Abstract
A mechanochemical Strecker reaction involving a wide range of aldehydes (aromatic, heteroaromatic and aliphatic), amines, and KCN afforded a library of α-aminonitriles upon mechanical activation. This multicomponent process was efficiently activated by lignocellulosic biomass as additives. Particularly, commercially available Kraft lignin was found to be the best activator for the addition of cyanide to the in situ formed imines. A comparative study of the 31P-NMR (Nuclear Magnetic Resonance) along with IR (Infrared) data analysis for the Kraft lignin and methylated Kraft lignin samples ascertained the importance of the free hydroxyl groups in the activation of the mechanochemical reaction. The solvent-free mechanochemical Strecker reaction was then coupled with a lactamization process leading to the formation of the N-benzylphthalimide (5a) and the isoindolinone derivative 6a.
Keywords: mechanochemistry, lignin, Strecker reaction, multicomponent reactions, ball milling
1. Introduction
Mechanochemistry—the use of mechanical energy to induce chemical transformation—has flourished in recent years, providing chemists with the means to improve synthetic chemical design in organic, inorganic, organometallic, and supramolecular chemistry, among others [1,2,3,4,5]. Commonly, mechanochemical reactions are conducted by milling, grinding, shearing, or pulling reactants in the absence of organic solvents or in the presence of a catalytic amount of them (liquid-assisted grinding, LAG) [6]. In organic chemistry, the use of electric ball mills—which until very recently had been used mostly to reduce the particle size of materials (rocks, inorganics, cellulose, lignin, etc.)—has enabled the study of numerous metal-catalyzed transformations [7], organocatalytic [8] and enzymatic processes [9], lignin [10] and cellulose depolymerizations [11,12,13,14], multicomponent reactions [15], and many others [16,17,18,19]. As a result of these investigations, mechanochemical activation and procedures have revealed several benefits, such as short reaction milling times, higher yield, reduced waste production, improved selectivity, and stoichiometry control, among others.
In the field of multicomponent reactions, our group very recently reported the use of inexpensive and readily available silica gel to mediate the mechanochemical multicomponent Strecker reaction (Figure 1a) [20]. The efficiency of this protocol enabled the high-yielding mechanosynthesis of diverse α-aminonitriles and some tetrahydroisoquinoline derivatives.
Figure 1.
(a) Mechanosynthesis of α-aminonitriles mediated by SiO2; (b) Structure of lignin; (c) General mechanochemical Strecker reaction; (d) Natural heterogeneous additives tested as activators for the mechanochemical Strecker reaction.
In the search for alternative materials capable of mediating the Strecker reaction under ball milling conditions, we focused our attention on the utilization of the main constituents of lignocellulosic feedstocks—such as cellulose or lignin—as potential activators for the reaction between aldehydes (or ketones), amines, and potassium cyanide in the ball mill. Cellulose is a readily available homopolymer of glucose consisting of β(1–4) bonds, which has been recently used as an additive and as a chiral inducer in organic reactions [21]. On the other hand, lignin is an inexpensive and abundant biopolymer containing phenylpropanoid units that are linked together through a variety of C-C and C-O bonds, resulting in the presence of aliphatic hydroxyl and phenolic hydroxyl groups (Figure 1b) [22]. The structural features present in cellulose and lignin are envisioned to confer these additives Brønsted acidic functionality, which could be useful for the activation of organic reactions [23]. Therefore, in order to evaluate the ability of natural heterogeneous activators to perform under mechanochemical conditions, we decided to test the catalytic activity of peanut shell powder (PSP) as a raw source of lignocellulosic biomass, cellulose, and several lignin samples in the three-component Strecker reaction to afford α-aminonitriles (Figure 1c–d).
2. Results and Discussion
2.1. Lignocellulosic Biomass as Activator of the Mechanochemical Strecker Reaction
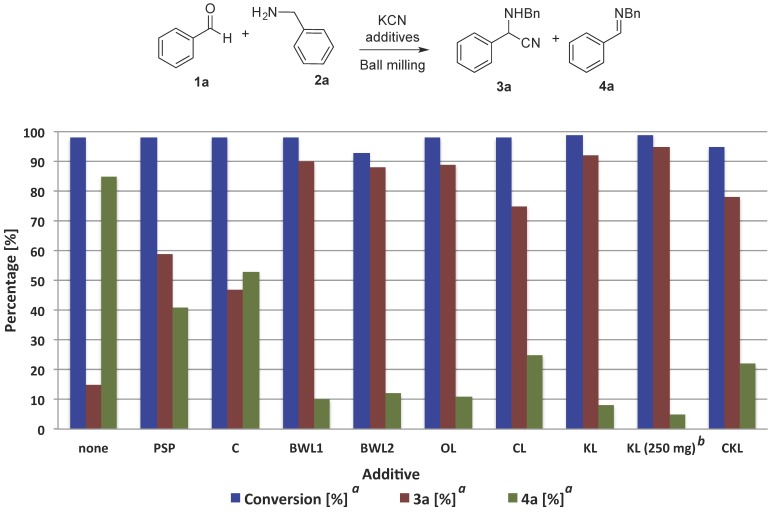
In order to evaluate the effect of the aforementioned solid additives on the Strecker reaction, we began ball milling a mixture of benzaldehyde (1a), benzylamine (2a), and potassium cyanide in a mixer ball mill. The analysis of the reaction mixture by 1H-NMR spectroscopy after two hours of milling at 30 Hz indicated full consumption of 1a and 2a. Additionally, the 1H-NMR analysis also revealed the presence of the expected α-aminonitrile 3a in minor quantity, along with the aldimine 4a as the major product preceding the milling process (conversion 98%, 3a/4a; 15/85; Figure 2). The formation of the α-aminonitrile 3a in 15% upon milling is remarkable, since the same uncatalyzed reaction attempted in solution (acetonitrile) afforded mainly imine 4a and traces of the product 3a. Next, the reaction was repeated in the presence of peanut shell powder (PSP) as an additive for the multicomponent reaction. To our delight, under these conditions, the amount of the α-aminonitrile 3a rose significantly after milling (3a/4a; 59/41; Figure 2). With the aim of examining the role of some of the major components present in PSP (cellulose, hemicellulose, lignin), crystalline cellulose and beechwood lignin (BWL1) were tested under mechanochemical conditions. After 2 h of milling, in comparison to cellulose, the lignin sample proved to be highly active in promoting the Strecker reaction, which resulted in a mixture of α-aminonitrile:imine of 90:10 (Figure 2).
Figure 2.
Conversion and selectivity of the mechanochemical Strecker reaction using biomass-derived additives. Reaction conditions: unless otherwise stated, 1a (53.1 mg, 0.5 mmol), 2a (53.6 mg, 0.5 mmol), KCN (35.8 mg, 0.55 mmol), and the additive (200 mg) were milled in a mixer mill for 2 h at 30 Hz using a stainless steel milling jar of 10 mL and one milling ball of the same material 10 mm in diameter. a Determined by 1H-NMR spectroscopy; b 3 h of milling. PSP = peanut shell powder; C = cellulose; BWL1 = beechwood lignin 1; BWL2 = beechwood lignin 2; OL = oak lignin; CL = cherry lignin; KL = Kraft lignin; CKL = capped Kraft lignin.
Subsequently, different lignin samples (cherry, beechwood, and Kraft) were examined as additives in the mechanochemical multicomponent reaction. In general, all the lignin samples clearly exhibited a positive effect favoring the formation of the product 3a over the imine 4a (Figure 2). Among the activators tested, commercial Kraft lignin showed the highest catalytic activity (3a/4a; 92/8; Figure 2) after 2 h at 30 Hz. An increase in the lignin loading (from 200 mg to 250 mg) and time (from 2 h to 3 h) led to higher 3a:4a ratio of 95:5. However, higher additive loading or longer milling times had a negligible impact on the output of the mechanochemical reaction. Finally, control experiments under conventional stirring in organic solvents (CD3CN and CDCl3) in the presence of Kraft lignin (KL) revealed incomplete conversion of starting materials 1a and 2a. Furthermore, the selectivity of the reaction in solution was found to mostly favor the formation of the imine 4a (in CD3CN: 3a/4a; 47/53 and in CDCl3: 3a/4a; 70/30).
2.2. Investigation of the Role of the Kraft Lignin in the Mechanochemical Reaction
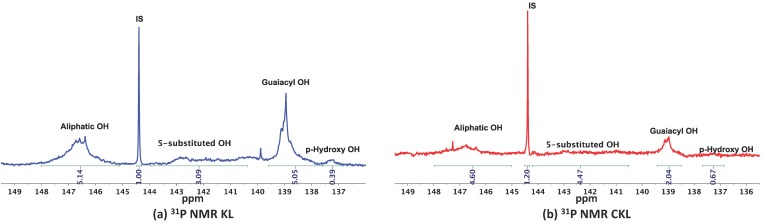
To determine the role of the Brønsted acidic sites of lignin on the activation of the multicomponent reaction, a sample of Kraft lignin was capped (methylated) following the procedure reported by Argyropoulos and coworkers using dimethyl carbonate (DMC) in the presence of NaOH in DMSO [24]. Under these conditions, the hydroxyl groups present in lignin are known to undergo methylation, which could have a direct impact on the overall functionality of the lignin, hence affecting its reactivity. Indeed, the mechanochemical reaction of 1a, 2a, and KCN in the presence of capped Kraft lignin (CKL) resulted in a slight decrease in the conversion of the reactants, and in lowering the amount of expected α-aminonitrile 3a (conv. 95%, 3a/4a; 78/22; Figure 2). Analysis by 31P-NMR [25] (Figure 3 and Table 1) and IR spectroscopy (Supplementary Material, Figure S1) of the lignin samples KL and CKL marked a clear reduction in the hydroxyl group content in the CKL compared to the raw KL. As observed in Figure 3, the content of aliphatic and phenolic OH groups decreased considerably after the capping reaction with DMC, which correlates well to the lower catalytic activity observed experimentally using CKL as additive.
Figure 3.
Functional group analysis of lignin by quantitative 31P-NMR measurements after phosphitylation; (a) Kraft Lignin (KL); (b) capped Kraft lignin (CKL) with dimethyl carbonate (DMC). IS = internal standard.
Table 1.
Hydroxyl content quantification by 31P-NMR spectroscopy of KL and CKL.
| OH | OH (mmol/g) | |||||
|---|---|---|---|---|---|---|
| Lignin | Aliphatic OH | 5-Substituted OH | Guaiacyl OH | p-Hydroxyphenyl OH | Total Phenolic OH | |
| KL | 1.79 | 0.90 | 1.77 | 0.17 | 2.84 | |
| CKL | 0.7 | 0.3 | 0.5 | 0.04 | 0.84 | |
Finally, the recyclability of Kraft lignin was evaluated. Both filtration and centrifugation of the reaction mixture after milling allowed the expedient separation of the lignin additive. This recovered lignin sample was then reused in the standard Strecker reaction. Again, the mechanochemical milling of 1a, 2a, KCN, and recycled KL showed full consumption of the benzaldehyde (1a) and the benzylamine (2a), although now the 1H-NMR analysis revealed a decrease in selectivity of the reaction (fresh KL: 3a/4a; 92/8, recycled KL: 3a/4a; 62/38).
2.3. Scope of the Mechanochemical Strecker Reaction
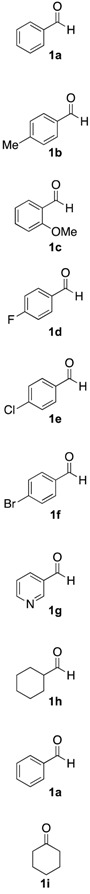
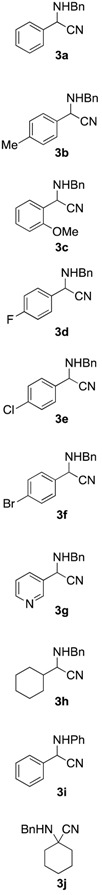
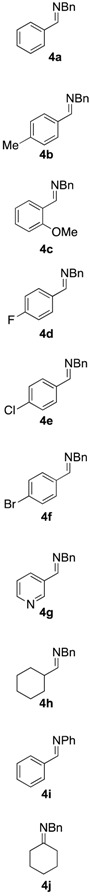
With the optimized conditions, we evaluated the scope of the lignin-mediated Strecker reaction in the ball mill. Starting with benzylamine (2a) as amine partner, benzaldehydes substituted either with electron-donating functional groups (1b–c) or aromatic aldehydes bearing electron-withdrawing substituents were tested, giving the corresponding α-aminonitriles 3b–f as the major products after three hours of milling (entries 2–6 in Table 2). Similarly, heterocyclic and aliphatic aldehydes such as 3-pyridinecarboxaldehyde (1g) (Supplementary Materials, Figure S2) and cyclohexanecarboxaldehyde (1h) also proved to be suitable for the multicomponent reaction in the ball mill, affording the products (4g–h) preferentially. Finally, a set of experiments using aniline (2b) as amino component with 1a (entry 9 in Table 2), and cyclohexanone (1i) as carbonyl compound together with benzylamine (2a) (entry 10 in Table 2) were also successful, highlighting the wide range of applicability for the mechanochemical method.
Table 2.
Mechanochemical Strecker reaction of aldehydes, ketones, and various amines a.

| Entry | Carbonyl Compound | Amine | α-aminonitrile 3a–j | Imine 4a–j | Yield [3:4] (%) b |
|---|---|---|---|---|---|
 |
 |
 |
 |
 |
 |
a Reaction conditions: carbonyl compound (0.5 mmol), amine (0.5 mmol), KCN (35.8 mg, 0.55 mmol), and Kraft lignin (250 mg) were milled in a mixer mill at 30 Hz for 3 h using a 10 mL stainless steel milling jar with 1 ball of 10 mm in diameter of the same material; b Determined by 1H-NMR spectroscopy with 1,3,5-trimethoxy-benzene as the internal standard.
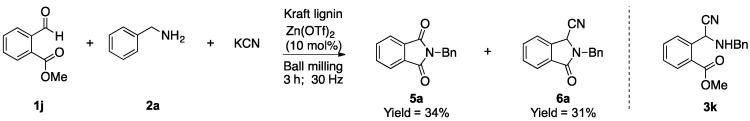
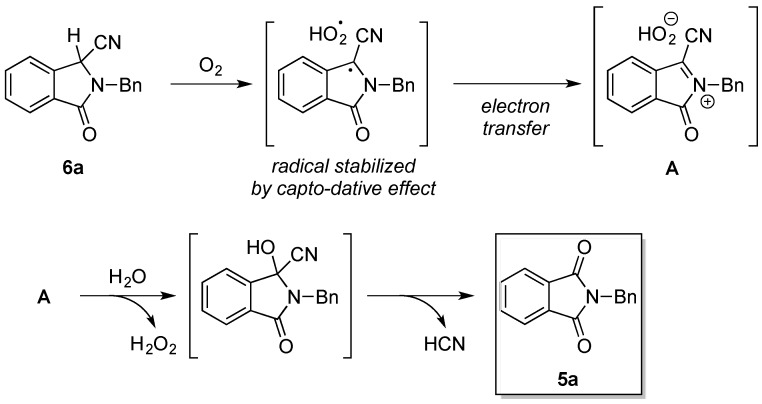
Next, in order to further exhibit the suitability of the mechanochemical Strecker reaction, we extended the protocol to the reaction between 2-(methoxycarbonyl)benzaldehyde (1j), 2a, and KCN. The expected α-aminonitrile 3k could in principle undergo a lactamization process in the milling vessel, leading to the formation of the isoindolinone derivative 6a (Scheme 1) [26].
Scheme 1.
Mechanosynthesis of N-benzylphthalimide (5a) and isoindolinone (6a).
In the presence of Kraft lignin, the milling process instead afforded the N-benzylphthalimide (5a) (33%). The formation of 5a can be explained as a result of a cyanide-catalyzed oxidative amidation of the isoindolinone 6a under the aerobic reaction conditions (ambient atmosphere) present in the ball mill (Scheme 2) [27,28,29]. Next, the same reaction was repeated, but this time Zn(OTf)2 (10 mol%) was added in addition to Kraft lignin in the milling vessel. Under these conditions, the mechanical milling led to the formation of the products 5a and 6a in 34% and 31% yield (Scheme 1). A control reaction using only Zn(OTf)2 (10 mol %) as a catalyst showed traces of the products 5a and 6a.
Scheme 2.
Possible reaction mechanism for the formation of 5a in the ball mill.
3. Materials and Methods
3.1. Materials
Aldehydes, ketone, amines, and KCN were purchased commercially and used without further purification. Cellulose (50 μm) was obtained from (J&K Scientific GmbH, Freiburgerstrasse 11, D-75179 Pforzheim, Germany).
Peanut shell powder was obtained after milling pieces of peanut shells (5 g) in a FRITSCH Planetary micro mill model “Pulverisette 7 classic line”, using milling vessels made of ZrO2 (45 mL) with five milling balls of the same material (10 mm in diameter) for 30 min at 600 rpm.
Dioxasolv cherry and oak lignins were extracted following a previously established literature procedure [30]. To cherry or oak sawdust (200 g) in a 2 L flask was added 1,4-dioxane (1.5 L) followed by the addition of 2 N HCl (0.16 L) solution. The following mixture was heated to a gentle reflux under an N2 atmosphere for 1 hour. The mixture was then cooled down to room temperature, and the lignin-containing liquor was collected by filtration. The collected liquor was concentrated under vacuum, resulting in a gummy residue, which was then taken up in acetone/water (9:1, 250 mL) and precipitated by the addition to rapidly stirring water (2.5 L). The crude lignin was collected by filtration and dried under vacuum. The dried crude lignin was taken up in acetone/methanol (9:1) and precipitated by dropwise addition to rapidly stirring Et2O (2 L). The precipitated lignin was collected by filtration and dried under vacuum to give purified samples of cherry/oak lignin (approximately 20 g). These lignins were used in subsequent experiments without further processing.
Organosolv beechwood Lignin (BWL1) was obtained after a pulping process using mixtures of ethanol and water.
Organosolv beechwood Lignin (BWL2) was extracted from beechwood chips using the following ethanol-based organosolv process. The lignin was extracted with aqueous ethanol (50% w/w) without the addition of an acid catalyst. Lignin was then precipitated with water and afterwards washed with water to remove the residual carbohydrates. The precipitate was sedimented by centrifugation, and the liquor above decanted. Finally, the lignin was dried and pulverized.
Kraft lignin-370959 was purchased from Sigma-Aldrich (Sigma-Aldrich Co., St. Louis, MO, USA).
3.2. Methods
3.2.1. Monitoring of the reactions and purification of the products
Reactions were monitored by thin-layer chromatography using TLC plates with silica gel 60 on aluminum with fluorescence indicator F254 from MERCK. Qualitative analysis of the TLC plates was carried out using UV light (λ = 254 nm and λ = 366 nm) or by immersion in an aqueous solution of potassium permanganate (KMnO4).
Column chromatography was performed using silica gel 60 (40−63 μm) and distilled grade solvents (n-pentane/ethyl acetate) as eluent.
3.2.2. Characterization of the Products 3, 4, 5 and 6 (NMR, IR Spectroscopy, MS Spectrometry)
All NMR spectra were recorded on 300, 400, and 600 MHz spectrometers (Varian Mercury 300, Varian V-NMRS 300 and 600). Proton chemical shifts are reported in parts per million on the δ scale and are calibrated using the residual non-deuterated solvent signal as an internal reference: CDCl3 (δ 7.26 ppm). Carbon chemical shifts are reported in parts per million on the δ scale and are referenced to the centermost carbon signal of the deuterated solvent: CDCl3 (δ 77.16 ppm). Spectral data is provided as follows: chemical shift in ppm (from downfield to upfield), multiplicity (s = singlet, d = doublet, dd = doublet of doublets, t = triplet, br = broad, m = multiplet), coupling constant J in Hz, and integration. IR-spectra were recorded on a Perkin Elmer 100 FT/IR spectrometer (PerkinElmer Life and Analytical Sciences, Shelton, 710 Bridgeport Avenue, CT, USA). Mass spectra were acquired on a Finnigan SSQ7000 (EI (Electron Ionization), 70 eV) spectrometer (Finningan Corporation, Waltham, MA, USA).
3.2.3. Mechanochemical Synthesis
Caution! Potassium cyanide is toxic and may also release highly toxic hydrogen cyanide. All operations involving KCN should be conducted in a well-ventilated fume hood.
Typical procedure for the Strecker reaction in the mixer mill: A mixture of the carbonyl compound (1; 0.50 mmol), amine (2; 0.50 mmol), KCN (35.8 mg, 0.55 mmol), and additive was milled in a 10 mL stainless steel milling vessel with one stainless steel milling ball of 10 mm in diameter at 30 Hz for 3 h. After the milling was complete, the content of one milling vessel was transferred into a beaker using a small amount of organic solvent (DCM or ethyl acetate). Then, a known amount of the internal standard (1,3,5-trimethoxy-benzene) was added, and the mixture was filtered to remove the insoluble material. Next, the filtrate was evaporated under reduced pressure. The residue was dissolved in CDCl3 and analyzed by 1H-NMR spectroscopy to determine the yield. The content of the second milling vessel was filtered with ethyl acetate, washed with aqueous saturated NaHCO3 solution and brine. The combined organic layers were dried over anhydrous Na2SO4, concentrated in vacuum, and further dried under reduced pressure to give the product.
3.2.4. Capping of Kraft Lignin with Dimethyl Carbonate
Kraft lignin (1 g) was dissolved in dimethyl sulfoxide (20 mL). Then, NaOH (240 mg) and DMC (6 mL) were added to the above mixture. The reaction mixture was heated at 150 °C for 5 h. After this time, the reaction mixture was cooled to room temperature, and then 2 N HCl (100 mL) was added to the mixture to precipitate the lignin. The precipitated lignin was transferred to a filter, and washed with water (3 × 100 mL). The solid residue was then dried overnight under vacuum at 50 °C.
3.2.5. 31P-NMR Spectroscopy
The quantitative 31P-NMR analysis for the phenolic and the aliphatic hydroxyl groups in the lignins—both before and after the DMC capping reaction—were conducted using a standard phosphorus pulse program following previous literature reports [25]. A weighed amount of vacuum-dried lignin sample (30 mg) was dissolved in 700 µL of an anhydrous solvent mixture (pyridine:CDCl3 1.6:1, v/v). A cyclohexanol solution (100 µL, 10.85 mg·mL−1) was added as an internal standard along with 100 µL of chromium (III) acetylacetonate solution (5.2 mg·mL−1) as the relaxation reagent. Finally, 100 µL of the phosphitylating agent (2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane) was added, and the mixture was transferred into a 5 mm-NMR tube for-NMR acquisition using 512 scans and a relaxation delay of 10 min on a 300 MHz spectrometer.
4. Conclusions
In summary, we first demonstrated the use of peanut shell powder followed by the cellulose and lignin components of lignocellulosic biomass as solid promoters for the mechanochemical Strecker reaction. Out of the set of lignin samples tested, commercial Kraft lignin was found to be the best activator to enhance the cyanation step of imines in the ball mill. The relationship between the molecular architecture of lignin and its catalytic activity is believed to be highly complex. In this regard, 31P-NMR and IR analysis of raw Kraft lignin and methylated (capped) lignin highlighted the role of the hydroxyl group content in lignin with its ability to facilitate the formation of the α-aminonitriles. Kraft lignin also proved to be very effective at promoting the multicomponent Strecker reaction between several aldehydes (aromatic, heteroaromatic, and aliphatic), amines, and KCN. Furthermore, improvement in the separation method of the lignin after the reaction—which enables higher recyclability of this additive—is currently ongoing in our group. Finally, the high efficiency of the mechanochemical approach was further demonstrated during the solvent-free synthesis of the N-benzylphthalimide (5a) and the isoindolinone (6a) through a one-pot three-component Strecker-lactamization catalyzed by the system Zn(OTf)2-Kraft lignin in the ball mill.
Acknowledgments
This research was supported by the Distinguished Professorship Program at RWTH Aachen University funded by the Excellence Initiative of the German federal and state governments (C.B., J.G.H.) and by the European Union (Marie Curie ITN ‘SuBiCat’ PITN-GA-2013-607044, S.D.). We are grateful to Nicholas J. Westwood for supplying CL and OL samples, and to Moritz Leschinsky for supplying the BWL1 sample.
Abbreviations
| BWL | Beachwood lignin |
| C | Cellulose |
| CKL | Capped Kraft lignin |
| CL | Cherry lignin |
| DMC | Dimethyl carbonate |
| IS | Internal standard |
| KL | Kraft lignin |
| LAG | Liquid assisted grinding |
| OL | Oak lignin |
| PSP | Peanut shell powder |
Supplementary Materials
The following are available online at: http://www.mdpi.com/1420-3049/22/1/146/s1, Figure S1–S2 and characterization of the products 3a–j, 5a and 6a.
Author Contributions
Saumya Dabral, Mathias Turberg, Andrea Wanninger, Carsten Bolm and José G. Hernández conceived and designed the experiments; Saumya Dabral and José G. Hernández performed the experiments; Saumya Dabral and José G. Hernández analyzed the data; Saumya Dabral, Mathias Turberg, Carsten Bolm and José G. Hernández wrote the paper. All the authors contributed and approved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interests.
Footnotes
Sample Availability: Not available.
References
- 1.James S.L., Adams C.J., Bolm C., Braga D., Collier P., Friščić T., Grepioni F., Harris K.D.M., Hyett G., Jones W., et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012;41:413–447. doi: 10.1039/C1CS15171A. [DOI] [PubMed] [Google Scholar]
- 2.Baláž P., Achimovičová M., Baláž M., Billik P., Cherkezova-Zheleva Z., Criado J.M., Delogu F., Dutková E., Gaffet E., Gotor F.J., et al. Hallmarks of mechanochemistry: from nanoparticles to technology. Chem. Soc. Rev. 2013;42:7571–7637. doi: 10.1039/c3cs35468g. [DOI] [PubMed] [Google Scholar]
- 3.Boldyreva E. Mechanochemistry of inorganic and organic systems: What is similar, what is different? Chem. Soc. Rev. 2013;42:7719–7738. doi: 10.1039/c3cs60052a. [DOI] [PubMed] [Google Scholar]
- 4.Rightmire N.R., Hanusa T.P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016;45:2352–2362. doi: 10.1039/C5DT03866A. [DOI] [PubMed] [Google Scholar]
- 5.Friščić T. Supramolecular concepts and new techniques in mechanochemistry: Cocrystals, cages, rotaxanes, open metal–organic frameworks. Chem. Soc. Rev. 2012;41:3493–3510. doi: 10.1039/c2cs15332g. [DOI] [PubMed] [Google Scholar]
- 6.Friščić T., Childs S.L., Rizvi S.A.A., Jones W. The role of solvent in mechanochemical and sonochemical cocrystal formation: A solubility-based approach for predicting cocrystallization outcome. CrystEngComm. 2009;11:418–426. doi: 10.1039/B815174A. [DOI] [Google Scholar]
- 7.Hernández J.G., Friščić T. Metal-catalyzed organic reactions using mechanochemistry. Tetrahedron Lett. 2015;56:4253–4265. doi: 10.1016/j.tetlet.2015.03.135. [DOI] [Google Scholar]
- 8.Hernández J.G., Juaristi E. Recent efforts directed to the development of more sustainable asymmetric organocatalysis. Chem. Commun. 2012;48:5396–5409. doi: 10.1039/c2cc30951c. [DOI] [PubMed] [Google Scholar]
- 9.Hernández J.G., Frings M., Bolm C. Mechanochemical enzymatic kinetic resolution of secondary alcohols under ball-milling conditions. ChemCatChem. 2016;8:1769–1772. doi: 10.1002/cctc.201600455. [DOI] [Google Scholar]
- 10.Kleine T., Buendia J., Bolm C. Mechanochemical degradation of lignin and wood by solvent-free grinding in a reactive medium. Green Chem. 2013;15:160–166. doi: 10.1039/C2GC36456E. [DOI] [Google Scholar]
- 11.Hick S.M., Griebel C., Restrepo D.T., Truitt J.H., Buker E.J., Bylda C., Blair R.G. Mechanocatalysis for biomass-derived chemicals and fuels. Green Chem. 2010;12:468–474. doi: 10.1039/b923079c. [DOI] [Google Scholar]
- 12.Meine N., Rinaldi R., Schüth F. Solvent-free catalytic depolymerization of cellulose to water-soluble oligosaccharides. ChemSusChem. 2012;5:1449–1454. doi: 10.1002/cssc.201100770. [DOI] [PubMed] [Google Scholar]
- 13.Boissou F., Sayoud N., de Oliveira Vigier K., Barakat A., Marinkovic S., Estrine B., Jérôme F. Acid-assisted ball milling of cellulose as an efficient pretreatment process for the production of butyl glycosides. ChemSusChem. 2015;8:3263–3269. doi: 10.1002/cssc.201500700. [DOI] [PubMed] [Google Scholar]
- 14.Rechulski M.D.K., Käldström M., Richter U., Schüth F., Rinaldi R. Mechanocatalytic depolymerization of lignocellulose performed on hectogram and kilogram scales. Ind. Eng. Chem. Res. 2015;54:4581–4592. doi: 10.1021/acs.iecr.5b00224. [DOI] [Google Scholar]
- 15.Polindara-García L.A., Juaristi E. Synthesis of Ugi 4-CR and Passerini 3-CR adducts under Mechanochemical activation. Eur. J. Org. Chem. 2016;2016:1095–1102. doi: 10.1002/ejoc.201501371. [DOI] [Google Scholar]
- 16.Rodríguez B., Bruckmann A., Rantanen T., Bolm C. Solvent-free carbon-carbon bond formations in ball mills. Adv. Synth. Catal. 2007;349:2213–2233. [Google Scholar]
- 17.Stolle A., Szuppa T., Leonhardt S.E.S., Ondruschka B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011;40:2317–2329. doi: 10.1039/c0cs00195c. [DOI] [PubMed] [Google Scholar]
- 18.Baig R.B.N., Varma R.S. Alternative energy input: Mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012;41:1559–1584. doi: 10.1039/C1CS15204A. [DOI] [PubMed] [Google Scholar]
- 19.Wang G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013;42:7668–7700. doi: 10.1039/c3cs35526h. [DOI] [PubMed] [Google Scholar]
- 20.Hernández J.G., Turberg M., Schiffers I., Bolm C. Mechanochemical Strecker reaction: Access to α-aminonitriles and tetrahydroisoquinolines under ball-milling conditions. Chem. Eur. J. 2016;22:14513–14517. doi: 10.1002/chem.201603057. [DOI] [PubMed] [Google Scholar]
- 21.Kaushik M., Basu K., Benoit C., Cirtiu C.M., Vali H., Moores A. Cellulose nanocrystals as chiral inducers: Enantioselective catalysis and transmission electron microscopy 3D characterization. J. Am. Chem. Soc. 2015;137:6124–6127. doi: 10.1021/jacs.5b02034. [DOI] [PubMed] [Google Scholar]
- 22.Upton B.M., Kasko A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016;116:2275–2306. doi: 10.1021/acs.chemrev.5b00345. [DOI] [PubMed] [Google Scholar]
- 23.Chen W., Peng X.-W., Zhong L.-X., Li Y., Sun R.-C. Lignosulfonic acid: A renewable and effective biomass-based catalyst for multicomponent reactions. ACS Sustain. Chem. Eng. 2015;3:1366–1373. doi: 10.1021/acssuschemeng.5b00091. [DOI] [Google Scholar]
- 24.Sen S., Patil S., Argyropoulos D. Methylation of softwood kraft lignin with dimethyl carbonate. Green Chem. 2015;17:1077–1087. doi: 10.1039/C4GC01759E. [DOI] [Google Scholar]
- 25.Constant S., Wienk H.L.J., Frissen A.E., de Peinder P., Boelens R., van Es D.S., Grisel R.J.H., Weckhuysen B.M., Huijgen W.J.J., Gosselink R.J.A., et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016;18:2651–2665. doi: 10.1039/C5GC03043A. [DOI] [Google Scholar]
- 26.Dhanasekaran S., Suneja A., Bisai V., Singh V.K. Approach to isoindolinones, isoquinolinones, and THIQs via Lewis acid-catalyzed domino Strecker-lactamization/alkylations. Org. Lett. 2016;18:634–637. doi: 10.1021/acs.orglett.5b03331. [DOI] [PubMed] [Google Scholar]
- 27.Seo H.-A., Cho Y.-H., Lee Y.-S., Cheon C.-H. Formation of Amides from Imines via Cyanide-Mediated Metal-Free Aerobic Oxidation. J. Org. Chem. 2015;80:11993–11998. doi: 10.1021/acs.joc.5b01922. [DOI] [PubMed] [Google Scholar]
- 28.Chen W., An W., Wang Y., Yu A. Mechanisms of Metal-Free Aerobic Oxidation To Prepare Benzoxazole Catalyzed by Cyanide: A Direct Cyclization or Stepwise Oxidative Dehydrogenation and Cyclization? J. Org. Chem. 2016;81:10857–10862. doi: 10.1021/acs.joc.6b01939. [DOI] [PubMed] [Google Scholar]
- 29.Beillard A., Métro T.-X., Bantreil X., Martinez J., Lamaty F. Cu(0), O2 and mechanical forces: A saving combination for efficient production of Cu–NHC complexes. Chem. Sci. doi: 10.1039/C6SC03182J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lancefield C.S., Ojo O.S., Tran F., Westwood N.J. Isolation of functionalized phenolic monomers through selective oxidation and C-O bond cleavage of the β-O-4 linkages in lignin. Angew. Chem. Int. Ed. 2015;54:258–262. doi: 10.1002/anie.201409408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.