Abstract
Tophaceous gout of the spine is an underappreciated source of back pain in patients with or without neurological decline. It has been reported to occur in the cervical, thoracic and lumbar spine. Rarely, does it occur at more than one region of the spine. Advanced imaging with magnetic resonance imaging and computed tomography are usually not helpful in differentiating between infection, malignancy and gout. Clinician should have a high suspicion of spinal gout in patients with history of gout who presents with renal insufficiency, presence of peripheral tophi on exam, with elevated serum uric acid and creatinine levels, erythrocyte sedimentation rate and C-reactive protein. Here we present a case of a 23-year-old male with history of gout and chronic renal disease with progressive weakness in his lower extremities with new urinary incontinence who was found to have spinal gout with epidural infection of both the cervical and thoracic spine. Our patient was successfully managed with surgical decompression followed by medical treatment with antibiotics and steroids.
Keywords: Spine, spinal gout, tophaceous gout, epidural infection, chronic kidney disease
Introduction
Gout is a common metabolic disease in which there is abnormal production or impaired excretion of uric acid resulting in the deposition of monosodium urate (MSU) crystals in joints and soft tissue causing acute and chronic inflammation. Common articular sites include the first metatarsophalangeal joint, knee, ankle, wrist and phalangeal joints (1). Tophi are nodules formed by the accumulation of MSU crystals in the soft tissue. MSU crystal deposition in the spine was once thought to be rare, however, several authors have speculated that the incidence is more common than previously believed (2-4). Kersley et al. reported the first case of tophaceous gout of the spine in 1950 when he identified post-mortem tophaceous destruction of the upper cervical spine (5). Since then there have been multiple case reports of tophaceous gout in the cervical (6,7), thoracic (8-10) and lumbar spine (11-14); with the lumbar spine being the most common site (2,3,14). There are currently only about three reports involving more than one region of the spine (1,6,15,16). MSU crystals can deposit on all spinal structures, such as facet joints (3,11), vertebral bodies (6), pedicles (9), intervertebral disc (7), ligamentum flavum (10), epidural space (17) and even intradural involvement of the filum terminale (18).
The presenting features of spinal gout include back pain (17), radiculopathy (11,12), myelopathic symptoms (10), paraparesis (8) or quadriplegia (6). In some cases, patients are asymptomatic and are not diagnosed until post-mortem (19,20). Clinical presentation and imaging studies can be nonspecific and can delay the diagnosis of spinal gout because they can resemble other more common pathologies, such as discitis (21), epidural abscess (13,14), and metastatic lesions (6,11). Most authors believe that the diagnosis of tophaceous gout of the spine is best made with high clinical suspicion and histopathological review of tissue samples obtain either by surgical decompression or image guided biopsy (2,14,22,23) in order to differentiate from infectious or malignant etiologies. Here we report an interesting case of a young patient with tophaceous gout of the cervical and thoracic spine with concomitant epidural infection. To our knowledge this is the first case report describing multilevel spinal gout involvement with associated epidural infection.
Case report
A 23-year-old male with past medical history significant for obesity, gout and early chronic kidney disease due to chronic interstitial nephritis attributed to heavy use of nonsteroidal anti-inflammatory drugs for gout management. Patient reported long standing back pain since age of 17 with gout flares as well as tophi in his fingers and great toe since the age of 20. He presented to our institution with 2–3 days of progressive lower extremity weakness with the inability to ambulate, numbness and paresthesia in his lower extremities and new urinary incontinence. He also complained of diffuse joint pain in his wrists, hands, hips, knees and ankles. Five months prior to presentation he was diagnosed with transverse myelitis secondary to coxsackievirus requiring one month of hospitalization in the intensive care unit. He subsequently recovered and progressed to his normal baseline function. He was discharged on prednisone taper which he completed 1 week prior to presenting at our institution.
On exam he was febrile and tachycardic and was found to have diffuse weakness in his upper and lower extremities that was worse in the left lower extremity. However, motor exam was limited by his multiple joint pains. He had negative upper motor neuron signs and intact rectal tone. Laboratory studies revealed leukocytosis [12.7×103/µL; normal range: (4.3–10) ×103/µL], elevated erythrocyte sedimentation rate (ESR: 97 mm/h; normal range: 0–15 mm/h) and C-reactive protein (CRP: 122.5 mg/L; normal range: 0–10 mg/L), elevated serum uric acid level (14.6 mg/dL; normal range: 3.9–7.6 mg/dL) and elevated creatinine (6.5 mg/dL; normal range: 0.51–1.18 mg/dL). Blood cultures were negative for bacterial or fungal growth.
Magnetic resonance imaging (MRI) of the cervical and thoracic spine showed epidural collection along the anterior thecal sac from C4 through T11 with enhancement of the facets and neural foramina at T8–9, T9–10, and T10–11 (Figures 1,2). There is also a right central disc protrusion at T6–7 and T7–8 causing mild anterior thecal effacement without cord signal changes. MRI of the cervical and lumbar spine demonstrated no epidural collection. However, there was enhancement of the right lumbar erector spinae muscle at the L1–2 level with perifacet enhancement of the right L2–3 facet and bilateral L3–4 and L4–5 facet. There was no evidence of osteomyelitis or discitis along the entire spine. Computed tomography of the entire spine showed normal alignment with bone erosion with well-defined sclerotic margins at the right L2–3 facet (Figure 3)
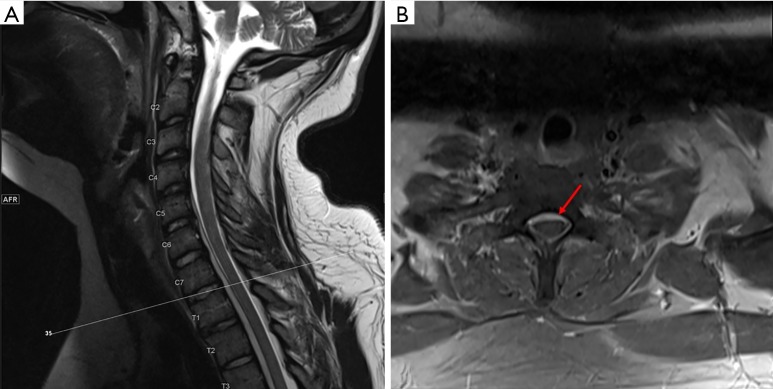
Figure 1.
MRI T2 sequences of the cervical spine. (A) Sagittal view of the cervical spine showing epidural collection along the ventral thecal sac from C4 to T3; (B) axial view at the level of C7–T1 showing epidural collection ventral to the thecal sac as indicated by the arrow.
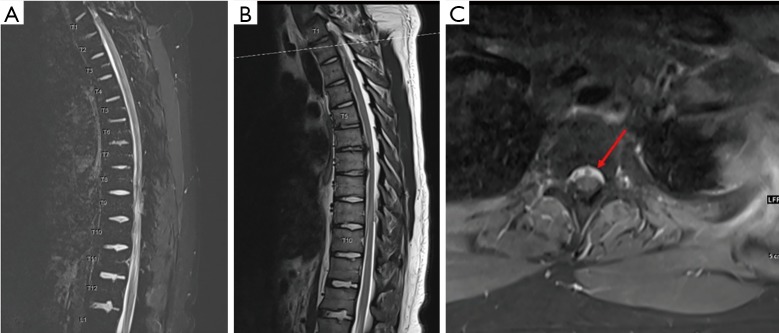
Figure 2.
MRI of the thoracic spine. (A,B) Sagittal cuts of the thoracic spine with T1 post contrast (A) and T2 sequences (B) showing epidural collection along the ventral thecal sac from T1 to T10; (C) axial view at the level of T1–T2 showing epidural collection ventral to the thecal sac with hyperintense material as indicated by the arrow.
Figure 3.
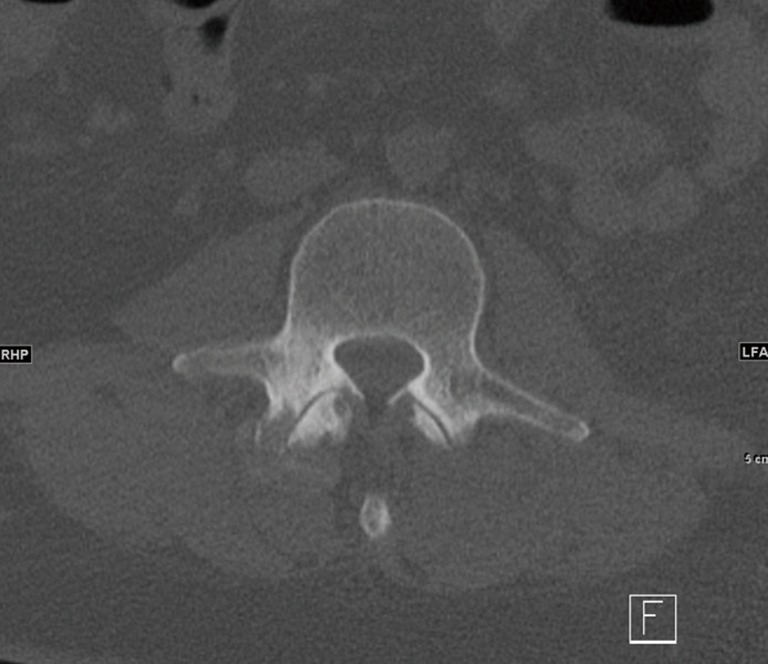
Right L2–3 facet joint with bony erosions commonly associated with gouty arthritis.
At admission, the patient was immediately started on vancomycin and zosyn for management of presumed epidural abscess. After 24 hours he noted subjective improvement in sensation, however, his motor exam did not change and continue to be limited by diffuse joint pain. Patient underwent multilevel laminotomy which revealed a whitish chalky granular substance within the epidural space (Figure 4). Histological exam revealed amorphous crystalline material associated with giant cell reaction consistent with gout (Figure 5). Cultures were positive for Staphylococcus warneri. Patient was subsequently treated with tapered prednisone and 6 weeks of vancomycin with improvement in strength and back pain by post-operative day four when he was discharged home.
Figure 4.
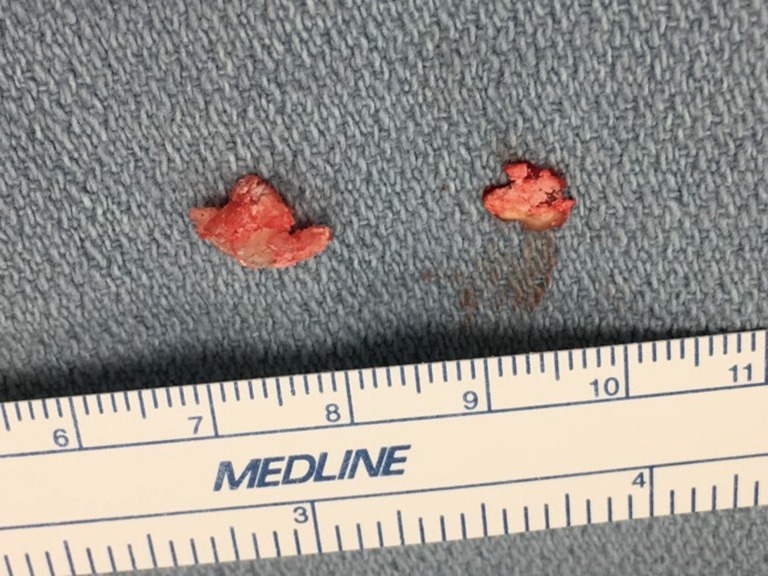
Chalky white material removed from epidural space resembling tophaceous gout.
Figure 5.
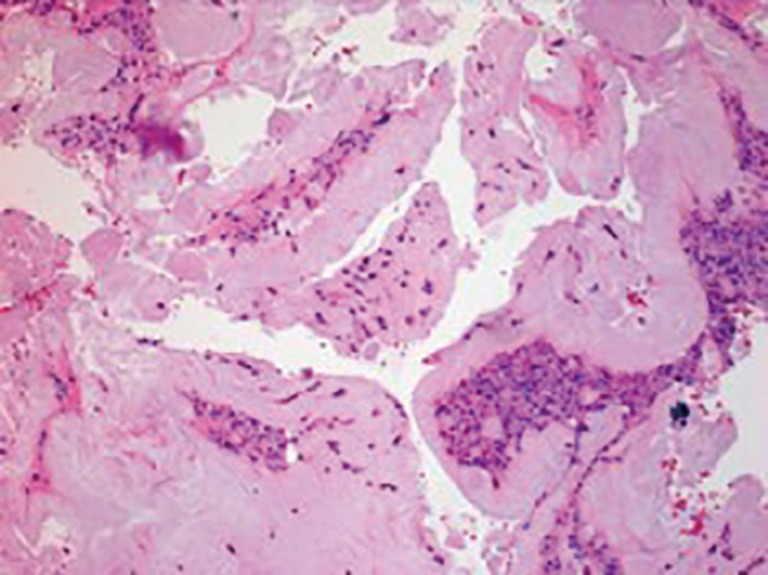
Histopathology section (HE staining, ×10) of tissue taken from the epidural space at T9–T10 showing fibrous tissue with amorphous crystalline material associated with giant cell reaction consistent with urate gout. However, due to processing of the sample, the crystals were washed out.
Discussion
Clinical presentation of spinal gout is not specific and can resemble that of epidural abscesses, osteomyelitis, discitis and metastasis (6,11,13,21). Symptoms can range from back pain to quadriparesis. Toprover et al. reviewed 131 cases of spinal gout and found that 75% had history of gout, 59% of those with gout have tophi on exam, and 16% have history of kidney disease (1). Abnormal laboratory studies commonly seen in patients with spinal gout include elevated serum uric acid, ESR, CRP, white blood count and serum creatinine (1). However, the presence of all these findings do not exclude infection from the differential diagnosis. Our patient had all the risk factors for spinal gout including elevated serum uric acid and creatinine levels and was found to have both spinal gout and epidural infection. This is the first case report of spinal gout with concomitant infection in more than one region of the spine.
Spinal gout is being increasingly reported. Between 1950 to 2015 there have been 133 case reports of spinal gout (1). Currently, there are no gold standard diagnostic tests or imaging that can identify spinal gout other than with biopsy. Findings on CT for spinal gout include bone or joint erosions with well-defined sclerotic margins, facet or intervertebral bone neoformation, or juxta- or intra-articular masses that were denser than the surrounding muscle (1,3). However, depending on the location of the crystal deposits it can be confused for tumor or abscess. Findings on MRIs can be quite variable and nonspecific because tophaceous lesions can appear hypointense or isointense on soft tissue densities on T1 and can range from hypointense to hyperintense on T2 images (1,7,15,24). Recently, newer methods of imaging gout with dual-energy CT (DECT) scanning is being developed (25,26). DECT can differentiate different materials based on their relative absorption of X-rays at different photon energy levels (25,27). There are currently a few promising cases of using DECT in the work up of spinal gout patients (26,28,29). In the absence of DECT, Hou et al. presents an algorithm that helps in the diagnosis and management of presumed spinal gout (2). Briefly, this includes obtaining a comprehensive history and physical exam with laboratory studies that include serum uric acid levels, CRP and ESR. Imaging studies with MRI or CT are warranted in patients with neurological deficit or have medically refractory pain. Patients with acute progressive neurological deterioration should undergo surgical decompression, while those without neurological deficits should obtain image-guided needle biopsy in order to confirm the diagnosis and receive medical treatment base on the biopsy results (2). Our patient was unique in that he had both spinal gout and infection making it difficult to diagnosis based on clinical presentation, laboratory and imaging studies alone. Given his neurologic decline we felt that surgery followed by medical treatment post operatively was the appropriate management.
Formation of gouty tophi is influenced by multiple factors including pH level, temperature and the presence of nucleating agent that allows crystallization of previously deposited MSU crystals (30). Similarly, the pathogenesis of gouty tophi formation in the spine may be related to local tissue changes, such as degenerative disease of the disc and facet joints or tissue necrosis, which serves as a nidus for MSU crystal deposition and accumulation (31). Our patient, with history of gout and chronic kidney disease, developed transverse myelitis which contribute to his development of symptomatic spinal gout. The history of gout with chronic kidney disease created an environment rich in MSU crystals, while the transverse myelitis created tissues changes that served as a nidus for the deposition and accumulation of MSU crystals to form gouty tophi along the spine. This may have become secondarily infected, similar to what we observe in peripheral joints (32).
Overall, tophaceous gout of the spine is more common than we expect and may be an underreported source of back pain. Spinal gout should be included in the differential diagnosis for patients with back pain or neurologic compromise, particularly in those with known history of gout. Additionally, we demonstrated in our case report that tophaceous gout can present anywhere within the spine at a given time with coexistence of more than one diagnosis. Although advance imaging is helpful in diagnosis, we feel that histopathologic review of tissue sample is diagnostic.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Toprover M, Krasnokutsky S, Pillinger MH. Gout in the spine: imaging, diagnosis, and outcomes. Curr Rheumatol Rep 2015;17:70. 10.1007/s11926-015-0547-7 [DOI] [PubMed] [Google Scholar]
- 2.Hou LC, Hsu AR, Veeravagu A, et al. Spinal gout in a renal transplant patient: a case report and literature review. Surg Neurol 2007;67:65-73. 10.1016/j.surneu.2006.03.038 [DOI] [PubMed] [Google Scholar]
- 3.Konatalapalli RM, Demarco PJ, Jelinek JS, et al. Gout in the axial skeleton. J Rheumatol 2009;36:609-13. 10.3899/jrheum.080374 [DOI] [PubMed] [Google Scholar]
- 4.Saketkoo LA, Robertson HJ, Dyer HR, et al. Axial gouty arthropathy. Am J Med Sci 2009;338:140-6. 10.1097/MAJ.0b013e3181a3dc14 [DOI] [PubMed] [Google Scholar]
- 5.Kersley GD, Mandel L, Jeffrey MR. Gout; an unusual case with softening and subluxation of the first cervical vertebra and splenomegaly. Ann Rheum Dis 1950;9:282-304. 10.1136/ard.9.4.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dharmadhikari R, Dildey P, Hide IG. A rare cause of spinal cord compression: imaging appearances of gout of the cervical spine. Skeletal Radiol 2006;35:942-5. 10.1007/s00256-006-0088-2 [DOI] [PubMed] [Google Scholar]
- 7.Cabot J, Blasco JL, Vidal Sarro N, et al. Cervical cord compression due to intradiscal gouty tophus. Spine (Phila Pa 1976) 2012;37:E1534-6. 10.1097/BRS.0b013e31826f2886 [DOI] [PubMed] [Google Scholar]
- 8.Liu T, Liu H, Zhu T. Thoracic spinal cord compression by extradural tophus: a case report and review of the literature. Spinal Cord Ser Cases 2015;1:15015. 10.1038/scsandc.2015.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan AT, Leung JL, Sy AN, et al. Thoracic spinal gout mimicking metastasis. Hong Kong Med J 2009;15:143-5. [PubMed] [Google Scholar]
- 10.Zheng ZF, Shi HL, Xing Y, et al. Thoracic cord compression due to ligamentum flavum gouty tophus: a case report and literature review. Spinal Cord 2015;53:881-6. 10.1038/sc.2015.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasturk AE, Basmaci M, Canby S, et al. Spinal gout tophus: a very rare cause of radiculopathy. Eur Spine J 2012;21:S400-S3. 10.1007/s00586-011-1847-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King JC, Nicholas C. Gouty arthropathy of the lumbar spine. A case report and review of the literature. Spine 1997;22:2309-12. 10.1097/00007632-199710010-00023 [DOI] [PubMed] [Google Scholar]
- 13.Bonaldi VM, Duong H, Starr MR, et al. Tophaceous gout of the lumbar spine mimicking an epidural abscess: MR features. AJNR 1996;17:1949-52. [PMC free article] [PubMed] [Google Scholar]
- 14.Volkov A, Rhoiney DL, Claybrooks R. Tophaceous gout of the lumbar spine: case report and review of the literature. Turk Neurosurg 2015;25:954-8. [DOI] [PubMed] [Google Scholar]
- 15.Tsai CH, Chen YJ, Hsu HC, et al. Bacteremia coexisting with tophaceous gout of the spine mimicking spondylodiscitis. Spine 2009;34:E106-9. 10.1097/BRS.0b013e31818d051a [DOI] [PubMed] [Google Scholar]
- 16.Bridges KJ, Bullis CL, Wanchu A, et al. Pseudogout of the cervical and thoracic spine mimicking infection after lumbar fusion: case report. J Neurosurg Spine 2017;27:145-9. 10.3171/2016.12.SPINE16979 [DOI] [PubMed] [Google Scholar]
- 17.Yoon JW, Park KB, Park H, et al. Tophaceous gout of the spine causing neural compression. Korean J Spine 2013;10:185-8. 10.14245/kjs.2013.10.3.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paquette S, Lach B, Guiot B. Lumbar radiculopathy secondary to gouty tophi in the filum terminale in a patient without systemic gout: case report. Neurosurgery 2000;46:986-8. [DOI] [PubMed] [Google Scholar]
- 19.Levin MH, Lichtenstein L, Scott HW. Pathologic changes in gout; survey of eleven necropsied cases. Am J Pathol 1956;32:871-95. [PMC free article] [PubMed] [Google Scholar]
- 20.Hall MC, Selin G. Spinal Involvement in Gout. J Bone Joint Surg Am 1960;42:341-3. 10.2106/00004623-196042020-00014 [DOI] [Google Scholar]
- 21.Ng W, Sin CH, Wong CH, et al. Unusual presentation of spinal gout: 2 cases report and literature review. J Orthop Case Rep 2017;7:50-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa EM, de Mello FM, Goldstein-Schainberg C, et al. Gout in the spine. Rev Bras Reumatol 2013;53:296-302. 10.1590/S0482-50042013000300008 [DOI] [PubMed] [Google Scholar]
- 23.Ahmad I, Tejada JG. Spinal gout: a great mimicker. A case report and literature review. Neuroradiol J 2012;25:621-5. 10.1177/197140091202500518 [DOI] [PubMed] [Google Scholar]
- 24.Hsu CY, Shih TT, Huang KM, et al. Tophaceous gout of the spine: MR imaging features. Clin Radiol 2002;57:919-25. 10.1053/crad.2001.1001 [DOI] [PubMed] [Google Scholar]
- 25.Chou H, Chin TY, Peh WCG. Dual-energy CT in gout - A review of current concepts and applications. J Med Radiat Sci 2017;64:41-51. 10.1002/jmrs.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chotard E, Sverzut JM, Liote F, et al. Gout at the spine: a retrospective study with dual-energy computed tomography. Ann Rheum Dis 2017;76:367-8. [Google Scholar]
- 27.Nicolaou S, Liang T, Murphy DT, et al. Dual-energy CT: a promising new technique for assessment of the musculoskeletal systems. AJR Am J Roentgenol 2012;199:S78-86. 10.2214/AJR.12.9117 [DOI] [PubMed] [Google Scholar]
- 28.Dhaese S, Stryckers M, Van Der Meersch H, et al. Gouty arthritis of the spine in a renal transplant patient: a clinical case report: an unusual presentation of a common disorder. Medicine (Baltimore) 2015;94:e676. 10.1097/MD.0000000000000676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parikh P, Butendieck R, Kransdorf M, et al. Detection of lumbar facet joint gouty arthritis using dual-energy computed tomography. J Rheumatol 2010;37:2190-1. 10.3899/jrheum.100492 [DOI] [PubMed] [Google Scholar]
- 30.McGill NW, Dieppe PA. Evidence for a promoter of urate crystal formation in gouty synovial fluid. Ann Rheum Dis 1991;50:558-61. 10.1136/ard.50.8.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tkach S. Gouty arthritis of the spine. Clin Orthop Relat Res 1970;71:81-6. [PubMed] [Google Scholar]
- 32.Papanicolas LE, Hakendorf P, Gordon DL. Concomitant septic arthritis in crystal monoarthritis. J Rheumatol 2012;39:157-60. 10.3899/jrheum.110368 [DOI] [PubMed] [Google Scholar]