Abstract
A tandem transformation of C-N coupling/C-H carbonylation has been developed for the synthesis of benzo-1,4-oxazepine pharmaceutically derivatives. Notably, this reaction was accomplished by various phenylamine with ally halides under carbon dioxide atmosphere employing 2-(2-dimethylamino-vinyl)-1H-inden-1-olcatalyzed. Furthermore, under the optimized conditions, various benzo-1,4-oxazepine derivatives were obtained in good yields. Finally, a plausible CuI/CuIII mechanism of C-N coupling/C-H carbonylation transformation was proposed.
Keywords: benzo-1,4-oxazepine; copper catalyst; tandem transformation; C-N coupling; C-H carbonylation
1. Introduction
The heterocycle benzoxazepines are privileged scaffolds in natural biologically products [1,2,3,4], pharmaceutical chemistry [5,6] and functionalized materials [7,8,9,10]. As such, Sintamilv (I) is an efficient antidepressant [11]; H1 receptor antagonist (II) is a selective antihistaminic agent [12]; and Sintamil (III) is a benzoxazepine analogue (Scheme 1) [13]. Furthermore, the therapeutic applications of benzoxazepines are for the central nervous system, along with anti-breast cancer activity and inhibitors of HIV [14,15].
Scheme 1.
The important benzo-1,4-oxazepine derivatives.
Currently, the challenge in organic synthesis is developing an efficient and eco-friendly protocol, especially in the area of drug discovery and natural products. Benzoxazepines are generally synthesized by condensation of 2-aryloxyethylamines with 2-formylbenzoic acid [16]. Others have also been synthesized from amides [17] and amino acids [18,19]. However, most of these methodologies are associated with several drawbacks, such as low synthetic efficiency and sensitivity. Thus, a remarkable gap remains in the search of economical synthesis methods. Tandem transformation is one of the most effective ways to achieve this goal. Considering the above points, herein we report the tandem reaction green protocol for the synthesis of benzo-1,4-oxazepine pharmaceutical derivatives.
The reaction conditions were screened based on a model reaction of phenylamine 1a and (1-chloro-vinyl)-benzene 2a (Table 1). The ligands were mainly based on the derivatives of 2-(2-dimethylamino-vinyl)-1H-inden-1-ol. It was discovered that ligand L1 was the ideal choice for this transformation (Entries 5–10). CuI exhibited superior catalytic efficiency over all other examined CuI catalysts (Entries 1–5), and Cs2CO3 turned out to be the proper base additive (Entries 11–12). Meanwhile, the reaction temperature was 100 °C (Entries 15–16).
Table 1.
Optimization of the reaction conditions a.

| Entry | Ligand | Cu Salt | Base | Yield (%) b |
|---|---|---|---|---|
| 1 | L1 | Cu(OAc)2 | Cs2CO3 | 8 |
| 2 | L1 | CuSO4 | Cs2CO3 | 0 |
| 3 | L1 | CuBr | Cs2CO3 | 23 |
| 4 | L1 | CuBr2 | Cs2CO3 | 19 |
| 5 | L1 | CuI | Cs2CO3 | 81 |
| 6 | L2 | CuI | Cs2CO3 | 29 |
| 7 | L3 | CuI | Cs2CO3 | 36 |
| 8 | L4 | CuI | Cs2CO3 | 47 |
| 9 | L5 | CuI | Cs2CO3 | 16 |
| 10 | L6 | CuI | Cs2CO3 | 38 |
| 11 | L1 | CuI | K2CO3 | 42 |
| 12 | L1 | CuI | K3PO4 | 0 |
| 13 | L1 | CuI | Cs2CO3 | 61 c |
| 14 | L1 | CuI | Cs2CO3 | 69 d |
 | ||||
a Unless otherwise noted, reactions conditions were 1a (0.5 mmol), 2a (0.6 mmol), Cu salt (10 mol %), ligand (10 mol %), base (2 eq.), DMSO (4 mL) reacted in CO2 at 100 °C for 12 h; b isolated yield; c reaction under 90 °C; d reaction under 110 °C.
With the optimal conditions established, the reaction scope was further investigated. A wide array of phenylamine 1 and ally halide 2 was subjected to this reaction in moderate to good yields (Table 2). Phenylamine derivatives bearing either an electron-withdrawing or electron-donating group reacted smoothly with 2. This transformation is applicable for para-substituted phenylamines. Chloroethylene bearing an electron-donating group showed better reactivity than those with an electron-withdrawing group (All the product spectrums, please see Supplementary Materials).
Table 2.
Synthesis of benzo-1,4-oxazepin-5-one 3 a.

| Entry | R1 | R2 | Product 3 | Yield (%) b |
|---|---|---|---|---|
| 1 | H | Ph |  |
81 |
| 2 | H | 4-CH3C6H4 |  |
78 |
| 3 | H | 4-ClC6H4 |  |
85 |
| 4 | H | CH3 |  |
74 |
| 5 | 4-Cl | Ph |  |
79 |
| 6 | 4-Cl | 4-CH3C6H4 |  |
76 |
| 7 | 4-Cl | 4-ClC6H4 |  |
86 |
| 8 | 4-Cl | CH3 |  |
84 |
| 9 | 4-CH3 | Ph |  |
76 |
| 10 | 4-CH3 | 4-CH3C6H4 |  |
75 |
| 11 | 4-CH3 | 4-ClC6H4 | 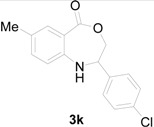 |
82 |
| 12 | 4-CH3 | CH3 |  |
72 |
a Reactions conditions were 1 (0.5 mmol), 2 (0.6 mmol), CuI (10 mol %), L1 (10 mol %), Cs2CO3 (2 equiv.), DMSO (4 mL) at 100 °C reacted in CO2 for 10 h; b isolated yield.
Interestingly, we found that 1-bromo-cyclohexene 4 has also been rapidly synthesized in good yields, and the results are summarized in Table 3. In addition, the reaction works well for both bearing electron-donating and electron-withdrawing groups.
Table 3.
Synthesis of benzo-1,4-oxazepin-5-one 5 a.

| Entry | R1 | Product 5 | Yield (%) b |
|---|---|---|---|
| 1 | H |  |
78 |
| 2 | 4-Cl |  |
84 |
| 3 | 4-CH3 |  |
75 |
a Reactions conditions were 1 (0.5 mmol), 2 (0.6 mmol), CuI (10 mol %), L1 (10 mol %), Cs2CO3 (2 equiv.), DMSO (4 mL) at 100 °C reacted in CO2 for 10 h; b isolated yield.
On the basis of the above experimental results, we tentatively proposed a reaction mechanism as shown in Scheme 2. At the beginning, CuI activate 6 was been formed through copper iodide coordinating with ligand. Next, complex 6 reacted with vinyl halides by oxidative addition produced a CuIII complex 7. The complex 7 reacted with aniline obtained the key intermediate complex 8 [20,21]. Selective ortho-carbonylation of the phenylamine was determined by Complex 9. Through the reductive elimination of Complex 9, Complex 10 was obtained, which regenerates Complex 6 for the next catalytic cycle [22,23]. However, how the ligand promotes this transformation is a part of ongoing study.
Scheme 2.
A plausible mechanism of the catalytic cycle.
2. Results and Discussion

2-Phenyl-2,3-dihydro-1H-benzo[e][1,4]oxazepin-5-one (3a): A mixture of phenylamine 1a (0.5 mmol, 46.5 mg), (1-chloro-vinyl)-benzene 2a (0.6 mmol, 83.4 mg), CuI (10 mol %, 9.5 mg), L1 (10 mol %, 20.1 mg) and Cs2CO3 (2 equiv., 325.8 mg) in DMSO (4 mL) was stirred in CO2 at 100 °C for 10 h. After completion of the reaction, the mixture was quenched with saturated salt water (10 mL); the solution was extracted with ethyl acetate (3 × 10 mL). The organic layers were combined and dried over sodium sulfate. The pure product was obtained by flash column chromatography on silica gel to afford 3a 96.8 mg in 81% yield. The spectroscopic data of all of the products are presented below. Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.63 (m, 1H), 7.43 (br, 1H), 7.08–7.43 (m, 8H), 5.07 (dd, J = 8.0, 5.7 Hz, 1H), 4.08 (dd, J = 12.3, 8.0 Hz, 1H), 3.96 (dd, J = 12.3, 5.6 Hz, 1H); 13C-NMR (100 MHz, CDCl3): 168.3, 147.7, 139.1, 132.9, 130.3, 128.6, 127.5, 126.6, 117.8, 116.4, 109.1, 77.6, 60.2; EIMS (m/z): 239 [M+]; Anal. Calcd. for C15H13NO2: C, 75.30; H, 5.48; N, 5.85; Found: C, 75.62; H, 5.13; N, 5.68.

2-p-Tolyl-2,3-dihydro-1H-benzo[e][1,4] xazepine-5-one (3b): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.61 (m, 1H), 7.44 (br, 1H), 7.04–7.31 (m, 7H), 5.07 (dd, J = 8.0, 5.7 Hz, 1H), 4.07 (dd, J = 12.3, 8.0 Hz, 1H), 3.95 (dd, J = 12.3, 5.7 Hz, 1H), 2.39 (s, 3H); 13C-NMR (100 MHz, CDCl3): 168.6, 147.8, 138.3, 135.3, 132.3, 130.5, 128.1, 127.6, 118.2, 115.9, 109.5, 77.5, 60.3, 25.2; EIMS (m/z): 253 [M+]; Anal. Calcd. for C16H15NO2: C, 75.87; H, 5.97; N, 5.53; Found: C, 75.50; H, 6.20; N, 5.88.

2-(4-Chloro-phenyl)-2,3-dihydro-1H-benzo[e][1,4] xazepine-5-one (3c): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.64 (m, 1H), 7.47 (br, 1H), 7.07–7.48 (m, 7H), 5.08 (dd, J = 8.1, 5.6 Hz, 1H), 4.09 (dd, J = 12.3, 8.1 Hz, 1H), 3.95 (dd, J = 12.3, 5.6 Hz, 1H); 13C-NMR (100 MHz, CDCl3): 168.3, 147.7, 139.3, 133.3, 132.4, 130.5, 128.6, 127.8, 118.4, 116.3, 110.1, 77.3, 60.9;EIMS (m/z): 273 [M+]; Anal. Calcd. for C15H12ClNO2: C, 65.82; H, 4.42; N, 5.12; Found: C, 65.51; H, 4.61; N, 5.33.

2-Methyl-2,3-dihydro-1H-benzo[e][1,4]oxazepin-5-one (3d): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.62 (m, 1H), 7.42 (br, 1H), 7.05–7.21 (m, 3H), 4.58 (dd, J = 12.3, 8.0 Hz, 1H), 3.96 (dd, J = 12.2, 5.6 Hz, 1H), 3.12–3.71 (m, 1H), 1.35 (d, J = 7.1 Hz, 3H); 13C-NMR (100 MHz, CDCl3): 168.2, 147.3, 132.8, 130.4, 118.7, 116.6, 109.7, 77.1, 53.1, 18.2; EIMS (m/z): 177.08 [M+]; Anal. Calcd. for C10H11NO2: C, 67.78; H, 6.26; N, 7.90; Found: C, 68.14; H, 6.55; N, 7.53.

7-Chloro-2-phenyl-2,3-dihydro-1H-benzo[e][1,4]oxazepin-5-one (3e): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.63 (m, 1H), 7.43 (br, 1H), 7.10–7.46 (m, 7H), 5.08 (dd, J = 8.1, 5.6 Hz, 1H), 4.10 (dd, J = 12.4, 8.1 Hz, 1H), 3.97 (dd, J = 12.4, 5.6 Hz, 1H); 13C-NMR (100 MHz, CDCl3): 168.3, 147.4, 139.5, 133.2, 130.2, 128.7, 127.5, 126.8, 123.8, 115.4, 109.2, 77.5, 60.2; EIMS (m/z): 273 [M+]; Anal. Calcd. for C15H12ClNO2: C, 65.82; H, 4.42; N, 5.12; Found: C, 65.70; H, 4.61; N, 5.44.

7-Chloro-2-p-tolyl-2,3-dihydro-1H-benzo[e][1,4]oxazepin-5-one (3f): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.64 (m, 1H), 7.43 (br, 1H), 7.07–7.38 (m, 6H), 5.08 (dd, J = 8.1, 5.9 Hz, 1H), 4.10 (dd, J = 12.4, 8.1 Hz, 1H), 3.96 (dd, J = 12.4, 5.9 Hz, 1H), 2.40 (s, 3H); 13C-NMR (100 MHz, CDCl3): 168.2, 147.1, 139.2, 135.8, 133.4, 130.5, 128.7, 126.9, 123.5, 115.5, 109.3, 77.2, 60.4, 25.7; EIMS (m/z): 287.07 [M+]; Anal. Calcd. for C16H14ClNO2: C, 66.79; H, 4.90; N, 4.87; Found: C, 66.95; H, 4.63; N, 5.23.
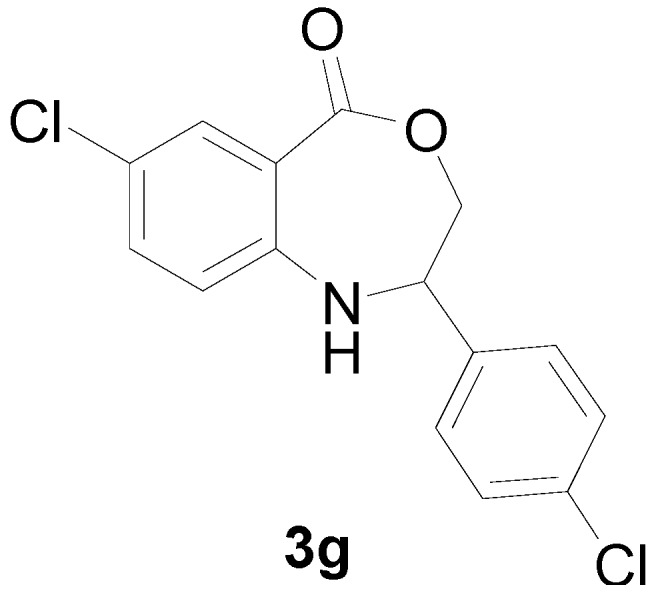
7-Chloro-2-(4-chloro-phenyl)-2,3-dihydro-1H-benzo[e][1,4]oxazepin-5-one (3g): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.66 (m, 1H), 7.46 (br, 1H), 7.09–7.50 (m, 6H), 5.10 (dd, J = 8.2, 5.6 Hz, 1H), 4.11 (dd, J = 12.4, 8.2 Hz, 1H), 3.96 (dd, J = 12.4, 5.6 Hz, 1H); 13C-NMR (100 MHz, CDCl3): 168.2, 147.4, 139.6, 133.2, 131.8, 130.2, 128.9, 126.7, 123.8, 115.2, 109.6, 77.5, 60.3; EIMS (m/z): 307 [M+]; Anal. Calcd. for C15H11Cl2NO2: C, 58.46; H, 3.60; N, 4.55; Found: C, 58.23; H, 3.92; N, 4.67.

7-Chloro-2-methyl-2,3-dihydro-1H-benzo[e][1,4]oxazepin-5-one (3h): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.64 (m, 1H), 7.45 (br, 1H), 7.06–7.23 (m, 2H), 4.6 (dd, J = 12.2, 8.1 Hz, 1H), 3.98 (dd, J = 12.2, 5.6 Hz, 1H), 3.12–3.71 (m, 1H), 1.36 (d, J = 7.2 Hz, 3H); 13C-NMR (100 MHz, CDCl3): 168.5, 147.3, 133.1, 130.2, 123.1, 116.8, 109.3, 77.5, 53.4, 18.3; EIMS (m/z): 211 [M+]; Anal. Calcd. for C10H10ClNO2: C, 56.75; H, 4.76; N, 6.62; Found: C, 56.89; H, 5.18; N, 6.34.

7-Methyl-2-phenyl-2,3-dihydro-1H-benzo[e][1,4]oxazepin-5-one (3i): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.58 (m, 1H), 7.41 (br, 1H), 7.06–7.40 (m, 7H), 5.00 (dd, J = 8.0, 5.6 Hz, 1H), 4.06 (dd, J = 12.2, 8.0 Hz, 1H), 3.92 (dd, J = 12.2, 5.6 Hz, 1H), 2.40 (s, 3H). 13C-NMR (100 MHz, CDCl3): 168.5, 147.2, 139.4, 133.3, 130.8, 128.9, 127.7, 126.9, 126.2, 116.7, 109.3, 77.8, 60.3, 25.3; EIMS (m/z): 253 [M+]; Anal. Calcd. for C16H15NO2: C, 75.87; H, 5.97; N, 5.53; Found: C, 75.65; H, 6.28; N, 5.33.

7-Methyl-2-p-tolyl-2,3-dihydro-1H-benzo[e][1,4]oxazepin-5-one (3j): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.56 (m, 1H), 7.44 (br, 1H), 7.06–7.36 (m, 6H), 4.98 (dd, J = 7.9, 5.6 Hz, 1H), 4.02 (dd, J = 12.2, 7.9 Hz, 1H), 3.90 (dd, J = 12.2, 5.6 Hz, 1H), 2.39 (s, 6H); 13C-NMR (100 MHz, CDCl3): 168.2, 147.5, 138.3, 135.1, 132.4, 130.8, 128.8, 127.5, 126.2, 116.2, 109.1, 77.2, 60.5, 25.8, 25.3; EIMS (m/z): 267 [M+]; Anal. Calcd. for C17H17NO2: C, 76.38; H, 6.41; N, 5.24; Found: C, 76.69; H, 6.24; N, 5.53.

2-(4-Chloro-phenyl)-7-methyl-2,3-dihydro-1H-benzo[e][1,4]oxazepin-5-one (3k): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.60 (m, 1H), 7.47 (br, 1H), 7.06-7.44 (m, 6H), 5.08 (dd, J = 8.0, 5.7 Hz, 1H), 4.10 (dd, J = 12.2, 8.0 Hz, 1H), 3.98 (dd, J = 12.2, 5.7 Hz, 1H), 2.42 (s, 3H); 13C-NMR (100 MHz, CDCl3): 168.1, 147.5, 139.6, 133.5, 132.2, 131.1, 128.3, 127.5, 126.4, 115.7, 109.7, 77.4, 60.7, 25.4; EIMS (m/z): 287 [M+]; Anal. Calcd. for C16H14ClNO2: C, 66.79; H, 4.90; N, 4.87; Found: C, 67.09; H, 4.99; N, 4.54.
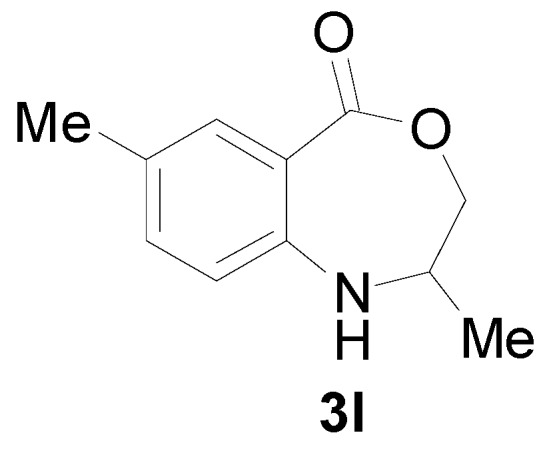
2,7-Dimethyl-2,3-dihydro-1H-benzo[e][1,4] xazepine-5-one (3l): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.62 (m, 1H), 7.43 (br, 1H), 7.04–7.20 (m, 2H), 4.56 (dd, J = 12.2, 8.0 Hz, 1H), 3.93 (dd, J = 12.2, 5.4 Hz, 1H), 3.10–3.70 (m, 1H), 2.41 (s, 3H), 1.34 (d, J = 7.0 Hz, 3H); 13C-NMR (100 MHz, CDCl3): 168.3, 147.1, 133.5, 130.9, 126.8, 115.8, 109.2, 77.5, 53.4, 25.3, 18.3; EIMS (m/z): 191 [M+]; Anal. Calcd. for C11H13NO2: C, 69.09; H, 6.85; N, 7.32; Found: C, 69.41; H, 6.55; N, 7.16.

5a,6,7,8,9,9a-Hexahydro-5H-10-oxa-5-aza-dibenzo[a,d]cyclohepten-11-one (5a): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.60 (m, 1H), 7.48 (br, 1H), 7.02–7.39 (m, 3H), 4.22 (dd, J = 11.3, 3.4 Hz, 1H), 3.11 (dd, J = 11.3, 3.5 Hz, 1H), 1.61–1.93 (m, 4H), 1.43–1.52 (m, 4H); 13C-NMR (100 MHz, CDCl3): 168.2, 147.6, 132.6, 130.1, 118.2, 115.9, 108.8, 85.8, 56.1, 28.5, 27.6, 22.9, 21.7; EIMS (m/z): 217 [M+]; Anal. Calcd. for C13H15NO2: C, 71.87; H, 6.96; N, 6.45; Found: C, 71.72; H, 6.66; N, 6.73.

2-Chloro-5a,6,7,8,9,9a-hexahydro-5H-10-oxa-5-aza-dibenzo[a,d]cyclohepten-11-one (5b): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.62 (m, 1H), 7.49 (br, 1H), 7.05–7.43 (m, 2H), 4.26 (dd, J = 11.3, 3.5 Hz, 1H), 3.11 (dd, J = 11.3, 3.7 Hz, 1H), 1.62–1.95 (m, 4H), 1.43–1.54 (m, 4H); 13C-NMR (100 MHz, CDCl3): 168.3, 147.1, 133.1, 130.4, 122.5, 116.1, 108.2, 85.6, 56.5, 28.8, 27.2, 22.7, 21.5; EIMS (m/z): 251 [M+]; Anal. Calcd. for C13H14ClNO2: C, 62.03; H, 5.61; N, 5.56; Found: C, 62.19; H, 5.31; N, 5.34.

2-Methyl-5a,6,7,8,9,9a-hexahydro-5H-10-oxa-5-aza-dibenzo[a,d]cyclohepten-11-one (5c): Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.58 (m, 1H), 7.46 (1H, br), 7.00–7.35 (m, 2H), 4.20 (dd, J = 11.2, 3.2 Hz, 1H), 3.09 (dd, J = 11.2, 3.4 Hz, 1H), 2.40 (s, 3H), 1.60–1.91 (m, 4H), 1.42–1.50 (m, 4H); 13C-NMR (100 MHz, CDCl3): 168.4, 147.3, 133.4, 130.8, 126.1, 116.1, 108.5, 85.4, 56.3, 28.7, 27.8, 22.8, 21.5; EIMS (m/z): 231 [M+]; Anal. Calcd. for C14H17NO2: C, 72.70; H, 7.41; N, 6.06; Found: C, 72.99; H, 7.28; N, 6.48.
3. Experimental Section
3.1. General Procedure for Preparation of L1–L6
Dimethylformamide dimethyl acetal (DMF-DMA) (10 mmol, 1.19 g) and 1-(1-hydroxy-1H-inden-2-yl)-ethanone (10 mmol, 1.74 g) were dissolved in p-xylene (5 mL). Additionally, the mixture was refluxed during a period of 5–12 h, during which time a yellow precipitate formed. The precipitate was filtered out and washed with petroleum ether three times. The solid was vacuum-dried, and 1.89 g (yield 94%) of a yellow solid were obtained, L1 2-(2-dimethylamino-vinyl)-1H-inden-1-ol. 1H-NMR (400 MHz, CDCl3): δ 7.23 (m, 2H), 7.17–7.07 (t, J = 8.0 Hz, 2H), 7.01–6.90 (t, J = 7.8 Hz, 1H), 6.60 (s, 1H), 6.07–6.05 (d, J = 12 Hz, 1H), 2.47 (s, 3H), 2.42 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 146.1, 141.2, 133.8, 130.2, 127.9, 126.9, 123.2,121.2, 120.6, 104.1, 75.4, 46.1, 38.6.
3.2. 2-Phenyl-2,3-dihydro-1H-benzo[e][1,4]oxazepin-5-one (3a)
A mixture of phenylamine 1a (0.5 mmol, 46.5 mg), (1-chloro-vinyl)-benzene 2a (0.6 mmol, 83.4 mg), CuI (10 mol %, 9.5 mg), L1 (10 mol %, 20.1 mg) and Cs2CO3 (2 equiv., 325.8 mg) in DMSO (4 mL) was stirred in CO2 at 100 °C for 10 h. After completion of the reaction, the mixture was quenched with saturated salt water (10 mL); the solution was extracted with ethyl acetate (3 × 10 mL). The organic layers were combined and dried over sodium sulfate. The pure product was obtained by flash column chromatography on silica gel to afford 3a 96.8 mg in 81% yield. The spectroscopic data of all of the products are represented below. Yellowish oil. 1H-NMR (400 MHz, CDCl3): 7.63 (m, 1H), 7.43 (br, 1H), 7.08–7.43 (m, 8H), 5.07 (dd, J = 8.0, 5.7 Hz, 1H), 4.08 (dd, J = 12.3, 8.0 Hz, 1H), 3.96 (dd, J = 12.3, 5.6 Hz, 1H); 13C-NMR (100 MHz, CDCl3): 168.3, 147.7, 139.1, 132.9, 130.3, 128.6, 127.5, 126.6, 117.8, 116.4, 109.1, 77.6, 60.2; EIMS (m/z): 239 [M+]; Anal. Calcd. for C15H13NO2: C, 75.30; H, 5.48; N, 5.85; Found: C, 75.62; H, 5.13; N, 5.68.
4. Conclusions
In conclusion, we have found a green protocol for the synthesis of benzo-1,4-oxazepine derivatives involving tandem transformation of C-N coupling/C-H carbonylation. The method was economically viable and relevant to green chemistry.
Acknowledgments
This work was supported by the Natural Science Foundation of Zhejiang Province (No. LQ15B020004), Natural Science Foundation ZA & FU (No. 04251700011).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/22/1/53 /s1.
Author Contributions
R.S.X. designed the experiments; J.Z. and Z.Z. analyzed the data and wrote the paper; X.J.Z. performed the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Maier M.E. Synthesis of Medium-Sized Rings by the Ring-Closing Metathesis Reaction. Angew. Chem. Int. Ed. 2000;39:2073–2077. doi: 10.1002/1521-3773(20000616)39:12<2073::AID-ANIE2073>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Nubbemeyer U. Intramolecular Staudinger Ligation: A Powerful Ring-Closure Method to Form Medium-Sized Lactams. Top. Curr. Chem. 2001;216:125–196. doi: 10.1002/anie.200351930. [DOI] [PubMed] [Google Scholar]
- 3.Goutham K., Ashok Kumar D., Suresh S., Sridhar B., Narender R., Karunakar G.V. Gold-Catalyzed Intramolecular Cyclization of N-Propargylic β-Enaminones for the Synthesis of 1,4-Oxazepine Derivatives. J. Org. Chem. 2015;80:11162–11168. doi: 10.1021/acs.joc.5b01733. [DOI] [PubMed] [Google Scholar]
- 4.Wan J.P., Jing Y.F., Hu C.F., Sheng S.R. Metal-Free Synthesis of Fully Substituted Pyridines via Ring Construction Based on the Domino Reactions of Enaminones and Aldehydes. J. Org. Chem. 2016;81:6826–6831. doi: 10.1021/acs.joc.6b01149. [DOI] [PubMed] [Google Scholar]
- 5.Taunton J., Collins J.L., Schreiber S.L. Synthesis of Natural and Modified Trapoxins, Useful Reagents for Exploring Histone Deacetylase Function. J. Am. Chem. Soc. 1996;118:10412–10422. doi: 10.1021/ja9615841. [DOI] [Google Scholar]
- 6.Karadeniz E., Zora M. One-pot Synthesis of 2-Ferrocenyl-substituted Pyridines. Tetrahedron Lett. 2016;57:4930–4934. doi: 10.1016/j.tetlet.2016.09.080. [DOI] [Google Scholar]
- 7.Sanchez-Quesada J., Ghadiri M.R., Bayley H., Braha O. Cyclic Peptides as Molecular Adapters for a Pore-Forming Protein. J. Am. Chem. Soc. 2000;122:11757–11766. doi: 10.1021/ja002436k. [DOI] [Google Scholar]
- 8.Bong D.T., Clark T.D., Granja J.R., Ghadiri M.R. Self-Assembling Organic Nanotubes. Angew. Chem. Int. Ed. 2001;40:988–1011. doi: 10.1002/1521-3773(20010316)40:6<988::AID-ANIE9880>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 9.Jarvo E.R., Miller S.J. Amino Acids and Peptides as Asymmetric Organocatalysts. Tetrahedron. 2002;58:2481–2495. doi: 10.1016/S0040-4020(02)00122-9. [DOI] [Google Scholar]
- 10.Mikušek J., Matouš P., Matoušová E., Janoušek M., Kuneš J., Pour M. Substrate Control in the Gold(I)-Catalyzed Cyclization of β-Propargylamino Acrylic Esters and Further Transformations of the Resultant Dihydropyridines. Adv. Synth. Catal. 2016;358:2912–2922. doi: 10.1002/adsc.201600412. [DOI] [Google Scholar]
- 11.Nagarajan K., David J., Kulkarni Y.S., Hendi S.B., Shenoy S.J., Upadhyaya P. Piperazinylbenzonaphthoxazepines with CNS Depressant Properties. Eur. J. Med. Chem. 1986;21:21–26. [Google Scholar]
- 12.Walther G., Daniel H., Bechtel W.D., Brandt K. New Tetracyclic Guanidine Derivatives with H1-antihistaminic Properties. Arzneim. Forsch. 1990;40:440–446. [PubMed] [Google Scholar]
- 13.Hadou A., Hamid A., Mathouet H., Deïda M.F., Daïch A. An Easy Access to The Exocyclic Lactams Analogous of The Central Nervous System Active Tricyclic Nitroxapine, Mianserine and Chlothiapine Agents using N-acyliminium Chemistry. Heterocycles. 2008;76:1017–1022. doi: 10.1002/chin.200918167. [DOI] [Google Scholar]
- 14.Díaz-Gavilán M., Rodríguez-Serrano F., Gómez-Vidal J.A., Marchal J.A., Aránega A., Gallo M.A., Espinosa A., Campos A.M. Synthesis of Tetrahydrobenzoxazepine Acetals with Electron-withdrawing Groups on The Nitrogen Atom. Novel Scaffolds Endowed with Anticancer Activity against Breast Cancer cells. Tetrahedron. 2004;60:11547–11557. doi: 10.1016/j.tet.2004.09.072. [DOI] [Google Scholar]
- 15.Shen J.H., Yang X.F., Wang F.Y., Wang Y., Cheng G.L., Cui X.L. Base-mediated Regiospecific Cascade Synthesis of N-(2-pyridyl) pyrroles from N-Propargylic β-Enaminones. RSC Adv. 2016;6:48905–48909. doi: 10.1039/C6RA08987A. [DOI] [Google Scholar]
- 16.Pecher J., Waefelaer A., Poultier P. Synthesis of Benzoxazepine Derivatives III. Isoindolinones. Bull. Soc. Chim. Belg. 1977;86:1003–1007. doi: 10.1002/bscb.19770861211. [DOI] [Google Scholar]
- 17.Katsuhide K., Noriko M., Ryoko O., Makoto K., Mika N., Toshio T., Tomochika O., Teruyoshi I. New 5-HT1A Receptor Agonists Possessing 1,4-Benzoxazepine Scaffold Exhibit Highly Potent Anti-ischemic Effects. Bioorg. Med. Chem. Lett. 2001;11:595–598. doi: 10.1016/s0960-894x(01)00008-7. [DOI] [PubMed] [Google Scholar]
- 18.Shen J.H., Xue L.L., Lin X., Cheng G.L., Cui X.L. The Base-promoted Synthesis of Multisubstituted Benzo[b][1,4]oxazepines. Chem. Commun. 2016;52:3292–3295. doi: 10.1039/C5CC09877G. [DOI] [PubMed] [Google Scholar]
- 19.Crosby S.H., Clarkson G.J., Deeth R.J., Rourke J.P. Platinum (IV) DMSO Complexes: Synthesis, Isomerization, and Agostic intermediates. Organometallics. 2010;29:1966–1976. doi: 10.1021/om901087m. [DOI] [Google Scholar]
- 20.Alvaro E., Hartwig J.F. Resting State and Elementary Steps of The Coupling of Aryl halides with Thiols Catalyzed by Alkylbisphosphine Complexes of Palladium. J. Am. Chem. Soc. 2009;131:7858–7868. doi: 10.1021/ja901793w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Rodriguez M.A., Shen Q., Hartwig J.F. A General and Long-lived Catalyst for The Palladium-catalyzed Coupling of Aryl Halides with Thiols. J. Am. Chem. Soc. 2006;128:2180–2181. doi: 10.1021/ja0580340. [DOI] [PubMed] [Google Scholar]
- 22.Dallinger D., Kappe C.O. Microwave-assisted Synthesis in Water as Solvent. Chem. Rev. 2007;107:2563–2591. doi: 10.1021/cr0509410. [DOI] [PubMed] [Google Scholar]
- 23.Mangina N., Suresh S., Sridhar B., Karunakar G.V. Gold (iii)-catalyzed Synthesis of Aroylbenzo [b] oxepin-3-ones from Ortho-O-propargyl-1-one Substituted Arylaldehydes. Org. Biomol. Chem. 2016;14:3526–3535. doi: 10.1039/C5OB02676H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




