of nine main chemical compounds that are beyond 1% in four cultivars of O. fragrans (Figure 3). The same chemical compound showed different changes to the same temperature change in different cultivars. For example, the relative release amount of (Z)-ocimene in most cultivars increased but decreased in YH when the temperature fell to 12 and 15 °C, which decreased in all cultivars with the treatment of 32 °C. Except for YH, the relative release amount of cis-linalool oxide and trans-linalool oxide increased with the fall of temperature to 12 and 15 °C and had no significant change or changed little with the increase in temperature to 32 °C. Only in JQ did the relative release amount of linalool significantly increase when the temperature fell to 12 and 15 °C. When the temperature increased to 32 °C, the relative release amount of linalool showed three different patterns: an increase in YD, a decrease in YL and YH, and no change in JQ. Whenever the temperature increased or decreased, the relative release amount of both α-ionone and β-ionone significantly decreased in all cultivars except for YH. The relative release amount of γ-decalactone significantly decreased in JQ, YL, and YD whenever the temperature increased or decreased; however, that significantly increased in YH whenever the temperature increased or decreased. The relative release amount of epoxy linalool increased in YL and YD when the temperature decreased to 12 and 15 °C, and it increased in JQ and YL with the treatment of 32 °C. The relative release amounts of 2H-β-ionone significantly increased in YL and YH when the temperature increased to 32 °C.
Figure 3.
Release amounts of nine volatile components in floral scent emitted from four cultivars of Osmanthus fragrans at different levels of temperature (12, 15, 19, and 32 °C). Error bars indicate SE (n = 3). Letters indicate significant differences (p < 0.05 by least significant difference) among the release amounts at different temperature treatments.
3. Discussion
Temperature is an important environmental factor to influence the emission of volatile compounds from plants [8,13,33]. In this study, the emissions of floral scent from four cultivars of O. fragrans treated with different levels of temperatures were investigated. Generally in most cultivars, the treatment of 32 °C resulted in lower numbers of the chemical volatile compounds than 19 °C (Table 3). Similarly, compared with 30 °C, the number of chemical compounds decreases with the increase of the temperature to 40 °C in Lilium ‘Siberia’ [8]. It was also found in Petunia axillaris [33] and Mediterranean plants [38] that the amount of floral scent greatly decreases with the enhancement of temperature.
Moreover, a high temperature negatively influences the release of chemical volatile compounds [7,8,33,38]. A high temperature of 40 °C significantly decreases most volatile components of Lilium ‘Siberia’, especially trans-ocimene, α-ocimene, and linalool [8]. In Petunia axillaris, 35 °C reduced not only release the amount of floral scent compounds, but also their endogenous levels compared with the other lower temperatures [33]. A high temperature (32 °C) shows influences similar to Lilium ‘Siberia’ and Petunia axillaris in O. fragrans, which was found to greatly decrease the relative release amount of α-ionone, β-ionone, γ-decalactone, and (Z)-ocimene in most cultivars (Table 2 and Figure 3). Two potential pathways contributed to the change in floral scent at different temperatures. On the one hand, the biosynthesis processes regulated by their synthesis enzymes. On the other hand, vaporization was also an important factor. It has been reported that temperature influences both metabolic and vaporization processes of the floral scent emission in Petunia axillaris [33]. What is more, in Petunia × hybrid, increasing ambient temperature leads to a decrease in phenylpropanoid-based floral scent production, which could be attributed to the downregulation of scent-related structural genes’ expression and the upregulation of a negative regulator of scent. A high temperature may downregulate volatile compound-related gene expression and reduce their enzyme activities, which would result in a lower release amount of many chemical volatile compounds in O. fragrans.
Downregulation of high temperature did not occur in the release of all chemical volatile compounds. For instance, 32 °C significantly increased the relative release amount of 2H-β-ionone in YL and YH (Table 2 and Figure 3), which generated an increase in the release amount of ketones, although 32 °C reduced the emission of other ketone compounds such as α-ionone and β-ionone in these two cultivars. A hypothesis was raised that gene expression related to some chemical volatile compounds such as 2H-β-ionone was probably upregulated by 32 °C. In addition, Farré-Armengol et al. revealed that the response of floral emissions to temperature differed among species and among different compounds within the species, after measuring the temperature responses of floral emissions of various common species of Mediterranean plants [38], suggesting that the response of floral emissions to temperature might differ among cultivars of O. fragrans.
As for lower temperatures, 12 °C resulted in lower numbers of the chemical volatile compounds than 19 °C in all four cultivars (Table 3), while 15 °C decreased numbers of the chemical volatile compounds in YL and YD and slightly affected numbers in JQ and YH (Table 3), which was similar to the results in Lilium ‘Siberia’ [8] that numbers of chemical compounds decrease with the decrease in temperature from 30 to 10 °C. In additional, the release amounts of ketone and ester compounds was significantly lower with the lower temperature treatments (10 and 20 °C) [8]. In accordance with the results of Lilium ‘Siberia’ lower temperatures (12 and 15 °C) resulted in lower release amounts of volatile categories ketones and esters in most cultivars (Figure 2). To be specific, the release amounts of α-ionone and β-ionone from ketones, and γ-decalactone from esters were greatly reduced with the lower temperature treatments in most cultivars (Figure 3). However, the release amounts of some volatile compounds were greatly increased with the lower temperature treatments, such as (Z)-ocimene, cis-linalool oxide, and trans-linalool oxide (Figure 3). As mentioned above, the release of a certain volatile compound is complex, which depends on the combined action of the biosynthesis processes and vaporization. Based on the endogenous levels of floral scent compounds at different temperatures in Petunia axillaris, the endogenous levels of iso-eugenol, benzyl benzoate, benzyl alcohol, and 2-phenylethanol gradually increases with the decrease in temperature from 30 to 20 °C [33]. The release amounts of iso-eugenol and benzyl benzoate gradually decreases with the decrease in temperature from 30 to 20 °C; however, the release amounts of benzyl alcohol and 2-phenylethanol increases first and then decreases from 30 to 20 °C [33], indicating that the increase in the endogenous amount by the decrease in temperature could be recovered by an increase in vapor pressure, which probably corresponds to the emission ratio.
4. Materials and Methods
4.1. Plant Material
Five-year-old potted plants of four O. fragrans cultivars, “Yu Linglong” (YL) from Albus group, “Jin Qiugui” (JQ) from Luteus group, “Yanhong Gui” (YH) and “Yingye Dangui” (YD) from Aurantiacus group (Figure 1), grown under the field condition in the germplasm repository of Zhejiang Agriculture and Forestry University, were employed as materials. When the developmental stages of flowers reached to Linggeng stage (the inflorescence burst through bracts and the florets closely crowded), potted plants were transported to the laboratory for treatments.
4.2. Temperature Treatments
Climate chambers were employed to provide different temperatures for the treatments. Treatments with low temperatures of 12 and 15 °C, medium temperature of 19 °C, and high temperature of 32 °C were used in this study. For each cultivar, three potted plants were cultured in a climate chamber with certain temperature (12, 15, 19, or 32 °C), and 12 potted plants were used for temperature treatments in total. Light intensity was maintained at 90–108 μmol·m−2·s−1 with a relative humidity of 80% in each climate chambers. Potted plants were continuously treated until the flowers were fully opened.
4.3. Floral Scent Collection
The samples were collected when the flowers were fully opened. A portion of 0.4 g of the entire flowers from all treatments were respectively detached and placed into brown glass vials (20 mL) capped with plastic caps having a polytetrafluoroethylene septum. Floral volatiles were collected using the solid-phase microextraction (SPME) method [24]. A fiber of 100 μm poly dimethyl-siloxane (PDMS, Supelco, Bellefonte, PA, USA) was used following the methods used in tree peony [24]. After 15 min equilibration, the aged PDMS fiber was exposed to each sample for 40 min and was then transferred into the injection port of the GC-MS systems. Before extraction, the fiber was aged for 1 h at 230 °C. Three biological replicate samples were collected from each treatment.
4.4. GC-MS Analysis
The samples were analyzed by gas chromatography-mass spectrometry (GC-MS QP2010 Plus, SHIMADZU, Kyoto, Japan) fitted with a gas chromatograph column Restek Rtx-Wax (30 m × 0.25 mm). Helium (99.999%) was used as the carrier gas at a flow rate of 1.0 mL·min−1. The column pressure was 49.5 Pa, and the desorption time was 1 min. The initial oven temperature of the column was maintained at 40 °C for 5 min and then ramped to 250 °C at 5 °C·min−1, and maintained for 5 min. The temperature of the ion source and interface temperature were 230 °C and 250 °C, respectively. The mass spectrometer was operated in electron ionization (EI) mode, and the ionization potential was 70 eV, scanning the range of 33–650 amu.
The identification of the compounds was done based on the retention index (RI) by comparison of the mass spectra with the NTST08 and NTST08s databases through a G1701DA ChemStation (Agilent, Palo Alto, CA, USA). The constituents were further confirmed by comparing with the published references [39,40,41,42,43,44]. Retention indices (RI) were calculated using retention times of n-alkanes that had been injected to the same instrument and under the same chromatographic conditions. Quantitative analysis in percent was performed by peak area normalization measurements [24,45,46,47].
4.5. Statistical Analysis
The statistical analysis was performed by one-way analysis of variance (ANOVA), using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as average ± standard errors of three biological replicates. Least significant difference (LSD) test was employed and differences of p < 0.05 were considered significant.
Acknowledgments
This research was supported by National Natural Science Foundation of China (Grant No. 31501790 and 31170656), Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ16C160003 and LQ15C160004), Zhejiang Provincial Major Program of New Cultivar Breeding (Grant No. 2016C02056-12) and Foundation of Zhejiang Educational Committee (Y201533133).
Author Contributions
Hongbo Zhao conceived and designed the experiments; Jianxin Fu and Dan Hou performed the experiments; Chao Zhang, Zhiyi Bao, and Shaoqing Hu analyzed the data; Jianxin Fu and Hongbo Zhao wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Knudsen J.T., Eriksson R., Gershenzon J., Ståhl B. Diversity and distribution of floral scent. Bot. Rev. 2006;72:1–120. doi: 10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2. [DOI] [Google Scholar]
- 2.Dudareva N., Negre F., Nagegowda D.A., Orlova I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006;25:417–440. doi: 10.1080/07352680600899973. [DOI] [Google Scholar]
- 3.Dudareva N., Pichersky E., Gershenzon J. Biochemistry of plant volatiles. Plant Physiol. 2004;135:1893–1902. doi: 10.1104/pp.104.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raguso R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008;39:549–569. doi: 10.1146/annurev.ecolsys.38.091206.095601. [DOI] [Google Scholar]
- 5.Lin C.Y., Chen Y.H., Chang T.C., Chen Y.J., Cheng S.S., Chang S.T. Characteristic aroma-active compounds of floral scent in situ from Barringtonia racemosa and their dynamic emission rates. J. Agric. Food Chem. 2013;61:12531–12538. doi: 10.1021/jf404505p. [DOI] [PubMed] [Google Scholar]
- 6.Bertin N., Staudt M., Hansen U., Seufert G., Ciccioli P., Foster P., Fugit J.L., Torres L. Diurnal and seasonal course of monoterpene emissions from Quercus ilex (L.) under natural conditions application of light and temperature algorithms. Atmos. Environ. 1997;31:135–144. doi: 10.1016/S1352-2310(97)00080-0. [DOI] [Google Scholar]
- 7.Cna’ani A., Muhlemann J.K., Ravid J., Masci T., Klempien A., Nguyen T.T.H., Dudareva N., Pichersky E., Vainstein A. Petunia × hybrida floral scent production is negatively affected by high-temperature growth conditions. Plant Cell Environ. 2015;38:1333–1346. doi: 10.1111/pce.12486. [DOI] [PubMed] [Google Scholar]
- 8.Hu Z., Zhang H., Leng P., Zhao J., Wang W., Wang S. The emission of floral scent from Lilium ‘Siberia’ in response to light intensity and temperature. Acta Physiol. Plant. 2013;35:1691–1700. doi: 10.1007/s11738-012-1211-8. [DOI] [Google Scholar]
- 9.Niinemets Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010;260:1623–1639. doi: 10.1016/j.foreco.2010.07.054. [DOI] [Google Scholar]
- 10.Penuelas J. An increasingly scented world. New Phytol. 2008;180:735–738. doi: 10.1111/j.1469-8137.2008.02658.x. [DOI] [PubMed] [Google Scholar]
- 11.Penuelas J., Staudt M. BVOCs and global change. Trends Plant Sci. 2010;15:133–144. doi: 10.1016/j.tplants.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Räisänen T., Ryyppö A., Kellomäki S. Effects of elevated CO2 and temperature on monoterpene emission of Scots pine (Pinus sylvestris L.) Atmos. Environ. 2008;42:4160–4171. [Google Scholar]
- 13.Staudt M., Bertin N. Light and temperature dependence of the emission of cyclic and acyclic monoterpenes from holm oak (Quercus ilex L.) leaves. Plant Cell Environ. 1998;21:385–395. doi: 10.1046/j.1365-3040.1998.00288.x. [DOI] [Google Scholar]
- 14.Staudt M., Bertin N., Hansen U., Seufert G., Cicciolij P., Foster P., Frenzel B., Fugit J.L. Seasonal and diurnal patterns of monoterpene emissions from Pinus pinea (L.) under field conditions. Atmos. Environ. 1997;31:145–156. doi: 10.1016/S1352-2310(97)00081-2. [DOI] [Google Scholar]
- 15.Rasulov B., Hüve K., Välbe M., Laisk A., Niinemets Ü. Evidence that light, carbon dioxide, and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiol. 2009;151:448–460. doi: 10.1104/pp.109.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Z., Copolovici L., Niinemets Ü. Can the capacity for isoprene emission acclimate to environmental modifications during autumn senescence in temperate deciduous tree species Populus tremula? J. Plant Res. 2012;125:263–274. doi: 10.1007/s10265-011-0429-7. [DOI] [PubMed] [Google Scholar]
- 17.Velikova V., Tsonev T., Barta C., Centritto M., Koleva D., Stefanova M., Busheva M., Loreto F. BVOC emissions, photosynthetic characteristics and changes in chloroplast ultrastructure of Platanus orientalis L. exposed to elevated CO2 and high temperature. Environ. Pollut. 2009;157:2629–2637. doi: 10.1016/j.envpol.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Nurzyńska-Wierdak R. Sweet basil essential oil composition: Relationship between cultivar, foliar feeding with nitrogen and oil content. J. Essent. Oil Res. 2012;24:217–227. doi: 10.1080/10412905.2012.676763. [DOI] [Google Scholar]
- 19.Jiang Y., Chen X., Lin H., Wang F., Chen F. Floral scent in wisteria: Chemical composition, emission pattern, and regulation. J. Am. Soc. Hortic. Sci. 2011;136:307–314. [Google Scholar]
- 20.Loivamäki M., Louis S., Cinege G., Zimmer I., Fischbach R.J., Schnitzler J.-P. Circadian rhythms of isoprene biosynthesis in grey poplar leaves. Plant Physiol. 2007;143:540–551. doi: 10.1104/pp.106.092759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson M.J., Owen S.M., Possell M., Hartwell J., Gould P., Hall A., Vickers C., Nicholas Hewitt C. Circadian control of isoprene emissions from oil palm Elaeis guineensis. Plant J. 2006;47:960–968. doi: 10.1111/j.1365-313X.2006.02847.x. [DOI] [PubMed] [Google Scholar]
- 22.Guenther A., Karl T., Harley P., Wiedinmyer C., Palmer P.I., Geron C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature) Atmos. Chem. Phys. 2006;6:3181–3210. doi: 10.5194/acp-6-3181-2006. [DOI] [Google Scholar]
- 23.Mayrhofer S., Teuber M., Zimmer I., Louis S., Fischbach R.J., Schnitzler J.-P. Diurnal and seasonal variation of isoprene biosynthesis-related genes in grey poplar leaves. Plant Physiol. 2005;139:474–484. doi: 10.1104/pp.105.066373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z.G., Lee M.R., Shen D.L. Analysis of volatile compounds emitted from fresh Syringa oblata flowers in different florescence by headspace solid-phase microextraction-gas chromatography-mass spectrometry. Anal. Chim. Acta. 2006;576:43–49. doi: 10.1016/j.aca.2006.01.074. [DOI] [PubMed] [Google Scholar]
- 25.Steenhuisen S.L., Raguso R.A., Jurgens A., Johnson S.D. Variation in scent emission among floral parts and inflorescence developmental stages in beetle-pollinated Protea species (Proteaceae) S. Afr. J. Bot. 2010;76:779–787. doi: 10.1016/j.sajb.2010.08.008. [DOI] [Google Scholar]
- 26.Shiojiri K., Karban R. Plant age, communication, and resistance to herbivores: Young sagebrush plants are better emitters and receivers. Oecologia. 2006;149:214–220. doi: 10.1007/s00442-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 27.Dixon J., Hewett E.W. Factors affecting apple aroma/flavour volatile concentration: A review. N. Z. J. Crop Hortic. 2000;28:155–173. doi: 10.1080/01140671.2000.9514136. [DOI] [Google Scholar]
- 28.Tingey D.T., Manning M., Grothaus L.C., Burns W.F. Influence of light and temperature on monoterpene emission rates from slash pine. Plant Physiol. 1980;65:797–801. doi: 10.1104/pp.65.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guenther A.B., Monson R.K., Fall R. Isoprene and monoterpene emission rate variability: Observations with eucalyptus and emission rate algorithm development. J. Geophys. Res. 1991;96:10799–10808. doi: 10.1029/91JD00960. [DOI] [Google Scholar]
- 30.Gouinguené S.P., Turlings T.C.J. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 2002;129:1296–1307. doi: 10.1104/pp.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang X., Alderson P.G., Hollowood T.A., Hewson L., Wright C.J. Flavour and aroma of fresh basil are affected by temperature. J. Sci. Food Agric. 2007;87:1381–1385. doi: 10.1002/jsfa.2869. [DOI] [Google Scholar]
- 32.Jakobsen H.B., Olsen C.E. Influence of climatic factors on emission of flower volatiles in situ. Planta. 1994;192:365–371. doi: 10.1007/BF00198572. [DOI] [Google Scholar]
- 33.Sagae M., Oyama-Okubo N., Ando T., Marchesi E., Nakayama M. Effect of temperature on the floral scent emission and endogenous volatile profile of Petunia axillaris. Biosci. Biotechnol. Biochem. 2008;72:110–115. doi: 10.1271/bbb.70490. [DOI] [PubMed] [Google Scholar]
- 34.Chandler S.F., Brugliera F. Genetic modification in floriculture. Biotechnol. Lett. 2013;33:207–214. doi: 10.1007/s10529-010-0424-4. [DOI] [PubMed] [Google Scholar]
- 35.Cai X., Mai R.Z., Zou J.J., Zhang H.Y., Zeng X.L., Zheng R.R., Wang C.Y. Analysis of aroma-active compounds in three sweet osmanthus (Osmanthus fragrans) cultivars by GC-olfactometry and GC-MS. Zhejiang Univ. Sci. B. 2014;15:638–648. doi: 10.1631/jzus.B1400058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu B., Guo X., Xiao P., Luo L. Chemical composition comparison of the essential oil from four groups of Osmanthus fragrans Lour. flowers. J. Essent. Oil Bear. Plants. 2012;15:832–838. doi: 10.1080/0972060X.2012.10644128. [DOI] [Google Scholar]
- 37.Xin H., Wu B., Zhang H., Wang C., Li J., Yang B., Li S. Characterization of volatile compounds in flowers from four groups of sweet osmanthus (Osmanthus fragrans) cultivars. Can. J. Plant Sci. 2013;93:923–931. doi: 10.4141/cjps2012-333. [DOI] [Google Scholar]
- 38.Farré-Armengol G., Filella I., Llusià J., Niinemets Ü., Peñuelas J. Changes in floral bouquets from compound-specific responses to increasing temperatures. Glob. Chang. Biol. 2014;20:3660–3669. doi: 10.1111/gcb.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao H., Li Z.G., Shen D.L. GC/MS fingerprint analysis of Osmanthus fragrans Lour. in different varieties. Acta Hortic. Sin. 2009;36:391–398. [Google Scholar]
- 40.Deng C.H., Song G.X., Hu Y.M. Application of HS-SPME and GC-MS to characterization of volatile compounds emitted from osmanthus flowers. Ann. Chim. 2004;94:921–927. doi: 10.1002/adic.200490114. [DOI] [PubMed] [Google Scholar]
- 41.Li Z.G., Cao H., Zhu G.H., Gao J.R., Shen D.L. Study on chemical constituents of fragrance released from fresh flowers of three different Osmanthus franrans Lour. during different florescences. Chem. Ind. For. Prod. 2008;28:75–80. [Google Scholar]
- 42.Lin F.P., Ma N., Zhou S., Zhang R.M., Gao Y. Tds-Gc-Ms analysis of volatile organic compounds from the fresh flowers of four Osmanthus fragrans varieties. J. Inner Mong. Agric. Univ. (Nat. Sci. Ed.) 2012;2:48–51. [Google Scholar]
- 43.Sun B.G., He J. Introduction to Perfume. Chemical Industry Press; Beijing, China: 1996. [Google Scholar]
- 44.Wang L.M., Li M.T., Jin W.W., Li S., Zhang S.Q., Yu L.J. Variations in the components of Osmanthus fragrans Lour. essential oil at different stages of flowering. Food Chem. 2009;114:233–236. doi: 10.1016/j.foodchem.2008.09.044. [DOI] [Google Scholar]
- 45.Dötterl S., Burkhardt D., Weißbecker B., Jürgens A., Schütz S., Mosandl A. Linalool and lilac aldehyde/alcohol in flower scents: Electrophysiological detection of lilac aldehyde stereoisomers by a moth. J. Chromatogr. A. 2006;1113:231–238. doi: 10.1016/j.chroma.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Larsen M., Poll L. Odour thresholds of some important aroma compounds in strawberries. Z. Lebensm. Unters. Forsch. 1992;195:120–123. doi: 10.1007/BF01201770. [DOI] [Google Scholar]
- 47.Palá-Paú J., Brophy J.J., Goldsack R.J., Fontaniella B. Analysis of the volatile components of Lavandula canariensis (L.) Mill., a Canary Islands endemic species, growing in Australia. Biochem. Syst. Ecol. 2004;32:55–62. [Google Scholar]