Abstract
The robotic system has several technical advantages over the manual video thoracoscopic approach. It offers a high definition three-dimensional view and robotic arms are more comfortable to use, because they allow more precise, flexible, and intuitive movements. This case report describes a locally advanced thymoma in a 75-year-old male patient, excised through a robotic-assisted thymectomy with atypical resection of the infiltrated left upper lobe, the preservation of the left phrenic nerve and partial resection of the left anonymous vein involved, without necessity of reconstruction. Clinical staging was thymoma T3 B1–2, while the postoperative histological classification and radiation was thymoma T3, B3, Masaoka-Koga stage IIB. The postoperative course was uneventful and the patient was discharged in second postoperative day. This case remarks that robotic devices are of great help in the intraoperative recognition and precise management of infiltrated structure, like important vessels and nerves, avoiding conversion to an open approach, which until now was the main surgical indication in these situations.
Keywords: Robotic surgical procedure, thymoma, thoracic surgery, mediastinum, brachiocephalic vein
Introduction
Numerous thymectomy techniques have been proposed according to the degree of disease invasiveness, and so, many debates have remained as to which is the best surgical technique to use (1). Generally, thymectomy is indicated in case of thymomas, thymic carcinomas or thymic cysts and in the management of myasthenia gravis (2). The resection must be complete in order to avoid the residue of ectopic tissue (2).
With the constant evolution of minimally invasive techniques, video-assisted thoracoscopy (VATS) of mediastinal masses has begun to spread, even in treating thymomas. In 1992, the first video-assisted thoracoscopic thymectomy was described by Landreneau and coworkers (3). However, the anatomic area of the mediastinum has narrow spaces that are difficult to reach with conventional thoracoscopic instruments, and poses potential risks, especially in case of neoplastic involvement of vulnerable large vessels and nerves (2). The following evolution of surgical technologies and the development of robotic technique has tried to overcome limits of conventional thoracoscopic approach. So that, in 2001 the first robot-assisted thymectomy was described by Yoshino et al. (4). Therefore, robotic surgery started to be evaluated in treating thymomas (2).
Here, we describe a case of a locally advanced thymoma with involvement of the left anonymous vein, partially resected with a robotic approach, and briefly discuss the advantages of the robotic technique for the treatment of mediastinal lesions, especially in awkward situations.
Case presentation
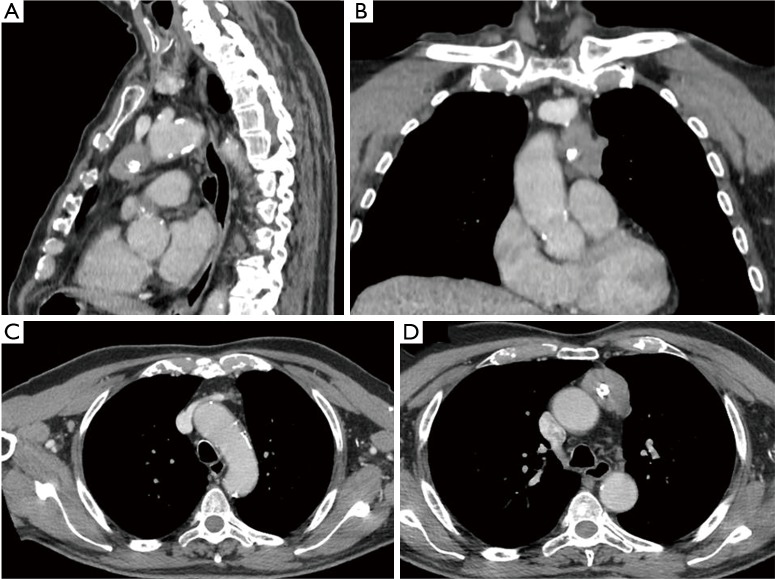
A 75-year-old male patient in good clinical condition referred to our hospital after a radiological finding of a mediastinal mass. The only symptom the patient reported was dysphonia for a few months. The patient underwent a chest-computed tomography (chest-CT) that confirmed the presence of a mass of 35 mm × 45 mm × 30 mm in the anterior mediastinum, with a main extension to the left and compatible with thymoma (Figure 1).
Figure 1.
CT-scan images. (A) Sagittal section: the relationship between the thymic mass and the caudal portion of the anonymous vein is visible; (B) coronal section: the mediastinal localization of the lesion is clearly visible; (C) frontal section: it is possible to see the contact between the innominate vein and the thymoma; (D) frontal section: it is shown the relationship between thymus and ascending aorta. CT, computed tomography.
Neurological examination, repetitive nerve stimulation, the dosage of circulating acetylcholine receptor antibodies and muscle-specific tyrosine kinase antibodies were performed. All these exams were negative for myasthenia gravis. Subsequently the patient underwent a CT-guided needle-biopsy of the lesion with a histological confirmation of diagnosis of thymoma B1–2, according to WHO classification.
Depending on the radiological imaging and the histological result, minimally invasive approach with robotic surgery had proposed to the patient. So, the informed consent was obtained. After general anesthesia the patient was intubated with a double-lumen tube for a selective lung ventilation. Patient positioning was standard, with an incomplete right lateral decubitus (sited up 30° angle). The left arm was positioned along his body as far back as possible to gain enough space for the robotic arms.
Intuitive da Vinci Robot Xi® with three robotic arms was installed. Three trocars were placed: the first one was positioned in the fifth intercostal space on the mid-axillary line and an 8mm thirty-degree camera was introduced. The second and the third trocars were positioned under vision in the third and sixth intercostal spaces respectively on the midclavicular line. CO2 was insufflated at 6–10 mmHg to enlarge the retrosternal mediastinal space and improving vision. A bipolar curved dissector was used to divide, whether a bipolar forceps was used both to trap the tissue and to coagulate when necessary (Figure 2).
Figure 2.

Instruments used for robotic thymectomy. (A) Curved bipolar dissector; (B) fenestrated bipolar forceps.
The mediastinal lesion was identified with the camera. It appeared to infiltrate the left upper lobe of the lung. An atypical lung resection of this area was performed, using a 60-mm stapler with two purple cartridges, introduced in the sixth intercostal space. The next structure detected was the left phrenic nerve. It was partially wrapped in the thymic tissue. It was patiently isolated with the bipolar dissector and spared. Afterwards, the mass was gently isolated by the pericardium and its vascular surface; some small adherences were coagulated with the bipolar dissector, and the right pleura was exposed.
The tissue dissection continued until the left anonymous vein was detected. One of its branches, the Keynes vein, was identified, closed using robotic Hem-o-lock® and cut. Just under this vessel there was a coin of neoplastic tissue of about 15 mm, involving the anonymous vein with suspicious of infiltration, because the vessel appeared to be retracted downward by the weight of the mass. So that, the isolation of the anonymous vein was completed on the posterior side of the vessel throughout its involved course to facilitate the passage of the vascular stapler jaws and make the procedure safer. According to the disposition of the neoplastic tissue coin, a vascular 30 mm stapler was introduced through the third intercostal space trocar, after removal of the robotic arm. The open jaws were collocated parallel to the tissue coin. In this way, when jaws closed, the resection of the vein tissue was limited, saving enough vessel tissue, without congestion or other vascular problems and without necessity of reconstruction.
The anatomical piece was removed with an endo-bag from the access at the sixth intercostal space. A frozen section confirmed free resection margins. Finally, a chest tube 28 Ch was positioned. The postoperative course was uneventful, and the patient was discharged on second postoperative day.
Final histology reported a thymoma B3, based on WHO classification, because the neoplasia infiltrated the perilesional capsule. The pathological stage was T3 according to TNM, and stage IIB on the basis of Masaoka-Koga classification.
Postoperative radiotherapy treatment was not performed, due to the age of the patient. A 6-month follow-up CT-scan was negative for local recurrence of neoplasia.
Discussion
The use of mini-invasive surgery to treat malignant thymic disease is now widely accepted, and it has advanced to include robotic-assisted thymectomy. The acceptance of this technique is growing, while the debate on which is the adequate technique is still opened (5). According to Manloy et al. (6), the conventional thoracoscopy should be the gold standard for the treatment of small thymic lesions (5-7). However, even for larger lesions, the use of VATS is showing numerous benefits associated with survival similar to that obtained through open surgery (8). Odaka and colleagues (8) recently reported the validity of the thoracoscopic approach in the treatment of thymic lesions with a diameter greater than 50 mm. Nevertheless, in their series, it was noted that a case of conversion was due to an infiltration of the left anonymous vein (8).
Robotic surgery using the da Vinci system represents a technological evolution of the minimally invasive platform in thoracic surgery. Its advantages over VATS consist of improved three-dimensional visualization, increased degrees of freedom of motion, and better ergonomics (9,10). Many surgery teams switched to robotic thymectomy, after extensive experience with video thoracoscopic thymectomy (11,12).
In this particular case, we chose a left robotic approach, because the lesion mainly extended to the left. Usually, the side we choose depends on which side the lesion develops most. In the case of median lesions, we normally prefer the left side for greater control on the phrenic nerve, according to the technique of Rueckert et al. (5). The choice of the side is variable, and there is not unanimous consensus on which is the more advantageous side. The proponents of the left-sided approach emphasize the superior visualization of a larger left-sided thymus, particularly in cases of complex overgrowth involving the phrenic nerve, as the main reason to obtain complete thymectomy (13,14). Conversely surgeons who favour easier conditions related to a larger space, and the landmark of the superior vena cava prefer the right-sided technique. They believe that trocar injuries could be best avoided by operating from the right side (15-20).
Furthermore, in this case, we performed a thymectomy and not a radical thymectomy. The extension of resection for malignant thymoma is still a matter of discussion. The National Comprehensive Cancer Network (NCCN) guidelines clearly state that surgical resection, which consists of radical thymectomy, is recommended for resectable thymic tumors (21). Conversely, the National Institute of Health (NIH) and the International Thymic Malignancy Interest Group (ITMIG) state that complete surgical resection is recommended for patients with either stage I or stage II thymoma, but they do not refer to the optimal extension of resection (22,23). In our experience, recently exposed at the oral presentation of the 2017 conference of Society of Thoracic Surgeons (STS), we treat 157 thymic malignancies, both radical thymectomy and conservative thymectomy (24). The two groups did not differ in terms of disease-free and overall survival after a mean follow up of 77 months, confirming the results of Tseng et al. (25).
Anyway, the peculiarity of this case is the easy management of the suspected vascular infiltration that was effortlessly overcome, thanks to the use of the surgical robot, as our video has shown (26) (Figure 3). Left anonymous vein infiltration is often a criterion that determines the need for conversion during mini-invasive thymectomy (27,28). Thanks to the limited extension of the contact with the vein and to the “EndoWrist” instruments that permit different degree of angle and motion, we managed to continue the minimally invasive approach and to pursue a conservative surgery.
Figure 3.

Main surgical passages of robotic resection of a thymoma involving the left anonymous vein, the left phrenic nerve and lung parenchyma of left upper lobe (26). Available online: http://www.asvide.com/articles/1747
The use of robotic surgery, thanks to the three-dimensionality and easy maneuverability of the instruments, offers the advantage to control situations that may appear technically more complex and dangerous, if conducted with conventional manual video-thoracoscopic surgery, despite we are aware that expert VATS surgeons can either afford complex situations after a long learning-curve.
Acknowledgements
The authors thank James Hughes for help with the English, and acknowledge support from the Umberto Veronesi Foundation for a fellowship to Pierluigi Novellis.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
G Veronesi is a consultant for ABI Medica SpA and Medtronic. Other authors have no conflicts of interest to declare.
References
- 1.Rea F, Marulli G, Bortolotti L, et al. Experience with the “da Vinci” robotic system for thymectomy in patients with myasthenia gravis: report of 33 cases. Ann Thorac Surg 2006;81:455-9. 10.1016/j.athoracsur.2005.08.030 [DOI] [PubMed] [Google Scholar]
- 2.Augustin F, Schmid T, Sieb M, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery thymectomy. Ann Thorac Surg 2008;85:S768-71. 10.1016/j.athoracsur.2007.11.079 [DOI] [PubMed] [Google Scholar]
- 3.Landreneau RJ, Dowling RD, Castillo WM, et al. Thoracoscopic resection of an anterior mediastinal tumor. Ann Thorac Surg 1992;54:142-4. 10.1016/0003-4975(92)91162-3 [DOI] [PubMed] [Google Scholar]
- 4.Yoshino I, Hashizume M, Shimada M, et al. Thoracoscopic thymomectomy with the da Vinci computer-enhanced surgical system. J Thorac Cardiovasc Surg 2001;122:783-5. 10.1067/mtc.2001.115231 [DOI] [PubMed] [Google Scholar]
- 5.Rueckert J, Swierzy M, Badakhshi H, et al. Robotic-assisted thymectomy: surgical procedure and results. Thorac Cardiovasc Surg 2015;63:194-200. 10.1055/s-0035-1549007 [DOI] [PubMed] [Google Scholar]
- 6.Manoly I, Whistance RN, Sreekumar R, et al. Early and mid-term outcomes of trans-sternal and video-assisted thoracoscopic surgery for thymoma. Eur J Cardiothorac Surg 2014;45:e187-93. 10.1093/ejcts/ezu077 [DOI] [PubMed] [Google Scholar]
- 7.Sugarbaker DJ. Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg 1993;56:653-6. 10.1016/0003-4975(93)90942-B [DOI] [PubMed] [Google Scholar]
- 8.Odaka M, Tsukamoto Y, Shibasaki T, et al. Thoracoscopic thymectomy is a feasible and less invasive alternative for the surgical treatment of large thymomas. Interact Cardiovasc Thorac Surg 2017;25:103-8. 10.1093/icvts/ivx048 [DOI] [PubMed] [Google Scholar]
- 9.Ashton RC, Jr, McGinnis KM, Connery CP, et al. Totally endoscopic robotic thymectomy for myasthenia gravis. Ann Thorac Surg 2003;75:569-71. 10.1016/S0003-4975(02)04296-0 [DOI] [PubMed] [Google Scholar]
- 10.Veronesi G, Cerfolio R, Cingolani R, et al. Report on first international workshop on robotic surgery in thoracic oncology. Front Oncol 2016;6:214. 10.3389/fonc.2016.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun Y, Hao L, Demin L, et al. Da Vinci robot-assisted system for thymectomy: experience of 55 patients in China. Int J Med Robot 2014;10:294-9. 10.1002/rcs.1577 [DOI] [PubMed] [Google Scholar]
- 12.Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. 10.1016/j.jtcvs.2012.12.031 [DOI] [PubMed] [Google Scholar]
- 13.Ismail M, Swierzy M, Rückert JC. State of the art of robotic thymectomy. World J Surg 2013;37:2740-6. 10.1007/s00268-013-2250-z [DOI] [PubMed] [Google Scholar]
- 14.Schneiter D, Tomaszek S, Kestenholz P, et al. Minimally invasive resection of thymomas with the da Vinci® surgical system. Eur J Cardiothorac Surg 2013;43:288-92. 10.1093/ejcts/ezs247 [DOI] [PubMed] [Google Scholar]
- 15.Marulli G, Rea F. Myasthenia gravis and thymectomy: many doubts and few certainties. Eur J Cardiothorac Surg 2015;48:46-7. 10.1093/ejcts/ezu398 [DOI] [PubMed] [Google Scholar]
- 16.Mussi A, Fanucchi O, Davini F, et al. Robotic extended thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2012;41:e43-6; discussion e47. [DOI] [PubMed]
- 17.Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicentre European study. J Thorac Cardiovasc Surg 2012;144:1125-30. 10.1016/j.jtcvs.2012.07.082 [DOI] [PubMed] [Google Scholar]
- 18.Cerfolio RJ, Bryant AS, Minnich DJ. Starting a robotic program in general thoracic surgery: why, how and lessons learned. Ann Thorac Surg 2011;91:1729-36; discussion 1736-7. [DOI] [PubMed]
- 19.Goldstein SD, Yang SC. Assessment of robotic thymectomy using the Myasthenia Gravis Foundation of America Guidelines. Ann Thorac Surg 2010;89:1080-5; discussion 1085-6. 10.1016/j.athoracsur.2010.01.038 [DOI] [PubMed] [Google Scholar]
- 20.Castle SL, Kernstine KH. Robotic-assisted thymectomy. Semin Thorac Cardiovasc Surg 2008;20:326-31. 10.1053/j.semtcvs.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 21.NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines®). Thymomas and thymic carcinomas. Version 1, 2017. March 2, 2017. Available online: www.nccn.org
- 22.National Institute of Health. Thymoma and Thymic Carcinoma Treatment (PDQ®). General information about thymoma and thymic carcinoma treatment. 2015;1-26. Available online: https://www.cancer.gov/cancertopics/pdq/treatment/thymoma/healthprofessional
- 23.International Thymic Malignancy Interest Group. Standard treatment options about thymoma. [cited in 2015 Feb 1]. Available online: https://www.itmig.org/node/11
- 24.Voulaz E, Veronesi G, Alloisio M. Oral presentation at STS 2017 conferences. 2017. [Google Scholar]
- 25.Tseng YC, Hsieh CC, Huang HY, et al. Is thymectomy necessary in nonmyasthenic patients with early thymoma? J Thorac Oncol 2013;8:952-8. 10.1097/JTO.0b013e31828cb3c2 [DOI] [PubMed] [Google Scholar]
- 26.Solinas M, Novellis P, Bottoni E, et al. Main surgical passages of robotic resection of a thymoma involving the left anonymous vein, the left phrenic nerve and lung parenchyma of left upper lobe. Asvide 2017;4:431. Available online: http://www.asvide.com/articles/1747
- 27.Keijzers M, Dingemans AM, Blaauwgeers H, et al. 8 years’ experience with robotic thymectomy for thymomas. Surg Endosc 2014;28:1202-8. 10.1007/s00464-013-3309-5 [DOI] [PubMed] [Google Scholar]
- 28.Deen S, Farivar AS, Louie BE. Thoracic techniques: robotic thymectomy for thymoma. Indian J Surg Oncol 2013;4:132-7. 10.1007/s13193-013-0211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]