Abstract
Although cultivated for over 7000 years, mainly for production of cotton fibre, the cotton plant has not been fully explored for potential uses of its other parts. Despite cotton containing many important chemical compounds, limited understanding of its phytochemical composition still exists. In order to add value to waste products of the cotton industry, such as cotton gin trash, this review focuses on phytochemicals associated with different parts of cotton plants and their biological activities. Three major classes of compounds and some primary metabolites have been previously identified in the plant. Among these compounds, most terpenoids and their derivatives (51), fatty acids (four), and phenolics (six), were found in the leaves, bolls, stalks, and stems. Biological activities, such as anti-microbial and anti-inflammatory activities, are associated with some of these phytochemicals. For example, β-bisabolol, a sesquiterpenoid enriched in the flowers of cotton plants, may have anti-inflammatory product application. Considering the abundance of biologically active compounds in the cotton plant, there is scope to develop a novel process within the current cotton fibre production system to separate these valuable phytochemicals, developing them into potentially high-value products. This scenario may present the cotton processing industry with an innovative pathway towards a waste-to-profit solution.
Keywords: cotton gin trash, cotton residues, Gossypium, terpenoids, phenolics, phytochemicals
1. Introduction
Cotton (Gossypium) is naturally a perennial plant that is now commercially cultivated as an annual plant in many parts of the world [1]. The cotton bud is the most utilized part of the plant and is the starting raw material for a wide range of products, such as textiles, edible oil, paper, livestock feed, and medicinal products, to name a few [2,3,4,5,6,7]. Cotton fibre has many positive characteristics (comfort, colour retention, absorbency, strength) [2] and, hence, global cultivation has increased to an estimated production of over 23 million tonnes in 2013–2014 [8]. This increase in cotton production has resulted in tonnes of waste remaining after harvesting and processing (ginning), which has contributed to a growing challenge of its disposal [9,10].
Non-cotton fibre biomass residues generated from cotton production and processing includes cotton gin trash (CGT), post-harvest field thrash (PHT), and crushed seeds from which oil has been extracted. Post-harvest trash (PHT) are the remaining parts of the plant left on the field, while CGT is centralised at gins and is comprised mainly of sticks, burrs (calyx), leaves, and soil [9,11]. These by-products of the cotton industry, although underutilized, are being used as soil composts and cottonseed meal nutritional supplements for livestock feed [10,12,13,14]. Other methods of utilization of cotton industry by-products have been explored, particularly as a low-cost biomass feedstock for commercial bioenergy/biofuel applications [15,16,17], mostly from CGT.
The entire cotton plant has the potential to be a source of valuable compounds, such as terpenes, phenolics, fatty acids, lipids, carbohydrates, and proteins [18,19,20,21,22,23]. These compounds, which are distributed in seeds, bolls, calyx, leaves, stalks, stems, and roots of the plant [20,23,24,25] play functional biological roles in humans and animals [21,26,27,28,29]. Gossypol, a poly-phenolic with potential contraceptive effects [30] and trans-caryophyllene, a terpenoid having anti-inflammatory and cytotoxic properties [31,32], are examples of compounds present in cotton with potential beneficial impact on humans and animals. Thus, by-products generated by the cotton industry (CGT, PHT, and crushed seeds) may represent a potential source of valuable extractives due to the distribution of chemical compounds throughout the whole cotton plant.
Agricultural products and by-products other than cotton are being exploited for generation of energy and materials [33], serving as a means of recycling and reducing organic wastes in the environment. Biomass from soybean, rice, and sugar cane are examples of agricultural by-products currently utilized for these purposes [34,35,36,37]. Valuable chemical extractives may also occur in some of this agricultural biomass [38], although these potential resources have not been fully exploited.
The multitude of potentially valuable chemicals that could be derived from cotton production by-products has gained little attention. Hence, exploiting the full potential of cotton waste will be beneficial to both the cotton industry and the local environment. A more detailed investigation is necessary to provide answers to such questions as: what is the nature of the extractives present in these by-products; how do the chemical profiles vary between cotton species and/or varieties and what potential uses do the waste products have as a source of high value compounds. To provide answers to some of these questions, this review begins with an overview of cotton production, the source of industry wastes, and its current utility. This review continues with a further discussion of current knowledge of chemical extractives present in the cotton plant and the distribution of these compounds within the plant highlighting their potential phytochemical properties which may be of value to both the pharmaceutical and agricultural sector.
2. Cotton
Cotton, the Gossypium genus in the tribe Gossypiae, in the family Malvaceae, can be generally divided into two types: cultivated and wild cotton. Of 50 known species, only four (4) are cultivated, with the remaining 46 growing wild in the tropics and sub-tropics [39,40]. The four common cultivated cotton species are G. hirsutum, G. herbaceum, G. barbadense, and G. arboreum. These species vary in terms of fibre quality [41] defined by length, maturity, strength, and micronaire (cell wall thickness) of the fibre. The differences in fibre quality, yield, and adaptation to certain climatic conditions, has contributed to the preference of some cotton species over others [39]. All four cultivated cotton species are used for other purposes, including food production and medicinal application [21,42,43,44].
G. hirsutum, sometimes referred to as “upland, American or Mexican cotton”, is the most commonly cultivated of all cotton species [41]. G. hirsutum, is widely grown in its transgenic form because of its high yield and adaptability to different environmental conditions [45], although high temperatures can result in sterility and boll shedding [46,47]. The other species are predominant in parts of Asia and Africa and are seldom cultivated outside these regions [48,49], due to their inability to adapt to different climatic conditions [46] and poor yields [45,49]. G. hirsutum has been manipulated to improve yield and there has been some effort made toward enhancing the other cultivated species using transgenic approaches [50,51].
2.1. Transgenic Cotton
Fungal infection, pests, weed infestation and unfavourable environmental conditions are some of the challenges which result in poor yields [52,53,54,55,56,57]. These challenges prompted transgenic manipulation of cotton plants, to equip them with the ability to withstand unfavourable conditions and promote higher yields [58,59,60]. Bt cotton carries transgenes derived from the soil dwelling bacteria Bacillus thuringiensis, hence the name Bt cotton. Bt cotton cultivars carry the Cry1Ac gene making it resistant to the tobacco bud worm [61], or a combination of Cry1Ac and Cry2Ab gene, making it resistant to a wider range of cotton pests [62]. A new type of Bt cotton, which is yet to be introduced in the market, contains Cry1Ac, Cry2Ab, and Vip3A genes, with the last gene promoting increased resistance to more challenging pests such as lepidopteran insects [63,64]. Roundup Ready cotton, another transgenic type, contains a gene that confers resistance to glyphosate herbicide, enabling weed control without destroying the cotton.
There have been other attempts to move favourable genes between species to enhance tolerance under unfavourable environmental conditions. For example, a heat shock protein gene GHSP26, thought to be responsible for drought tolerance in G. arboretum, has been transferred to G. hirsutum, thereby enhancing its ability to withstand drought [65].
3. Cotton Industry and Processing
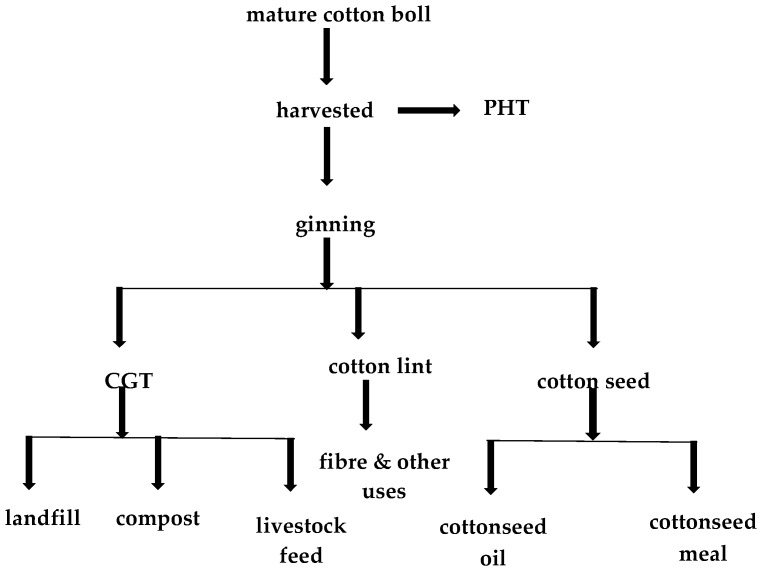
When fully matured, cotton bolls are picked and transported for processing, leaving the remaining plant as field trash. During the refining process or ginning of the harvested cotton, impurities are removed from the cotton fibres and are recovered as a processing by-product (CGT). Moreover, cotton seed is also processed to recover cotton seed oils and cotton seed meals. Cotton production generates three categories of waste products: (i) field trash (stems, flowers, leaves, and stalks); (ii) CGT (leaves, fibre, flowers, immature seeds, sticks and soil) [9] and cotton seed meal (from which oil has been extracted) (Figure 1).
Figure 1.
Flowchart of cotton processing from field to cotton gin products.
3.1. Cotton Waste
Cotton by-products have provided producers with additional value, mainly in the form of livestock feed supplements and soil amendments [6,66]. Despite its abundance, field trash is generally viewed as having little value-added potential and is, therefore, not a resource that is utilised in standard farming practices. Field trash is currently slashed and left in the field where it provides some benefits through improving soil carbon and reducing soil erosion. The high cost associated with harvesting field trash for other uses is considered a major economic hurdle.
Cotton seeds constitute 55% of the total ginned cotton by weight, whereas cotton fibre and CGT make up about 35%–40% and 10% respectively [67]. Although historically viewed as a waste by-product of cotton processing, cotton seed is now considered a high value co-product and an important part of the cotton processing value chain. Cotton seed is fractionated into high value oils and high protein meals, both with applications in food and feed industries. In contrast, CGT is considered a lower value waste with little value adding potential and the management of CGT is regarded as a financial burden to most ginning operations. CGT is generally disposed of in one of four ways: as solid waste (landfilling), composting and land application, incineration and, to a lesser extent, fed to livestock as a supplement [9,10]. Moreover, disposal options are tightly regulated by local environmental laws which add further restrictions.
CGT has a reasonable nutritional profile composed of dry matter (90%), crude protein (12%), total digestible nutrients (47%), calcium (11%), sodium (121 ppm), and iron (963 ppm) [66], and has been proven to contribute to the wellbeing of livestock [6,12]. Despite this, its use has been discouraged (and banned altogether in some countries) owing to the presence of residual chemicals which are used during cultivation [68]. This has resulted in CGT being widely used as fertilizer supplements [69,70] and composts to maintain/conserve soil moisture and composition that improve crop production [71,72].
Waste generated from cotton harvesting and cotton ginning mills are used as replacement components for inorganic-based filler materials and additives, for the production of thermoplastic composites poly(lactic acid) (PLA) and low-density polyethylene (LDPE) [73]. By-products from the cotton industry have also been processed to produce fulvic acid and silica [74]. Cotton trash has been investigated in numerous studies as a renewable feedstock in bioethanol production [15,16,75,76]. CGT is well suited as a biofuel feedstock because its composition has the attribute of high polysaccharide content (up to 50%) for effective and scalable conversion to biofuels.
A promising, yet less well documented use of CGT is in the manufacturing of biologically active compounds. There are a variety of chemical compounds which occur naturally in cotton plants with wide ranging activities. Given that such compounds are present in the cotton plant, it is plausible that the remaining trash also contains a proportion of biologically active molecules. These compounds and their uses are examined and discussed in the following sections of this review.
4. Chemical Compounds in Cotton
Different compounds present in cotton play important roles during metabolism or interaction with the environment. Naturally-occurring compounds in cotton include terpenes, phenols, proteins, carbohydrates, fatty acids, and lipids [19] (Table 1). As with most plants, the distribution of these compounds vary between different parts of the cotton plant with some compounds concentrated in specific parts of the plant [77] (Figure 2). The distribution of these chemical compounds is related to their different properties and functionality in the plant. The various compounds found in cotton plant will be discussed, highlighting the chemistry, as well as their distribution within the plant.
Table 1.
Chemical compounds isolated from cotton (Gossypium).
| Compounds | Molecular Formula | Molecular Weight (g/mol) | References |
|---|---|---|---|
| Terpenes | |||
| Monoterpenes | C10H16 | 136.24 | |
| Camphene | [21] | ||
| Limonene | [21,23] | ||
| Myrcene | [23] | ||
| Ocimene | [21,23] | ||
| α-pinene | [23] | ||
| β-pinene | [23] | ||
| Sabinene | [21,23] | ||
| α-Thujene | [21] | ||
| Sesquiterpenes | C15H24 | 204.35 | |
| α-Bergamotene | [78] | ||
| Bisabolene | [78] | ||
| 1(10),4-Cadinadiene; (6β,7β)-form | [79,80] | ||
| Caryophyllene | [78] | ||
| Copaene | [81] | ||
| Guaiadiene: (4β,5α,7β)-form | [81] | ||
| Farnesene | [78] | ||
| Humulene | [81,82] | ||
| Terpene derivatives | |||
| α and β-Amyrin | C30H50O | 426.72 | [83] |
| Bisabolol | C15H26O | 222.37 | [82,84] |
| 1,3,5,10-Bisabolatetraen-7-ol | C15H22O | 218.34 | [82] |
| Bisabolene oxide | C15H24O | 220.35 | [85] |
| 1(10),4-Cadinadien-2-ol; (2ξ,6β,7β)-form | C15H24O | 220.35 | [86] |
| 1,3,5,7,9-Cadinapentaene-3,9-diol | C15H18O2 | 230.31 | [86] |
| 1,3,5,7,9-Cadinapentaene-3,9-diol; 3-Me ether | C16H20O2 | 244.33 | [86] |
| 1,3,5,9-Cadinatetraene; 7αH-form, 3-Hydroxy | C15H20O | 216.32 | [87] |
| 1,3,5-Cadinatriene-3,9-diol; (7α,9α,10α)-form, 9-Ketone | C15H20O2 | 232.32 | [88] |
| 1,3,5-Cadinatriene-3,9-diol; (7β,10α)-form, 9-Ketone | C15H20O2 | 232.32 | [88] |
| 1,3,5-Cadinatriene-3,9,10-triol; (7β,9β,10α)-form, 9-O-β-d-Glucopyranoside | C21H32O8 | 412.48 | [89] |
| 3(15),6-Caryophylladien-12-ol; (6E)-form | C15H24O | 220.35 | [90] |
| 3(15),6-Caryophylladien-12-ol; (6E)-form, 6α,7β-Epoxide, Ac | C17H26O3 | 278.39 | [90] |
| Caryophyllene oxide | C15H24O | 220.35 | [82] |
| 3,10-Dihydroxy-1,3,5,7-cadinatetraen-9-one | C15H18O3 | 246.31 | [91] |
| 8,9-Dihydroxy-2,5-dioxo-1(6),3,7,9-cadinatetraen-14-al | C15H14O5 | 274.27 | [91] |
| 2,14-Epoxy-1,3,5,7,9-cadinapentaene-8,9-diol | C15H16O3 | 244.29 | [92] |
| 2,14-Epoxy-1,3,5,7,9-cadinapentaene-8,9,12-triol; 15-Hydroxy, 9-O-(6-O-sulfo-β-d-glucopyranoside) | C21H26O13S | 518.50 | [93] |
| Heliocide H1 | C25H30O5 | 410.51 | [93,94] |
| Heliocide H1; 7-Me ether | C26H32O5 | 424.54 | [95] |
| Heliocide H2 | C25H30O5 | 410.51 | [93] |
| Heliocide H2; 3-Me ether | C26H32O5 | 424.54 | [95] |
| Heliocide H3 | C25H30O5 | 410.51 | [93] |
| Heliocide H3; 3-Me ether | C26H32O5 | 424.54 | [95] |
| Heliocide H4 | C25H30O5 | 410.51 | [93] |
| Heliocide H4; 3-Me ether | C26H32O5 | 424.54 | [95] |
| β-Sitosterol | C29H50O | 414.71 | [83] |
| Strigol | C19H22O6 | 346.38 | [96,97] |
| 2,3,8,9-Tetrahydroxy-1,3,5,7,9-cadinapentaen-14-al; 3-Me ether | C16H18O5 | 290.32 | [98] |
| 2,3,9-Trihydroxy-1,3,5,7,9-cadinapentaen-14-al; 3-Me ether | C16H18O4 | 274.32 | [99] |
| 2,8,9-Trihydroxy-1,3,5,7,9-cadinapentaen-14-al | C15H16O4 | 260.29 | [100] |
| 2,8,9-Trihydroxy-1,3,5,7,9-cadinapentaen-14-al; 8-Deoxy | C15H16O3 | 244.29 | [100] |
| 2,8,9-Trihydroxy-1,3,5,7,9-cadinapentaen-14-al; 8-Me ether | C16H18O4 | 274.32 | [100] |
| 3,8,9-Trihydroxy-2,5-dioxo-1(6),3,7,9-cadinatetraen-14-al; 3-Me ether | C16H16O6 | 304.30 | [101] |
| Phytol | C20H40O | 296.54 | [18] |
| Phenols | |||
| Phenolic acids | |||
| Benzoic acid | C7H6O2 | 122.12 | [22] |
| Chlorogenic acid | C16H18O9 | 354.31 | [22] |
| Ferrulic acid | C10H10O4 | 194.18 | [22] |
| Gallic acid | C7H6O5 | 170.12 | [22] |
| Gentisic acid | C7H6O4 | 154.12 | [22] |
| P-coumaric acid | C9H8O3 | 164.16 | [22] |
| 4-hydroxybenzoic acid | C7H6O3 | 138.12 | [22] |
| 3,4-Dihydroxybenzoic acid | C7H6O4 | 154.12 | [22] |
| Syringic acid | C9H10O5 | 198.17 | [22] |
| Phenolic acid analogs | |||
| Gossypol; (±)-form, 6-Me ether | C31H32O8 | 532.59 | [102] |
| Gossypol; (±)-form, 6,6′-di-Me ether | C32H34O8 | 546.62 | [102] |
| Gossypol; (+)-form | C30H30O8 | 518.56 | [83] |
| Gossypurpurin | C60H56N2O13 | 1013.11 | [103] |
| Gossyrubilone | C20H25NO4 | 343.42 | [95] |
| Flavonoids | |||
| α-2′,3,3′,4,4′,6-Heptahydroxychalcone; 2′-O-d-Glucopyranoside | C12H22O13 | 482.40 | [104] |
| 3,3′,4′,5,7,8-Hexahydroxyflavone; 3-O-β-d-Glucopyranoside | C21H20O13 | 480.38 | [19] |
| Gossypetin 7-glucoside | C21H20O13 | 480.38 | [19] |
| 3,3′,4′,5,7,8-Hexahydroxyflavone; 8-O-α-l-Rhamnopyranoside | C21H20O12 | 464.38 | [105] |
| Kaempferol 3-glycosides; Monoglycosides, 3-O-α-d-Glucopyranoside | C21H20O11 | 448.38 | [19] |
| 3,3′,4′,5,7-Pentahydroxyflavan; (2S,3R)-form | C15H14O6 | 290.27 | [106] |
| 3,3′,4′,5,7-Pentahydroxyflavone; 3′-O-β-d-Glucopyranoside | C21H20O12 | 464.38 | [105] |
| 3,4′,5,7,8-Pentahydroxyflavone | C15H10O7 | 302.24 | [107] |
| Quercetin 3-glycosides; Disaccharides, 3-O-[β-d-Galactopyranosyl-(1→6)-β-d-glucopyranoside] | C27H30O17 | 626.52 | [105] |
| Quercetin 3-glycosides; Tetra- and higher saccharides, 3-O-[α-d-Apiofuranosyl-(1→5)-β-d-apiofuranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→6)]-β-d-glucopyranoside] | C37H46O24 | 874.76 | [108] |
| 3,3′,5,7-Tetrahydroxy-4′-methoxyflavone | C16H12O7 | 316.27 | [19] |
| 3,4′,5,7-Tetrahydroxy-8-methoxyflavone; 3-O-β-d-Glucopyranoside, 7-O-α-l-rhamnopyranoside | C28H32O16 | 624.55 | [109] |
| Other Phenols | |||
| Scopoletin | C10H8O4 | 192.17 | [110] |
| Fatty acids and Lipids | |||
| 11,14-Eicosadienoic acid | C20H36O2 | 308.50 | [24] |
| Hexadecanoic acid | C16H32O2 | 256.43 | [24] |
| 9-Hexadecanoic acid; (Z)-form | C16H30O2 | 254.41 | |
| Octadecanoic acid | C18H36O2 | 284.48 | [24] |
| 9-Octadecenoic acid; (Z)-form | C18H34O2 | 282.47 | [24] |
| 9,12-Octadecadienoic acid; (Z,Z)-form | C18H32O2 | 280.45 | [18] |
| 9,12,15-Octadecatrienoic acid; (Z,Z,Z)-form | C18H30O2 | 278.43 | [18] |
| Tetradecanoic acid (myristic acid) | C14H28O2 | 228.37 | [24] |
| Triacontanoic acid | C30H60O2 | 452.80 | [111] |
| Carbohydrates | |||
| Cellulose | C6H10O5 | 162.14 | [25] |
| Cyanidin 3-glycosides; Disaccharides, 3-O-[β-d-Xylopyranosyl-(1→4)-β-d-glucopyranoside] | C26H29O15 | 581.51 | [112] |
| 6-O-α-d-Galactopyranosyl-d-glucose | C12H22O11 | 342.30 | [113,114] |
| Glycerol 1-alkanoates; Glycerol 1-(22-hydroxydocosanoate), 22′-O-(3,4-Dihydroxycinnamoyl) | C34H56O8 | 592.81 | [115,116] |
| Raffinose | C18H32O16 | 504.44 | [117,118] |
| Proteins | |||
| 3-Phosphoglycerate phosphatase | [119] | ||
| Vicilin A and B | [20] | ||
| Legumin Aand B | [20] | ||
| Hydrocarbons | |||
| 1H-Indole-3-carboxaldehyde | C12H7NO | 145.16 | [120,121] |
| 1-Methyl-2-propylbenzene | C10H14 | 134.22 | [122] |
| Octatriacontane | C38H78 | 535.03 | [123] |
| Alcohols | |||
| Dotriacontanol | C32H66O | 466.88 | [18] |
| 1-Tetratriacontanol | C34H70O | 494.93 | [18] |
| Triacontanol | C30H62O | 438.81 | [18] |
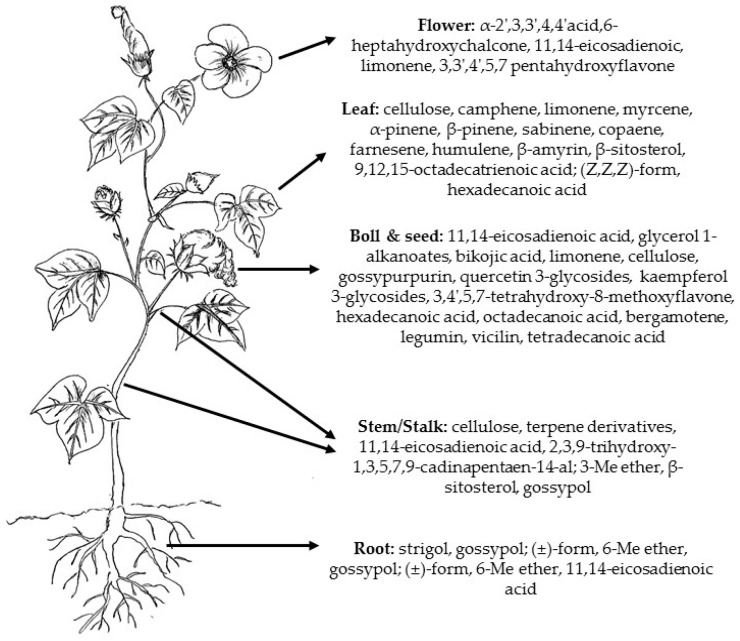
Figure 2.
Distribution of common secondary metabolites in cotton plant.
4.1. Terpenes
Like most plants, the cotton plant is susceptible to insect, herbivore, and pathogen attack. In a bid to ward off these predators, compounds are produced by the plant as a defence mechanism. Terpenes are an important class of defence compounds synthesized in the cotton plant and are also the largest group of plant defence compounds [124,125]. They are major constituents of essential oils found in most plants and, as such, have been applied in the food, chemical, and cosmetic industry [126]. Terpenes are composed of units of a five-carbon compound, isoprene (Figure 3), linked together in a head to tail fashion [127], forming long chains or rings. They are classified into seven classes by the number of isoprene units they contain and include hemiterpenes, monoterpenes, sesquiterpenes, diterpenes, triterpenes, tetraterpenes, and polyterpenes [125,127,128,129]. Generally, hemiterpenes do not occur as free compounds but are bound to other non-terpene compounds [126], while terpenes modified by oxidation or a re-arrangement of the carbon skeleton are referred to as terpenoids.
Figure 3.
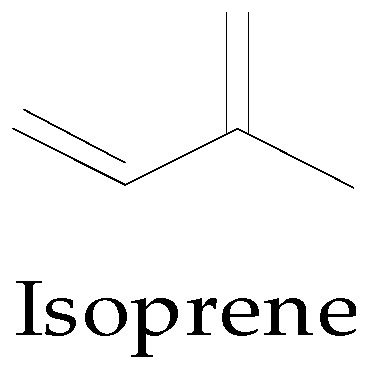
Chemical structure of isoprene (building block of terpenes).
According to Pare and Tumlinson [130] and Rose and Tumlinson [131], terpenes in cotton can be divided into two groups. The first are constitutive compounds that are present in the storage compartments of the cotton plant and are released immediately after insect feeding or damage. Some of these terpenes include α-pinene, β-pinene, limonene, caryophyllene, α-humulene, and myrcene. The second group of terpenes are referred to as inducible compounds which are synthesized de novo several hours after exposure to pests and herbivores and include β-ocimene, α-farnesene, β-farnesene, and linalool. Some of these terpenes occur in their enantiomeric forms in the plant with a reported occurrence of the negative forms e.g., -α-farnesene, -β-farnesene and -β-ocimene [131,132]. Monoterpenes, sesquiterpenes, triterpenes, and terpene derivatives mostly occur in the cotton plant, with monoterpenes, sesquiterpenes, and their derivatives being the most common [133]. The total concentration of terpenes in cotton plant is unclear, although accumulation of terpenes in cotton plant parts varies with up to 15.5 mg terpenoids reportedly accrued per fresh weight of cotton leaves [124]; 2.81 mg and 2.49 mg per foliage weight reported for monoterpenes and sesquiterpenes, respectively.
4.1.1. Terpene Biosynthesis
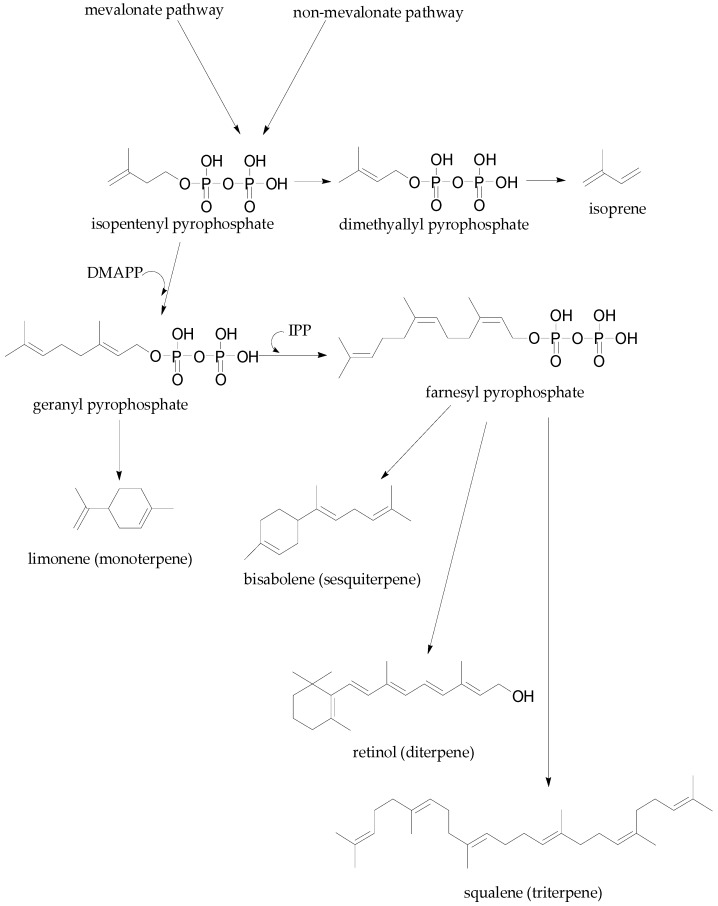
Terpenes are synthesized via the acetate/mevalonate pathway [133] and mevalonate independent pathway [127,128]. The non-mevalonate pathway is also referred to as the deoxyxylulose phosphate (DXP) pathway or the methyl erythritol phosphate (MEP) pathway. Although terpene synthesis begins with photosynthesis, most studies identify the combination of three acetyl CoA molecules as the starting point of terpene or terpenoid biosynthesis via the acetate/mevalonate pathway [134].
The mevalonate and non-mevalonate pathway result in the formation of isopentenyl pyrophosphate (IPP) and dimethyl allyl pyrophosphate (DMAPP) which forms isoprene catalysed by isoprene synthase. Monoterpenes are synthesized in the plastids of plant cells from geranyl pyrophosphate (GPP) (Figure 4) which is formed from the combination of DMAPP and IPP catalysed by isoprenyl diphosphate synthases [135]. Sesquiterpenes (Figure 4) are synthesized in the cytosol from farnesyl pyrophosphate (FPP), which is formed from one molecule of GPP and IPP joined in a head to tail combination. The activity of sesquiterpene synthase enzymes converts FPP to sesquiterpenes via ionization reactions [136]. Other terpenes, diterpenes, and triterpenes are synthesized from FPP via the formation of geranyl geranyl diphosphate (GGDP) and squalene, respectively (Figure 4). Both pathways of terpene biosynthesis can, thus, be summarised into a four step process: (1) synthesis of IPP and isomerization to DMAPP; (2) addition of more IPP compounds; (3) terpene backbone formation by terpene synthase activity; and (4) enzymatic modification to induce specific functions of the terpenes [129].
Figure 4.
Biosynthesis of terpenes from isopentenyl pyrophosphate, a product of the mevalonate and non-mevalonate pathway.
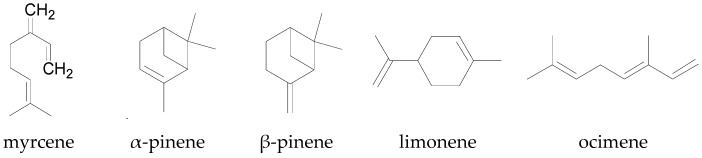
4.1.2. Monoterpenes (C10)
The monoterpenes (C10H16) are a class of terpenes that consist of two isoprene units and can be linear (acyclic), monocyclic (containing one ring), or bicyclic (containing two rings) [137]. There are over 1000 monoterpenes known to occur in nature and examples of common monoterpenes in plants include myrcene (acyclic), limonene (monocyclic), and pinene (bicyclic) (Figure 5) [137]. Together with the sesquiterpenes, monoterpenes are major constituents of essential oils extracted from various plant materials [138]. Biochemical modifications of monoterpenes such as oxidation, hydroxylation and rearrangement of atoms result in the formation of monoterpenoids such as geraniol and linalool [135,139]. In the cotton plant, there are some acyclic monoterpenes which belong to the group of constitutive compounds, such as α-pinene, β-pinene, and limonene amongst others, as well as herbivore-induced monoterpenes [130,132]. Although monoterpenes found in cotton are distributed in different parts of the plant, including leaves, seeds, flowers, stems, and roots, they are predominantly concentrated within the leaves and flowers (Figure 2) [23,124,140,141].
Figure 5.
Chemical structures of some monoterpenes in plants, including cotton.
4.1.3. Sesquiterpenes (C15)
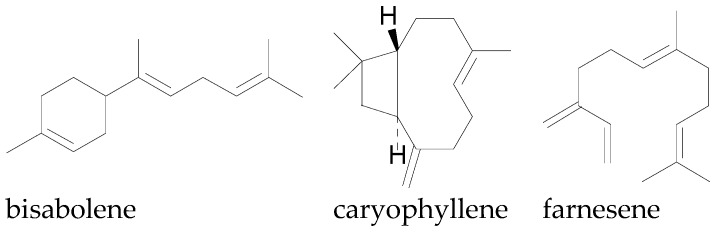
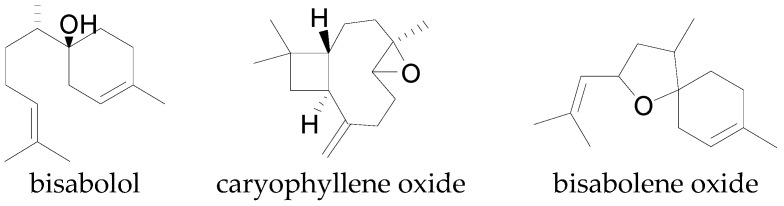
Sesquiterpenes (C15H24) are composed of three isoprene units either in acyclic or cyclic form and occur in most plants. Sesquiterpenes are not limited to higher plants; they have been discovered in micro-organisms, such as bacteria, fungi, and marine organisms [135]. The sesquiterpenes occur in many cotton species and have been extracted from the leaves, flowers, seeds, and bolls of cotton plants [21,142]. Bell [19] reported the total concentration of some sesquiterpenes in essential oil extracted from whole cotton plants up to 26.12% and 30.1% for G. hirsutum and G. barbadense respectively. The sesquiterpenes, α-bergamotene, caryophyllene, bisabolene, farnesene, humulene and copanene are some of the sesquiterpenes commonly associated with cotton (Figure 6), while oxidized forms such as bisabolol, bisabolene oxide, caryophyllene oxide, and other sesquiterpenoids also occur in the cotton plant [19] (Figure 7).
Figure 6.
Chemical structures of some sesquiterpenes in cotton.
Figure 7.
Some sesquiterpenoids isolated from cotton.
4.1.4. Triterpenes (C30)
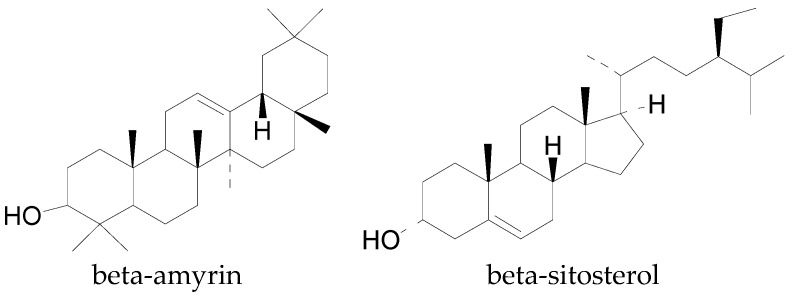
There are no reports of diterpenes, tetraterpenes, and polyterpenes in cotton plants, however, two triterpene derivatives, β-sitosterol and β-amyrin montanate (Figure 8), were reported to occur in cotton leaves by Shakhidoyatov et al. [18]. Triterpenes are generally made of six (6) isoprene units and contain 30 carbon atoms with a molecular formula of C30H48.
Figure 8.
Triterpene derivatives in cotton.
4.2. Phenols
Phenolic compounds are secondary metabolites found in most plants and normally comprise of one or more hydroxyl groups directly attached to one or more aromatic hydrocarbons [143]. Phenols occur in many lower and higher plants, medicinal plants/herbs, and dietary herbs [144], and their distribution is mainly governed by the physiological roles they play within the plant [143,145].
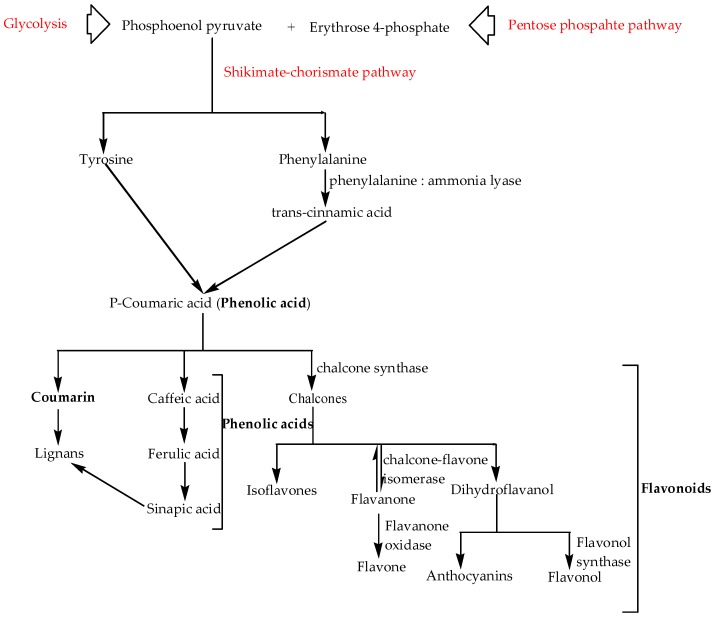
There are up to nine (9) groups of compounds classified as phenols, including phenolic acids, phenolic acid analogs, flavonoids, tannins, stilbenes, curcuminoids, coumarins, lignans, and quinones [144,146]. Despite the wide occurrence of phenols in higher plants, only phenolic acids, phenolic acid analogs, flavonoids, tannins, and coumarins have been reported to occur in cotton seeds (41 ppm), bracts (22.6 ppm), leaves (21.6 ppm), and roots [19]. Phenolic compounds are synthesized within the chloroplast of plant cells through a series of reactions which are preceded by the synthesis of aromatic amino acids tyrosine and phenylalanine via the shikimate-chorismate pathway. This pathway involves reactions between phosphoenol pyruvate (a by-product of glycolysis) and erythrose 4-phosphate (a by-product of the oxidative pentose phosphate pathway). These two aromatic amino acids, regarded as the major precursors in the synthesis of phenolic compounds, undergo a series of reactions via the phenylpropanoid pathway resulting in different classes of phenolic compounds (Figure 9). Several other key enzymes are implicated in the synthesis of phenols from one class to another.
Figure 9.
The generalised biosynthetic pathway of phenolic compounds.
4.2.1. Flavonoids
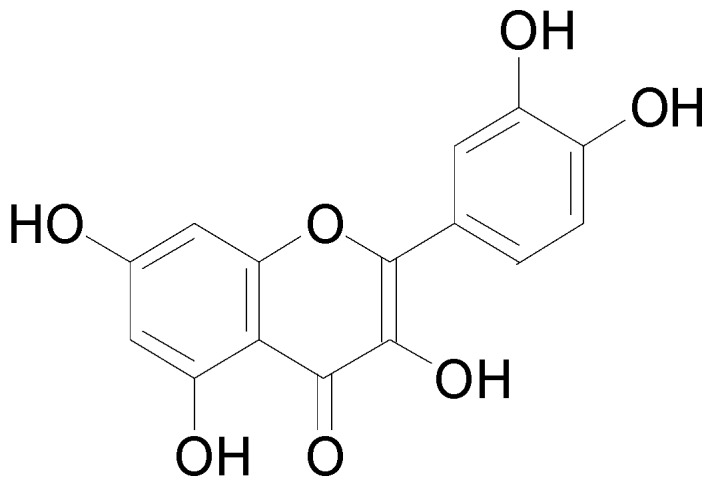
Flavonoids are the most abundant class of phenolic compounds. Huang, Cai and Zhang [144] reported that over 4000 flavonoids occur in nature while Cheynier [143] suggested that the number is closer to 8000. Flavonoids derive their name from the latin word “flavus” which means “yellow”, because of the prevalent yellow colour and are largely responsible for the colours of flowers, leaves, barks, fruits, and seeds of most plant species [134]. Flavonoids have a basic skeletal structure of phenyl benzopyrone (C6-C3-C6) comprised of two aromatic rings linked by three carbon atoms. Flavonoids occur as free compounds e.g., quercetin (Figure 10) or as glycosides combined with different sugars [144] e.g., kaempferol 3-glycosides, and quercetin 3-glycosides.
Figure 10.
Quercetin, a flavonoid with the basic skeletal structure of flavonoids.
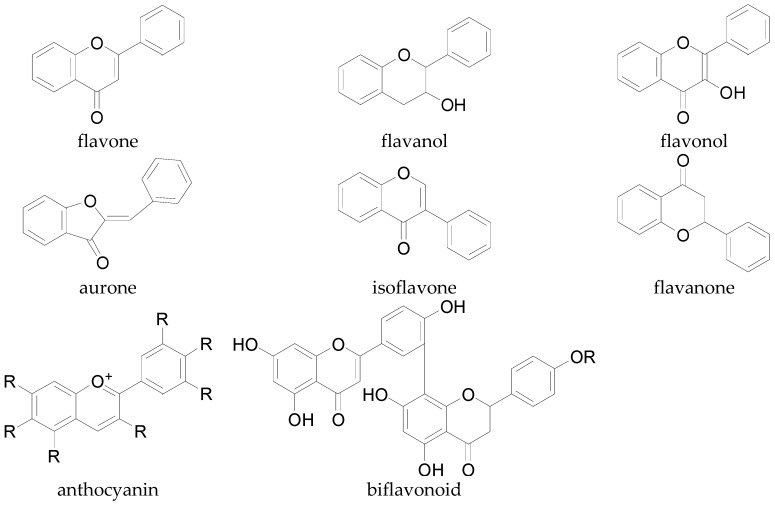
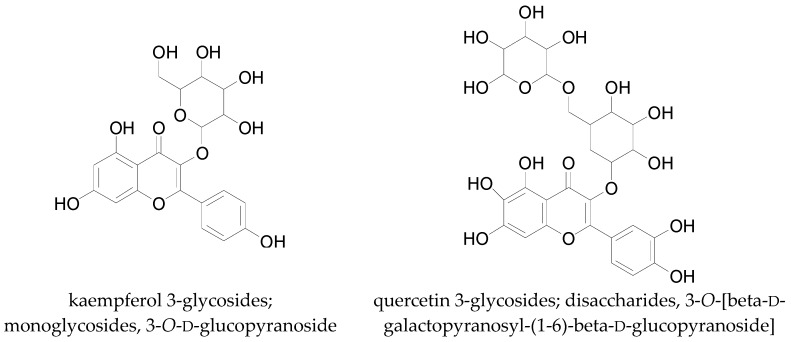
There are several different classes of flavonoids such as the flavones, flavonols, isoflavones, aurones, anthocyanins, biflavonoids, flavanols, and flavanones [134,147] (Figure 11). These flavonoids differ slightly in their chemical structures. The flavonols possess hydroxyl side groups, which distinguishes them from the flavones. Isoflavones differ from flavones by the location of the phenyl group, whereas the anthocyanins differ from other flavonoids by possessing a positive charge. Biflavonoids have a general formula of (C6-C3-C6)2 and aurones possess a chalcone-like group instead of the six-membered ring typical of flavonoids. Several of these flavonoids have been identified in cotton including flavones, and flavonols which mostly occur as glycosides located in flowers, leaves, and seeds [19]. The most common flavonoids in cotton are glycosides of kaempferol, quercetin, and herbacetin (Figure 12). Flavonoid glycosides are water- and ethanol-soluble, while free flavonoids are only soluble in organic solvents [134].
Figure 11.
Base structures of the different classes of flavonoids.
Figure 12.
Chemical structures of some flavonoids in cotton.
4.2.2. Phenolic Acids and Analogs
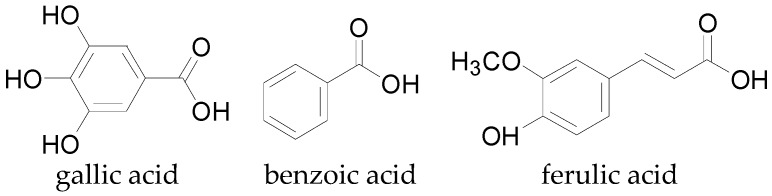
Phenolic acids and their analogs are another group of phenolics that occur in cotton. The hydroxybenzoic acids (HDBA), gallic acid (Figure 13), p-hydroxybenzoic acid, protocatechiuc acid, and others listed in Table 1 are common secondary metabolites in cotton, as well as being the predominant phenolic acids in nature. The hydroxycinnamic acids (HDCA) are hydroxyl derivatives of cinnamic acids with a basic C6-C3 structure. Some HDCA identified in cotton plants include chlorogenic acid, ferulic acid (Figure 13), and p-coumaric acid which are precursors in the biosynthetic pathway to other phenolic compounds such as the lignins, coumarins, and flavonoids [144].
Figure 13.
Chemical structures of some phenolic acids present in cotton.
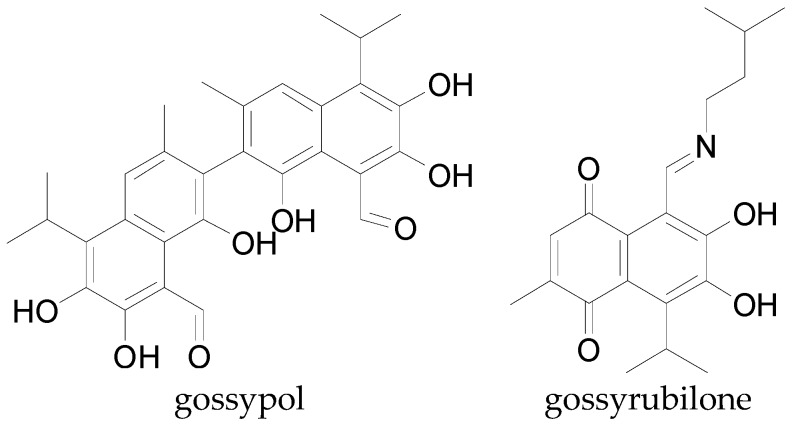
Most phenolic acids have a bitter taste and presumably contribute to the bitter taste of cottonseed products [19]. Gossypol, gossypurpurin, gossyrubilone, and other phenolic acid analogs [30,144] presented in Figure 14 are common secondary compounds isolated from cotton seeds and it is believed they occur in other parts of the cotton plant.
Figure 14.
Chemical structures of some phenolic acid analogs present in cotton.
4.2.3. Tannins and Coumarins
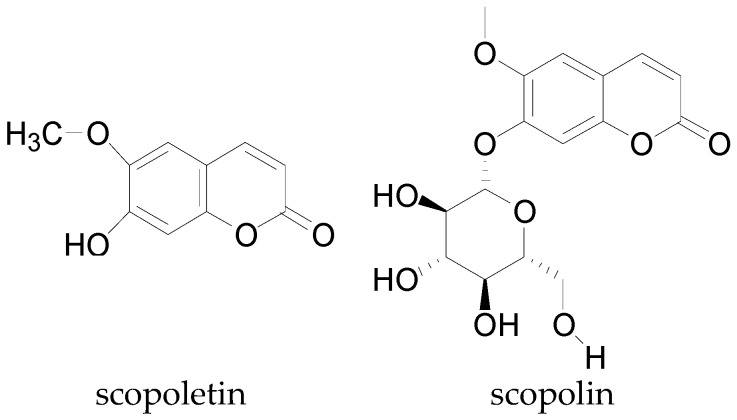
Tannins are a large class of poly phenolic water-soluble compounds which have molecular weights in the range of 500–4000 g/mol. Plant tannins are divided into two classes, the hydrolysable tannins which derive their base unit from gallic acid, and condensed tannins, which arise from proanthocyanidins (condensed flavonols), as well as flavonoid and non-hydrolyzable tannins [144]. Condensed tannins are normally found in combination with alkaloids, polysaccharides, or proteins. These are the class of tannins reported to occur in cotton [148] and act as pesticides, protecting the cotton plant against predators [19]. The coumarins are another group of phenolic acids isolated from cotton. Scopoletin, a coumarin derivative and its glycoside, scopolin presented in Figure 15 have been identified in cotton plant tissue confirming the report that coumarins occur in the free form and as glycosides in cotton, as well as other plants [144].
Figure 15.
Chemical structures of scopoletin and its glycoside scopolin.
4.3. Fatty Acids, Carbohydrates and Proteins
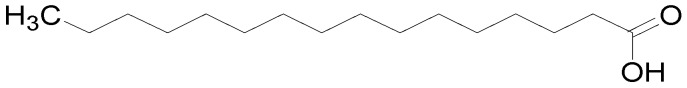
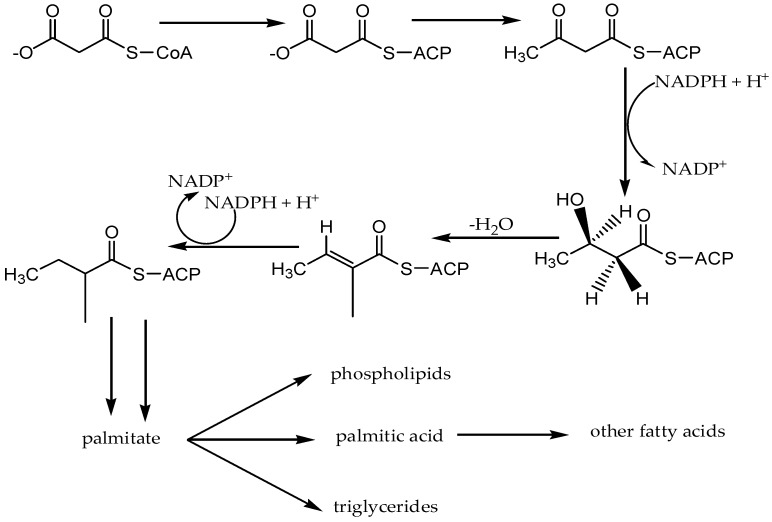
Fatty acids are carboxylic acids with long aliphatic chains that are synthesized in the cytosol of plant cells from malonyl-CoA, which in turn is derived from acetyl-CoA. Palmitic acid (Figure 16) is a base fatty acid from which other fatty acids are formed by 2-carbon increments or reduction. The synthesis of palmitic acid (Figure 17) from the precursor malonyl-CoA follows a five step repeating cycle of acylation, condensation, reduction, dehydration, and reduction, which is catalyzed by the fatty acid synthase complex [149,150].
Figure 16.
Palmitic acid, a base fatty acid from which other fatty acids are formed.
Figure 17.
Fatty acid biosynthetic pathway in plants.
Saturated fatty acids which occur in the cotton plant include myristic acid (tetradecanoic acid), melissic acid (triacontanoic acid), palmitic acid (hexadecanoic acid), stearic acid (octadecanoic acid), and palmitoleic acid (9-hexadecanoic acid) [18,19,151]. Unsaturated fatty acids identified in cotton include eicosadienoic acid, linoleic acid (octadecadienoic acid), linolenic (octadecatrienoic acid), and elaidic acid (octadecenoic acid) [18,19]. Most fatty acids identified in cotton are free fatty acids (not linked to any molecules) and play functional roles as a source of energy for plant growth [19].
Cotton, like all plants, is comprised of cellulose and hemicelluloses, proportions of which vary between different parts of the plant. Cotton fibre itself is comprised mainly of cellulose at levels greater than 94% by weight [25]. Raffinose is a unique minor sugar found in cotton plants predominantly in the seed [117,118].
Alkali and water soluble proteins are also found in cotton [152], including water soluble globular proteins vicilin and legumin (Table 1) present in the seeds of cotton [20]. Proline-rich protein H6 is involved in the development of the cell wall structure of cotton fibre [153,154].
4.4. Variation in Cotton Chemical Composition
4.4.1. Genotypes and Varieties
Cotton plants can be categorised as glanded and glandless cotton. Glanded cotton contains pigment glands distributed in tissues and organs of the cotton plant which are rich in gossypol and terpenoid aldehydes [155]. Glandless cotton was developed from the wild-type glanded cotton by McMichael [156] in order to tackle the challenge of gossypol extraction from cottonseed and cotton seed oil [157]. Since then, different varieties of glandless and glanded cotton have been developed, but the absence of pigment glands has made glandless cotton susceptible to infection and pest infestation [155,158]. Glanded cotton contains more proteins, fatty acids, sugars, and terpenoids in comparison with glandless cotton [159], with very little variation between varieties within each group when cultivated under the same environmental conditions [151,160], although Dowd et al. [151] found variation in fatty acid composition was influenced more by genotype than environmental factors, with up to 62.4% of palmitoleic acid content being controlled by genotype and only 5.4% of the variation in linoleic acid induced by environment.
4.4.2. Non-Transgenic and Cotton Transgenic Cotton Differences
Cultivation of transgenic Bt cotton has been widely practised [161,162] which has led to interest in the possibility of induced alterations in the chemical composition, as well as nutritional value of Bt cotton. Yan, et al. [163] reported that all chemical compounds present in non-transgenic cotton were also present in transgenic cotton and indicated that Bt cotton contained higher concentration of some monoterpenes e.g., alpha and beta pinene and lesser concentrations of myrcene and ocimene when compared to non-transgenic cotton. It has been suggested that the increased production of pinene in Bt cotton can be attributed to the activity of genes which cause the plant to repel insects/pests [164,165,166]. Nutritional evaluation of Bt cotton relative to non-transgenic cotton by Mohanta, et al. [167] revealed slight variations in concentration of proteins and carbohydrates in both types of cotton. Overall, these findings suggest transgenic cotton differs slightly from non-transgenic cotton by the general composition of proximate constituents (moisture content, crude fat, and total ash), fibres, minerals, and secondary metabolites.
5. Pharmacological Properties of Compounds in Cotton
Several studies have emphasized the importance of plants to the pharmaceutical and medical industry [168,169,170]. Cotton is described as a medicinal plant because of the chemical compounds that have been isolated from it [21,83]. A number of compounds found in cotton play pharmacological roles in nature (Table 2) including anti-microbial, anti-inflammatory, cytotoxic, anti-cancer, and contraceptive roles in both humans and animals. Monoterpenes such as myrcene, pinene, camphene, limonene, and sabinene isolated from cotton possess anti- microbial, anti-inflammatory, anti-cancer, anti-oxidant, and gastro-protective properties [28,171,172].
Table 2.
Biological activities of different compounds present in cotton.
| Compounds | Biological Activity | References |
|---|---|---|
| Terpenes | ||
| camphene | Aromatic properties, antioxidants effects | [21] |
| limonene | Flavouring properties. gastro-protective effects, anti-cancer and anti-inflammatory activity | [28,142] |
| myrcene | Analgesic effects, anti-microbial activity, anti-inflammatory activity, anti-catabolic activity | [171,172] |
| α and β-pinene | Gastro-protective effects, anti- microbial and ant-inflammatory effects | [28,174,180] |
| sabinene | Anti-microbial activity, anti-oxidant activity | [21] |
| α-thujene | Pungent activity | [21] |
| caryophyllene | Ant-inflammatory effects, anti-microbial activity, regulation of cellular lipid metabolism, flavouring properties | [27,32,181,182] |
| farnesene | Anti-oxidant effects | [179] |
| humulene | Anti-inflammatory properties, aromatic properties and cytotoxic activity | [29,32] |
| bisabolol | Aromatic properties, anti-inflammatory effects, anti-carcinogenic activity, anti-microbial and anti-oxidative properties | [28,183,184] |
| caryophyllene oxide | Cytotoxic activity, phytogrowth inhibition, analgesic and anti-inflammatory activity | [177] [26,185] |
| 3,10-dihydroxy-1,3,5,7-cadinatetraen-9-one | Phytoalexin, antifungal agent | [92,186] |
| β-sitosterol | Antimicrobial activity, anti-hypercholesteraemic and anti-inflammatory activity | [18,28] |
| strigol | Germination stimulant | [96,187,188] |
| 2,3,9-trihydroxy-1,3,5,7,9-cadinapentaen-14-al; 3-Me ether | Phytoalexin | [99] |
| 2,8,9-trihydroxy-1,3,5,7,9-cadinapentaen-14-al; 8-deoxy | Antifungal activity | [92] |
| Phenols | ||
| chlorogenic acid | Anti-oxidant and anti-mutagenic activity | [144] |
| gallic acid | Antioxidant activity, cytotoxic activity | [189,190] |
| 4-hydroxybenzoic acid | Anti-microbial activity, used as preservative, oestrogenic activity, anti-inflammatory and anti-oxidant activity | [175,176,191] |
| gossypol; (+)-form | Contraceptive and hypokalemic activity | [30,192,193] |
| 3,3′,4′,5,7-pentahydroxyflavan; (2S,3R)-form | Cytotoxic and phytotoxic activity | [194,195] |
| 3,3′,4′,5,7-pentahydroxyflavone; 3′-O-β-d-glucopyranoside | Enzyme inhibitor, cytotoxic, anti-oxidant activity | [196] |
| scopoletin | Anti-spasmodic and anti-inflammatory activity | [19] |
| Fatty acids | ||
| 11,14-eicosadienoic acid | Hormonal activity | [18] |
| hexadecanoic acid | Anti-microbial and anti-inflammatory activity | [197] |
| octadecanoic acid | Pharmaceutical excipient, surfactant and softening activity | [198] |
| 9-octadecenoic acid; (Z)-form | Insecticidal, anti-bacterial and fungicidal activity | [199,200,201] |
| tetradecanoic acid | Defoaming agent, flavour adjuvant used in food processing | [202] |
| Carbohydrates | ||
| cellulose | Capsule and tablet diluent | [203] |
| Proteins | ||
| 3-phosphoglycerate phosphatase | Enzyme activity | [119] |
| vicilin | Anti-hypertensive activity | [204] |
5.1. Anti-Microbial Properties
In vitro and in vivo studies with compounds derived from cotton have found they elicit various effects in most experimental cells and animals. Monoterpenes such as pinene present in the leaves of cotton possess anti-microbial activity against fungi and bacteria. Concentrations as low as 5 µg/mL and 117 µg/mL were reported to have anti-microbial activity towards bacteria and fungi, respectively [173,174]. Only positive enantiomers of the compound induced this effect. The phenolic acid 4-hydroxybenzoic acid which has anti-microbial properties against gram positive and gram-negative bacteria at IC50 value of 160 µg/mL [175] is another compound present in the leaves of cotton. The degree of anti-microbial activity of these compounds varies across micro-organisms. This was observed in fungal toxicity assays with 4-hydroxybenzoic acid on Ganoderma boninense at concentrations as low as 0.5–2.5 µg/mL [176].
5.2. Anti-Inflammatory and Anti-Oxidant Properties
Chemical compounds, such as trans-caryophyllene, caryophyllene oxide, α-humulene, and β-amyrin, are compounds which exert different anti-inflammatory properties. α-Humulene and trans-caryophyllene are reported to prevent chemical-induced paw oedema in rats with 50 mg/kg of both compounds inducing the same anti-inflammatory effects as 0.5 mg/kg of dexamethasone (a steroid anti-inflammatory medication) [32]. At doses of 12 mg/kg and 25 mg/kg body weight of experimental mice, caryophyllene oxide induced anti-inflammatory and analgesic properties almost equivalent to that of an aspirin at a dose of 100 mg/kg body weight of the experimental animals [177]. In humans, studies using peripheral blood mononuclear cells (PMBCs), 1, 2, and 5 µg/mL of β-amyrin promoted the secretion of IL-6 cytokine [178] which is actively involved in pro-inflammatory and anti-inflammatory immune responses. Anti-oxidant properties of β-amyrin and farnesene from “in vitro” studies using human blood cells showed that doses as low as 1 µg/mL [178] and 100 µg/mL [179], respectively, induced anti-oxidant activities in a time-dependent manner.
5.3. Cytotoxic and Contraceptive Properties
Cytotoxic activities associated with compounds isolated from cotton are mostly reported in relation to cancer cell lines. α-Bisabolol, a common compound present in cotton possesses the ability to induce apoptosis in malignant carcinoma cell lines without affecting the viability of healthy cells [184]. A dose of 2 µM of α-bisabolol is reported to be effective against cancer cell lines, but an increase in dosage from 50 to 250 µM can induce cytotoxicity in normal cells. Another sesquiterpene, caryophyllene oxide, also exhibits cytotoxic properties against cancer cell lines with a minimum dose of 3.125 µM resulting in reduction in viabilities of the target cells, with this effect more pronounced as the dosage increased [185]. Gossypol is a major compound present in cottonseed oil and other parts of the cotton plant and has been found to have contraceptive properties in mammals. In human males, a concentration of 0.3 mg/kg of body weight can induce azoospermia in a time-dependent manner, whereas in male rats, a concentration of 30 mg/kg will induce the equivalent effect [192]. The contraceptive property of gossypol is not restricted to males alone as a study by Randel, Chase, and Wyse [193] indicated that this compound, if administered at a dose of 40 mg/kg body weight of female mammals, induces abnormal oestrous cycles and reduced pregnancy rates.
6. Conclusions
In this review, it has been shown that the whole cotton plant is a reservoir of a wide variety of compounds which have a range of biological functions and exploitable applications. The distribution of compounds in the cotton plant provides knowledge of the chemical content of cotton waste derived from harvesting and cotton ginning operations. Potentially valuable chemical compounds with application in food manufacturing, perfumery, and pharmaceutical industries are found in components of these cotton processing by-products (burr, leaves, crushed seeds, sticks, roots, and flowers of the cotton plant). Gossypol, which is known to have contraceptive properties is not only concentrated in the seeds of cotton, but occurs in the roots and possibly in other parts of the plant. Phenolic compounds and terpenes present in the cotton burr stem, leaves, flowers, stalks, and roots have insecticidal, herbicidal, and phytotoxic properties that could be exploited. This review has highlighted that cotton waste products can be sources of biologically valuable compounds. Special consideration should be given to CGT as a low cost resource because it is centrally stockpiled and collocated with existing infrastructure. Therefore, investigating the occurrence of these chemical compounds in cotton by-products can contribute to recycling and value adding of waste generated from cotton ginning.
Acknowledgments
The authors wish to thank Cotton Research Development Corporation (CRDC) Australia for funding the study and Southern Cross Plant Science, Southern Cross University and New South Wales DPI Agriculture for providing the academic environment to prepare this review.
Conflicts of Interest
The authors declare that there are no conflicts of interest. Furthermore, the funding sponsors had a role in the decision to publish this review.
References
- 1.Cotton Australia The Cotton Plant. [(accessed on 11 May 2015)]. Available online: http://cottonaustralia.com.au/cotton-library/fact-sheets/cotton-fact-file-the-cotton-plant.
- 2.Hegde R.R., Dahiya A., Kamath M.G., Gao X., Jangala P.K. Cotton Fibres. Tickle college of Engineering, University of Tennessee; Knoxville, TN, USA: 2004. [Google Scholar]
- 3.Raju S.A.J., Jonathan H.K., Rao P.S. Traditional extraction of bark tannin from the mangrove tree Ceriops decantra (Griff.) Ding Hou and its use in treating cotton fishing nets. Nat. Prod. Radiance. 2008;7:173–175. [Google Scholar]
- 4.Cotton Australia Uses of Cotton. [(accessed on 11 May 2015)]. Available online: http://cottonaustralia.com.au/australian-cotton/basics/uses-of-cotton.
- 5.Blackwood I. White Cottonseed—A Supplementary Feed for Beef Cattle. NSW DPI; Orange, New South Wales, Australia: 2007. [Google Scholar]
- 6.Rogers G.M., Poore M.H., Paschal J.C. Feeding cotton products to cattle. Verterinary Clin. N. Am. Food Anim. Pract. 2002;18:267–294. doi: 10.1016/S0749-0720(02)00020-8. [DOI] [PubMed] [Google Scholar]
- 7.Ezuruike U.F., Prieto J.M. The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. J. Ethnopharmacol. 2014;155:857–924. doi: 10.1016/j.jep.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 8.Statistica World’s 10 Leading Cotton Producing Countries in 2013/2104 (in Metric Tons) [(accessed on 22 April 2015)]. Available online: http://www.statista.com/statistics/263055/cotton-production-worldwide-by-top-countries/
- 9.Buser M. Extruding Cotton Gin byproducts to reduce chemical residues. J. Cotton Sci. 2001;5:92–102. [Google Scholar]
- 10.Knox O., Rochester I., Vadakattu G., Lawrence L. Composting in Australian cotton production. Aust. Cotton Grow. 2006:46–48. [Google Scholar]
- 11.Stanley A.W. Production and ginning of cotton. In: Lee I.A., Neefus J.D., Stellman J.M., editors. Encyclopedia of Occupational Health and Safety. 89th ed. International Labor Organization; Geneva, Switzerland: 2011. [Google Scholar]
- 12.Kennedy J.B., Rankins D.L., Jr. Comparison of cotton gin trash and peanut hulls as low-cost roughage sources for growing beef cattle. Prof. Anim. Sci. 2008;24:40–46. doi: 10.15232/S1080-7446(15)30808-1. [DOI] [Google Scholar]
- 13.Wilde C., Johnson J., Farmer M. Inventory of cotton gin trash on the Texas high plains and bio-energy feedstock potentials. Tex. J. Agric. Natl. Resour. 2010;23:42–49. [Google Scholar]
- 14.Packham R., Royal A., Payne C. Cottonseed meal in broiler diets. 1. The use of cottonseed meal as a replacement for soya bean meal in broiler starter diets. Aust. J. Exp. Agric. 1973;13:649–655. doi: 10.1071/EA9730649. [DOI] [Google Scholar]
- 15.Jeoh T. Master’s Thesis. Virginia Polytechnic Institute and State University; Blacksburg, VA, USA: 1998. Steam Explosion Pretreatment of Cotton Gin Waste for Fuel Ethanol Production. [Google Scholar]
- 16.McIntosh S., Vancov T., Palmer J., Morris S. Ethanol production from cotton gin trash using optimised dilute acid pretreatment and whole slurry fermentation processes. Bioresour. Technol. 2014;173:42–51. doi: 10.1016/j.biortech.2014.09.063. [DOI] [PubMed] [Google Scholar]
- 17.Sharma-Shivappa R., Chen Y. Conversion of cotton wastes to bioenergy and value-added products. Am. Soc. Agric. Biol. Eng. 2008;51:2239–2246. doi: 10.13031/2013.25377. [DOI] [Google Scholar]
- 18.Shakhidoyatov K.M., Rashkes A.M., Khidyrova N.K. Components of cottonplant leaves, their functional role and biological activity. Chem. Nat. Compd. 1997;33:605–616. doi: 10.1007/BF02249623. [DOI] [Google Scholar]
- 19.Bell A.A. Physiology of secondary products. In: Mauney J.R., Stewart J.M., editors. Cotton Physiology. The Cotton Foundation; Memphis, TN, USA: 1986. pp. 597–621. [Google Scholar]
- 20.Hu G., Houston N.L., Pathak D., Schmidt L., Thelen J.J., Wendel J.F. Genomically biased accumulation of seed storage proteins in allopolyploid cotton. Genetics. 2011;189:1103–1115. doi: 10.1534/genetics.111.132407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Essien E.E., Aboaba S.O., Ogunwande I.A. Constituents and antimicrobial properties of the leaf essential oil of Gossypium barbadense (Linn.) J. Med. Plant Res. 2011;5:702–705. [Google Scholar]
- 22.Perveen S.S., Qaisrani T.M., Bhutta S., Perveen R., Naqvi S.H.M. HPLC analysis of cotton phenols and their contribution in bollworm resistance. J. Biol. Sci. 2001;1:587–590. [Google Scholar]
- 23.Perveen S.S., Qaisrani T.M., Siddiqui F., Perveen R., Naqvi S.H.M. Cotton plant volatiles and insect’s behavior. Pak. J. Biol. Sci. 2001;4:554–558. [Google Scholar]
- 24.Iskhakov N.I., Sadykov A.S., Ismailov A.I. The fatty acid composition of the phospholipids of cottonseed oil. Chem. Nat. Compd. 1965;1:152–154. doi: 10.1007/BF00568352. [DOI] [Google Scholar]
- 25.Haleem N., Arshad M., Shahid M., Tahir M.A. Synthesis of carboxymethyl cellulose from waste of cotton ginning industry. Carbohydr. Polym. 2014;113:249–255. doi: 10.1016/j.carbpol.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez-Muñoz B.A., Aguilar M.I., King-Díaz B., Rivero J.F., Lotina-Hennsen B. The sesquiterpenes β-caryophyllene and caryophyllene oxide isolated from Senecio salignus act as phytogrowth and photosynthesis inhibitors. Molecules. 2012;17:1437. doi: 10.3390/molecules17021437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S., Yang Z., Gao Y., Wang Y. A role for trans-caryophyllene in the moderation of insulin secretion. Biochem. Biophys. Res. Commun. 2014;444:451–454. doi: 10.1016/j.bbrc.2013.11.136. [DOI] [PubMed] [Google Scholar]
- 28.Ku C.-M., Lin J.-Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013;141:1104–1113. doi: 10.1016/j.foodchem.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Rogerio A.P., Andrade E.L., Leite D.F.P., Figueiredo C.P., Calixto J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene α-humulene in experimental airways allergic inflammation. Br. J. Pharmacol. 2009;158:1074–1087. doi: 10.1111/j.1476-5381.2009.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han X., Shen T., Lou H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007;8:950. doi: 10.3390/i8090950. [DOI] [Google Scholar]
- 31.Amiel E., Ofir R., Dudai N., Soloway E., Rabinsky T., Rachmilevitch S. Caryophyllene, a compound isolated from the biblical balm of gilead (Commiphora gileadensis), is a selective apoptosis inducer for tumor cell lines. Evid. Based Complement. Altern. Med. 2012;2012:872394. doi: 10.1155/2012/872394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandes E.S., Passos G.F., Medeiros R., da Cunha F.M., Ferreira J., Campos M.M., Pianowski L.F., Calixto J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007;569:228–236. doi: 10.1016/j.ejphar.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 33.United Nations Environment Programme (UNEP) Compendium of Technologies. United Nations Environment Programme; Osaka/Shiga, Japan: 2009. Converting waste agricultural biomass into a resource. [Google Scholar]
- 34.Hossain A.B.M.S., Al-saif A.M. Biodiesel fuel production from soybean oil waste as agricultural bio-resource. Aust. J. Crop Sci. 2010;4:538–542. [Google Scholar]
- 35.Brentin R.P. ACS Symposiums. Amercian Chemical Soceity; Washington, DC, USA: 2014. Soy-Based Chemicals and Materials: Growing the Value Chain. [Google Scholar]
- 36.Franco H.C.J., Pimenta M.T.B., Carvalho J.L.N., Magalhães P.S.G., Rossell C.E.V., Braunbeck O.A., Vitti A.C., Kölln O.T., Rossi Neto J. Assessment of sugarcane trash for agronomic and energy purposes in Brazil. Sci. Agricola. 2013;70:305–312. doi: 10.1590/S0103-90162013000500004. [DOI] [Google Scholar]
- 37.Pode R., Diouf B., Pode G. Sustainable rural electrification using rice husk biomass energy: A case study of Cambodia. Renew. Sust. Energy Rev. 2015;44:530–542. doi: 10.1016/j.rser.2015.01.018. [DOI] [Google Scholar]
- 38.Kuchelmeister C., Bauer S. Rapid small-scale determination of extractives in biomass. Bioenergy Res. 2014;8:68–76. doi: 10.1007/s12155-014-9493-x. [DOI] [Google Scholar]
- 39.Gotmare V., Singh P., Tule B. Technical Bulletin. Volume 5 Central Institute for Cotton Research; Nagpur, India: 2000. Wild and cultivated species of Cotton. [Google Scholar]
- 40.The Columbia Electronic Encyclopedia. Columbia University Press; New York, NY, USA: 2013. Columbia Encyclopedia, cotton plant. [Google Scholar]
- 41.Liu C., Yuan D., Zhang X., Lin Z. Isolation, characterization and mapping of genes differentially expressed during fibre development between Gossypium hirsutum and G. barbadense by cDNA-SRAP. J. Genet. 2013;92:175–181. doi: 10.1007/s12041-013-0238-y. [DOI] [PubMed] [Google Scholar]
- 42.Khaleequr R., Arshiya S., Shafeequr R. Gossypium herbaceum Linn: An ethnological review. J. Pharm. Sci. Innov. 2012;1:1–5. [Google Scholar]
- 43.Ayeni M.J., Oyeyemi S.D., Kayode J., Peter G.P. Phytochemical, proximate and mineral analyses of the leaves of Gossypium hirsutum L. and Momordica charantia L. J. Nat. Sci. Res. 2015;5:99–107. [Google Scholar]
- 44.Kazeem M.I., Abimbola S.G., Ashafa A.O.T. Inhibitory potential of Gossypium arboreum leaf extracts on diabetes key enzymes, α-amylase and α-glucosidase. Bangladesh J. Pharmacol. 2013;8 doi: 10.3329/bjp.v8i2.14152. [DOI] [Google Scholar]
- 45.Avci U., Pattathil S., Singh B., Brown V.L., Hahn M.G., Haigler C.H. Cotton fiber cell walls of Gossypium hirsutum and Gossypium barbadense have differences related to loosely-bound xyloglucan. PLoS ONE. 2013;8:e56315. doi: 10.1371/journal.pone.0056315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawan Z.M., Hanna L.I., Gad El Karim G.A., McCuistion W.L. Relationships between climatic factors and flower and boll production in Egyptian cotton (Gossypium barbadense) J. Arid Environ. 2002;52:499–516. doi: 10.1006/jare.2002.1012. [DOI] [Google Scholar]
- 47.Gipson J.R. Temperature effects on growth, development and fiber properties. In: Mauney J.R., Stewart J.M., editors. Cotton Physiology. Cotton Foundation; Memphis, TN, USA: 1986. pp. 47–56. [Google Scholar]
- 48.Wu Z., Soliman K.M., Zipf A., Saha S., Sharma G.C., Jenkins J.N. Isolation and Characterization of Genes Differentially Expressed in Fiber of Gossypium barbadense L. J. Cotton Sci. 2005;9:166–174. [Google Scholar]
- 49.Wendel J.F., Brubaker C.L., Seelanan T. The origin and evolution of Gossypium. In: Mauney J.R., Stewart J.M., editors. Cotton Physiology. Cotton Foundation; Memphis, TN, USA: 1986. pp. 1–18. [Google Scholar]
- 50.Nandeshwar S.B., Moghe S., Chakrabarty P.K., Deshattiwar M.K., Kranthi K., Anandkumar P., Mayee C.D., Khadi B.M. Agrobacterium-mediated transformation of Cry1Ac gene into shoot-tip meristem of diploid cotton Gossypium arboreum cv. RG8 and regeneration of transgenic plants. Plant Mol. Biol. Rep. 2009;27:549–557. doi: 10.1007/s11105-009-0102-7. [DOI] [Google Scholar]
- 51.Momtaz O.A., Diab A.A., Madkour M.A. Development of Transgenic Egyptian Cotton (Gossypium barbadense) Varieties from Meristematic Tissue; Proceedings of the Beltwide Cotton Conferences; San Antonio, TX, USA. 4–8 January 2000. [Google Scholar]
- 52.Gomez S.K., Oosterhuis D.M., Hendrix D.L., Johnson D.R., Steinkraus D.C. Diurnal pattern of aphid feeding and its effect on cotton leaf physiology. Environ. Exp. Bot. 2006;55:77–86. doi: 10.1016/j.envexpbot.2004.10.001. [DOI] [Google Scholar]
- 53.Wilson L. Cotton Aphids (Exotic Species) [(accessed on 27 April 2015)]. Available online: http://www.planthealthaustralia.com.au/wp-content/uploads/2013/01/Cotton-aphid-FS.pdf.
- 54.Cook D.R. Ph.D. Thesis. Louisiana State University; Baton Rouge, LA, USA: 2003. Thrips Species Composition in Louisiana Cotton and Associated Management Strategies. [Google Scholar]
- 55.Cook D.R., Leonard R.B., Burris E., Gore J. Impact of thrips infesting cotton seedlings on cotton yield distribution and maturity. J. Cotton Sci. 2013;17:23–33. [Google Scholar]
- 56.Stuart R.R., Gao Y.-L., Lei Z.-R. Thrips: Pests of Concern to China and the United States. Agric. Sci. China. 2011;10:867–892. doi: 10.1016/S1671-2927(11)60073-4. [DOI] [Google Scholar]
- 57.Badii K.B., Asante S.K. Efficacy of some synthetic insecticides for control of cotton bollworms in northern Ghana. Afr. Crop Sci. J. 2012;20:59–66. [Google Scholar]
- 58.Kranthi S., Dhawad C.S., Naidu S., Bharose A., Chaudhary A., Sangode V., Nehare S.K., Bajaj S.R., Kranthi K.R. Susceptibility of the cotton bollworm, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) to the Bacillus thuringiensis toxin Cry2Ab before and after the introduction of Bollgard-II. Crop Protect. 2009;28:371–375. doi: 10.1016/j.cropro.2008.12.001. [DOI] [Google Scholar]
- 59.Zhang B. Transgenic Cotton: Methods and Protocols. Humana Press; New York, NY, USA: 2013. p. 277. [Google Scholar]
- 60.Krishna V.V., Qaim M. Bt cotton and sustainability of pesticide reductions in India. Agric. Syst. 2012;107:47–55. doi: 10.1016/j.agsy.2011.11.005. [DOI] [Google Scholar]
- 61.Perlak F.J., Oppenhuizen M., Gustafson K., Voth R., Sivasupramaniam S., Heering D., Carey B., Ihrig R.A., Roberts J.K. Development and commercial use of Bollgard® cotton in the USA—Early promises versus today’s reality. Plant J. 2001;27:489–501. doi: 10.1046/j.1365-313X.2001.01120.x. [DOI] [PubMed] [Google Scholar]
- 62.Kouser S., Qaim M. Impact of Bt cotton on pesticide poisoning in smallholder agriculture: A panel data analysis. Ecol. Econ. 2011;70:2105–2113. doi: 10.1016/j.ecolecon.2011.06.008. [DOI] [Google Scholar]
- 63.Monsanto Cotton Research and Development. [(accessed on 27 April 2015)]. Available online: http://www.monsanto.com/global/au/products/pages/cotton-research-and-development.aspx.
- 64.Ceeney S., Kauter G., Leven T. World’s-Best Science: The basis of the Bollgard III RMP. [(accessed on 28 April 2015)]. Available online: http://www.cottongrower.com.au/images/articles/073f23c5c9b963000f807344a6115923.pdf.
- 65.Maqbool A., Abbas W., Rao A.Q., Irfan M., Zahur M., Bakhsh A., Riazuddin S., Husnain T. Gossypium arboreum GHSP26 enhances drought tolerance in Gossypium hirsutum. Biotechnol. Prog. 2010;26:21–25. doi: 10.1002/btpr.306. [DOI] [PubMed] [Google Scholar]
- 66.Stewart L., Rossi J. Using Cotton Byproducts in Beef Cattle Diets. University of Georgia; Athens, GA, USA: 2010. [Google Scholar]
- 67.Cotton Australia . Cotton Library. Cotton Australia; Sydney, NSW, Australia: 2015. Processing, Exporting and Marketing. [Google Scholar]
- 68.Crossan A.N., Kennedy I.R. Calculation of pesticide degradation in decaying cotton gin trash. Bull. Environ. Contam. Toxicol. 2008;81:355–359. doi: 10.1007/s00128-008-9414-9. [DOI] [PubMed] [Google Scholar]
- 69.Díaz M.J., Madejón E., López F., López R., Cabrera F. Composting of vinasse and cotton gin waste by using two different systems. Resour. Conserv. Recycl. 2002;34:235–248. doi: 10.1016/S0921-3449(01)00109-4. [DOI] [Google Scholar]
- 70.Tejada M., Gonzalez J.L. Crushed cotton gin compost on soil biological properties and rice yield. Eur. J. Agron. 2006;25:22–29. doi: 10.1016/j.eja.2006.01.007. [DOI] [Google Scholar]
- 71.Papafotiou M., Vagena A. Cotton gin trash compost in the substrate reduces the daminozide spray dose needed to produce compact potted chrysanthemum. Sci. Hort. 2012;143:102–108. doi: 10.1016/j.scienta.2012.06.004. [DOI] [Google Scholar]
- 72.Duan R., Fedler C.B., Pearson P.R. Environmental effects of using cotton burr compost mulch to establish roadside vegetation. Ecol. Eng. 2012;39:90–94. doi: 10.1016/j.ecoleng.2011.11.015. [DOI] [Google Scholar]
- 73.Sutivisedsak N., Cheng H.N., Dowd M.K., Selling G.W., Biswas A. Evaluation of cotton by-products as fillers for poly(lactic acid) and low density polyethylene. Ind. Crops Prod. 2012;36:127–134. doi: 10.1016/j.indcrop.2011.08.016. [DOI] [Google Scholar]
- 74.Plácido J., Capareda S. Production of silicon compounds and fulvic acids from cotton wastes biochar using chemical depolymerization. Ind. Crops Prod. 2015;67:270–280. doi: 10.1016/j.indcrop.2015.01.027. [DOI] [Google Scholar]
- 75.Agblevor F.A., Batz S., Trumbo J. Composition and ethanol production potential of cotton gin residues. Appl. Biochem. Biotechnol. 2003;105:219–230. doi: 10.1385/ABAB:105:1-3:219. [DOI] [PubMed] [Google Scholar]
- 76.Placido J., Imam T., Capareda S. Evaluation of ligninolytic enzymes, ultrasonication and liquid hot water as pretreatments for bioethanol production from cotton gin trash. Bioresour. Technol. 2013;139:203–208. doi: 10.1016/j.biortech.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 77.Yoav B. Phenols in cotton seedlings resistant and susceptible to Alternaria macrospora. J. Phytopathol. 1986;116:1–10. doi: 10.1111/j.1439-0434.1986.tb00888.x. [DOI] [Google Scholar]
- 78.Thompson A.C., Baker D.N., Gueldner R.C., Hedin P.A. Identification and quantitative analysis of the volatile substances emitted by maturing cotton in the field. Plant Physiol. 1971;48:50–52. doi: 10.1104/pp.48.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davis G.D., Essenberg M. (+)-δ-Cadinene is a product of sesquiterpene cyclase activity in cotton. Phytochemistry. 1995;39:553–567. doi: 10.1016/0031-9422(95)00067-H. [DOI] [Google Scholar]
- 80.Alchanati I., Patel J.A.A., Liu J., Benedict C.R., Stipanovic R.D., Bell A.A., Cui Y., Magill C.W. The enzymatic cyclization of nerolidyl diphosphate by δ-cadinene synthase from cotton stele tissue infected with Verticillium dahliae. Phytochemistry. 1998;47:961–967. doi: 10.1016/S0031-9422(98)80054-X. [DOI] [Google Scholar]
- 81.Minyard J.P., Tumlinson J.H., Thompson A.C., Hedin P.A. Constituents of the Cotton Bud. Sesquiterpene Hydrocarbons. J. Agric. Food Chem. 1966;14:332–336. doi: 10.1021/jf60146a001. [DOI] [Google Scholar]
- 82.Elzen G.W., Williams H.J., Vinson S.B. Isolation and identification of cotton synomones mediating searching behavior by parasitoidCampoletis sonorensis. J. Chem. Ecol. 1984;10:1251–1264. doi: 10.1007/BF00988552. [DOI] [PubMed] [Google Scholar]
- 83.Daniel M. Medicinal Plants: Chemistry and Properties. Science Publishers; Enfield, NH, USA: 2006. [Google Scholar]
- 84.Minyard J.P., Thompson A.C., Hedin P.A. Constituents of the cotton bud. VIII. .beta.-Bisabolol, a new sesquiterpene alcohol. J. Org. Chem. 1968;33:909–911. doi: 10.1021/jo01266a116. [DOI] [Google Scholar]
- 85.Hedin P.A., Thompson A.C., Gueldner R.C., Ruth J.M. Isolation of bisabolene oxide from the cotton bud. Phytochemistry. 1972;11:2118–2119. doi: 10.1016/S0031-9422(00)90190-0. [DOI] [Google Scholar]
- 86.McCormick J.P., Shinmyozu T., Pachlatko J.P., Schafer T.R., Gardner J.W., Stipanovic R.D. Gossypium cadinanes and their analogs: Synthesis of lacinilene C, 2,7-dihydroxycadalene, and their methyl ethers. J. Org. Chem. 1984;49:34–40. doi: 10.1021/jo00175a007. [DOI] [Google Scholar]
- 87.Stipanovic R.D., Puckhaber L.S., Reibenspies J.H., Williams H.J. The absolute configuration of (−)-3-hydroxy-α-calacorene. Phytochemistry. 2006;67:1304–1308. doi: 10.1016/j.phytochem.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 88.Davila-Huerta G., Hamada H., Davis G.D., Stipanovic R.D., Adams C.M., Essenberg M. Cadinane-type sesquiterpenes induced in Gossypium cotyledons by bacterial inoculation. Phytochemistry. 1995;39:531–536. doi: 10.1016/0031-9422(94)00958-V. [DOI] [Google Scholar]
- 89.Zhang H.L., Nagatsu A., Okuyama H., Mizukami H., Sakakibara J. Sesquiterpene glycosides from cotton oil cake. Phytochemistry. 1998;48:665–668. doi: 10.1016/S0031-9422(98)00075-2. [DOI] [Google Scholar]
- 90.Williams H.J., Moyna G., Vinson S.B., Scott A.I., Bell A.A., Stipanovic R.D. β-Caryophyllene derivatives from the wild cottons. Nat. Prod. Lett. 1997;11:25–30. doi: 10.1080/10575639708043753. [DOI] [Google Scholar]
- 91.Stipanovic R.D., Greenblatt G.A., Beier R.C., Bell A.A. 2-Hydroxy-7-methoxycadalene. The precursor of lacinilene C 7-methyl ether in Gossypium. Phytochemistry. 1981;20:729–730. doi: 10.1016/0031-9422(81)85162-X. [DOI] [Google Scholar]
- 92.Abraham K.J., Pierce M.L., Essenberg M. The phytoalexins desoxyhemigossypol and hemigossypol are elicited by Xanthomonas in Gossypium cotyledons. Phytochemistry. 1999;52:829–836. doi: 10.1016/S0031-9422(99)00331-3. [DOI] [Google Scholar]
- 93.Piccinelli A.L., Lotti C., Severino L., Luongo D., Rastrelli L. Unusual cytotoxic sulfated cadinene-type sesquiterpene glycosides from cottonseed (Gossypium hirsutum) Tetrahedron. 2008;64:5449–5453. doi: 10.1016/j.tet.2008.04.013. [DOI] [Google Scholar]
- 94.Stipanovic R.D., Bell A.A., O’Brien D.H., Lukefahr M.J. Heliocide H1. A new insecticidal C25 terpenoid from cotton (Gossypium hirsutum) J. Agric. Food Chem. 1978;26:115–118. doi: 10.1021/jf60215a042. [DOI] [Google Scholar]
- 95.Bell A.A., Stipanovic R.D., O’Brien D.H., Fryxell P.A. Sesquiterpenoid aldehyde quinones and derivatives in pigment glands of Gossypium. Phytochemistry. 1978;17:1297–1305. doi: 10.1016/S0031-9422(00)94578-3. [DOI] [Google Scholar]
- 96.Cook C.E., Whichard L.P., Wall M., Egley G.H., Coggon P., Luhan P.A., McPhail A.T. Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea) J. Am. Chem. Soc. 1972;94:6198–6199. doi: 10.1021/ja00772a048. [DOI] [Google Scholar]
- 97.Sato D., Awad A.A., Takeuchi Y., Yoneyama K. Confirmation and quantification of strigolactones, germination stimulants for root parasitic plants striga and orobanche, produced by cotton. Biosci. Biotechnol. Biochem. 2005;69:98–102. doi: 10.1271/bbb.69.98. [DOI] [PubMed] [Google Scholar]
- 98.Stipanovic R.D., Bell A.A., O’Brien D.H. Raimondal, a new sesquiterpenoid from pigment glands of Gossypium raimondii. Phytochemistry. 1980;19:1735–1738. doi: 10.1016/S0031-9422(00)83804-2. [DOI] [Google Scholar]
- 99.Karimdzhanov A.K., Ismailov A.I., Abdullaev Z.S., Islambekov S.Y., Kamaev F.G., Sadykov A.S. Structure of gossyvertin—A new phytoalexin of the cotton plant. Chem. Nat. Compd. 1976;12:211–214. doi: 10.1007/BF00566347. [DOI] [Google Scholar]
- 100.Bell A.A., Stipanovic R.D., Howell C.R., Fryxell P.A. Antimicrobial terpenoids of Gossypium: Hemigossypol, 6-methoxyhemigossypol and 6-deoxyhemigossypol. Phytochemistry. 1975;14:225–231. doi: 10.1016/0031-9422(75)85044-8. [DOI] [Google Scholar]
- 101.Stipanovic R.D., Kim H.L., Altman D.W., Bell A.A., Kohel R.J. Raimondalone, a sesquiterpene from a cotton interspecific hybrid. Phytochemistry. 1994;36:953–956. doi: 10.1016/S0031-9422(00)90470-9. [DOI] [Google Scholar]
- 102.Stipanovic R.D., Bell A.A., Mace M.E., Howell C.R. Antimicrobial terpenoids of Gossypium: 6-methoxygossypol and 6,6′-dimethoxygossypol. Phytochemistry. 1975;14:1077–1081. doi: 10.1016/0031-9422(75)85190-9. [DOI] [Google Scholar]
- 103.Nazarova I.P., Glushenkova A.I., Ul’chenko N.T., Moiseeva G.P. Influence of wilt infection on the gossypol pigments of seeds and roots of a cotton plant of the variety Tashkent-1. Chem. Nat. Compd. 1989;25:54–57. doi: 10.1007/BF00596700. [DOI] [Google Scholar]
- 104.Pakudina Z.P., Rakhimov A.A., Sadykov A.S. A chalcone from cotton plant flowers. Chem. Nat. Compd. 1969;5:109–110. doi: 10.1007/BF00633299. [DOI] [Google Scholar]
- 105.Hedin P.A., Jenkins J.N., Parrott W.L. Evaluation of flavonoids in Gossypium arboreum (L.) cottons as potential source of resistance to tobacco budworm. J. Chem. Ecol. 1992;18:105–114. doi: 10.1007/BF00993746. [DOI] [PubMed] [Google Scholar]
- 106.Bell A.A., Howell C.R., Stipanovic R.D. Cotton Host-microbe interactions. In: Stewart J.M., Oosterhuis D., Heitholt J.J., Mauney J.R., editors. Physiology of Cotton. Springer; Berlin/Heidelberg, Germany: 2009. pp. 187–205. [Google Scholar]
- 107.Rangaswami S., Rao P.S., Seshadri T.R. Pigments of cotton flowers. Proc. Indian Acad. Sci. (Math. Sci.) 1939;9:133–135. [Google Scholar]
- 108.Yuan S., Yang M., Zhao Y. A new flavonol glycoside from glandless cotton seeds. Acta Pharm. Sin. B. 2012;2:42–45. doi: 10.1016/j.apsb.2011.11.001. [DOI] [Google Scholar]
- 109.Elliger C.A. Sexangularetin 3-glucoside-7-rhamnoside from Gossypium hirsutum. Phytochemistry. 1984;23:1199–1201. doi: 10.1016/S0031-9422(00)82647-3. [DOI] [Google Scholar]
- 110.Wakelyn P.J., Stipanovic R.D., Bell A.A. Identification of scopoletin in dried bract of the cotton plant. J. Agric. Food Chem. 1974;22:567–568. doi: 10.1021/jf60194a049. [DOI] [PubMed] [Google Scholar]
- 111.Tonn W.H., Schoch E.P. Cotton Wax. Ind. Eng. Chem. 1946;38:413–415. doi: 10.1021/ie50436a021. [DOI] [Google Scholar]
- 112.Rakhimkhanov Z.B., Karimdzhanov A.K., Ismailov A.I., Sadykov A.S. A study of the anthocyanins of the cotton plant. Chem. Nat. Compd. 1968;4:164. doi: 10.1007/BF00565755. [DOI] [Google Scholar]
- 113.Jaquet J.-P., Buchala A., Meier H. Changes in the non-structural carbohydrate content of cotton (Gossypium spp.) fibres at different stages of development. Planta. 1982;156:481–486. doi: 10.1007/BF00393321. [DOI] [PubMed] [Google Scholar]
- 114.Tang F., Wang T., Zhu J. Carbohydrate profiles during cotton (Gossypium hirsutum L.) boll development and their relationships to boll characters. Field Crops Res. 2014;164:98–106. doi: 10.1016/j.fcr.2014.06.002. [DOI] [Google Scholar]
- 115.Ryser U., Meier H., Holloway P.J. Identification and localization of suberin in the cell walls of green cotton fibres (Gossypium hirsutum L. var. green lint) Protoplasma. 1983;117:196–205. doi: 10.1007/BF01281823. [DOI] [Google Scholar]
- 116.Schmutz A., Buchala A.J., Ryser U. Changing the dimensions of suberin lamellae of green cotton fibers with a specific inhibitor of the endoplasmic reticulum-associated fatty acid elongases. Plant Physiol. 1996;110:403–411. doi: 10.1104/pp.110.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ivanova I.A., Turakhozhaev M.T., Shakirov T.T. Isolation of raffinose from cottonseed meal. Chem. Nat. Compd. 1984;20:657–660. doi: 10.1007/BF00580014. [DOI] [Google Scholar]
- 118.Kuo T.M., VanMiddlesworth J.F., Wolf W.J. Content of raffinose oligosaccharides and sucrose in various plant seeds. J. Agric. Food Chem. 1988;36:32–36. doi: 10.1021/jf00079a008. [DOI] [Google Scholar]
- 119.Randall D.D., Tolbert N.E., Gremel D. 3-Phosphoglycerate phosphatase in plants: II. Distribution, physiological considerations, and comparison with P-glycolate phosphatase. Plant Physiol. 1971;48:480–487. doi: 10.1104/pp.48.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sankar S.S., Gilbert R.D., Fornes R.E. Isolation of indole-3 carboxaldehyde from cotton plant parts. Text. Res. J. 1983;53:51–53. doi: 10.1177/004051758305300109. [DOI] [Google Scholar]
- 121.Hong Y.K., Gilbert R.D., Fornes R.E. Quantitative determination of indole-3 carboxyaldehyde in aqueous extracts of cotton bracts by HPLC. Text. Res. J. 1985;55:17–19. doi: 10.1177/004051758505500104. [DOI] [Google Scholar]
- 122.Hedin P.A., Thompson A.C., Gueldner R.C. Constituents of cotton bud essential oil. Phytochemistry. 1975;14:2087–2088. doi: 10.1016/0031-9422(75)83137-2. [DOI] [Google Scholar]
- 123.Hanny B.W., Gueldner R.C. Surface lipids of the glabrous cotton (Gossypium hirsutum) strain, Bayou SM1. J. Agric. Food Chem. 1976;24:401–403. doi: 10.1021/jf60204a029. [DOI] [Google Scholar]
- 124.Opitz S., Kunert G., Gershenzon J. Increased terpenoid accumulation in cotton (Gossypium hirsutum) foliage is a general wound response. J. Chem. Ecol. 2008;34:508–522. doi: 10.1007/s10886-008-9453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Degenhardt J., Köllner T.G., Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry. 2009;70:1621–1637. doi: 10.1016/j.phytochem.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 126.Dornelas M.C., Mazzafera P. A genomic approach to characterization of the Citrus terpene synthase gene family. Genet. Mol. Biol. 2007;30:832–840. doi: 10.1590/S1415-47572007000500011. [DOI] [Google Scholar]
- 127.Dubey V.S., Bhalla R., Luthra R. An overview of the non-mevalonate pathway for terpenoid biosynthesis in plants. J. Biosci. 2003;28:637–646. doi: 10.1007/BF02703339. [DOI] [PubMed] [Google Scholar]
- 128.Dewick P.M. The biosynthesis of C5–C25 terpenoid compounds. Nat. Prod. Rep. 2002;19:181–222. doi: 10.1039/b002685i. [DOI] [PubMed] [Google Scholar]
- 129.McGarvey D.J., Croteau R. Terpenoid metabolism. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pare P.W., Tumlinson J.H. De Novo Biosynthesis of Volatiles Induced by Insect Herbivory in Cotton Plants. Plant Physiol. 1997;114:1161–1167. doi: 10.1104/pp.114.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rose U.S.R., Tumlinson J.H. Systemic induction of volatile release in cotton: How specific is the signal to herbivory? Planta. 2005;222:327–335. doi: 10.1007/s00425-005-1528-2. [DOI] [PubMed] [Google Scholar]
- 132.Loughrin J.H., Manukian A., Heath R.R., Turlings T.C., Tumlinson J.H. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc. Natl. Acad. Sci. USA. 1994;91:11836–11840. doi: 10.1073/pnas.91.25.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang C.-Q., Wu X.-M., Ruan J.-X., Hu W.-L., Mao Y.-B., Chen X.-Y., Wang L.-J. Isolation and characterization of terpene synthases in cotton (Gossypium hirsutum) Phytochemistry. 2013;96:46–56. doi: 10.1016/j.phytochem.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 134.Bhat S.V., Nagasampagi B.A., Sivakumar M. Chemistry of Natural Products. Narosa publishing house; Delhi, India: 2005. [Google Scholar]
- 135.Chizzola R. Regular monoterpenes and sesquiterpenes (essential oils) In: Ramawat K.G., Mérillon J.-M., editors. Natural Products. Springer; Berlin/Heidelberg, Germany: 2013. pp. 2973–3008. [Google Scholar]
- 136.Sallaud C., Rontein D., Onillon S., Jabès F., Duffé P., Giacalone C., Thoraval S., Escoffier C., Herbette G., Leonhardt N., et al. A novel pathway for sesquiterpene biosynthesis from Z,Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. Plant Cell. 2009;21:301–317. doi: 10.1105/tpc.107.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Santos M.R.V., Moreira F.V., Fraga B.P., Souza D.P.D., Bonjardim L.R., Quintans-Junior L.J. Cardiovascular effects of monoterpenes: A review. Rev. Bras. Farmacogn. 2011;21:764–771. doi: 10.1590/S0102-695X2011005000119. [DOI] [Google Scholar]
- 138.Angioni A., Barra A., Coroneo V., Dessi S., Cabras P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006;54:4364–4370. doi: 10.1021/jf0603329. [DOI] [PubMed] [Google Scholar]
- 139.Banthorpe D.V., Charlwood B.V., Francis M.J.O. Biosynthesis of monoterpenes. Chem. Rev. 1972;72:115–155. doi: 10.1021/cr60276a002. [DOI] [PubMed] [Google Scholar]
- 140.Yang C.-Q., Ruan J.-X., Wang L.-J., Chen X.-Y. Isolation and characterization of volatile terpene synthases in cotton; Proceedings of the Plant and Animal Genome XXII; San Diego, CA, USA. 11–15 January 2014. [Google Scholar]
- 141.Opitz S., Nes W.D., Gershenzon J. Both methylerythritol phosphate and mevalonate pathways contribute to biosynthesis of each of the major isoprenoid classes in young cotton seedlings. Phytochemistry. 2014;98:110–119. doi: 10.1016/j.phytochem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 142.Moraes T.M., Kushima H., Moleiro F.C., Santos R.C., Machado Rocha L.R., Marques M.O., Vilegas W., Hiruma-Lima C.A. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: Role of prostaglandins and gastric mucus secretion. Chem. Biol. Interact. 2009;180:499–505. doi: 10.1016/j.cbi.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 143.Cheynier V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012;11:153–177. doi: 10.1007/s11101-012-9242-8. [DOI] [Google Scholar]
- 144.Huang W.-Y., Cai Y.-Z., Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer. 2009;62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 145.Ignat I., Volf I., Popa V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 146.Balasundram N., Sundram K., Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- 147.Kumar S., Pandey A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013;2013:16. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Legé K.E. Tannins in Cotton. In: Bajaj Y.P.S., editor. Cotton. Vol. 42. Springer; Berlin/Heidelberg, Germany: 1998. pp. 350–363. [Google Scholar]
- 149.Berg J.M., Tymoczko J.L., Stryer L. Biochemistry. 5th ed. W.H. Freeman; New York, NY, USA: 2002. [Google Scholar]
- 150.Bressler R., Wakil S.J. Studies on the mechanism of fatty acid synthesis: IX. The conversion of malonyl coenzyme a to long chain fatty acids. J. Biol. Chem. 1961;236:1643–1651. [Google Scholar]
- 151.Dowd M.K., Boykin D.L., Meredith W.R., Jr., Campbell B.T., Bourland F.M., Gannaway J.R., Glass K.M., Zhang J. Fatty acid profiles of cottonseed genotypes from the national cotton variety trials. J. Cotton Sci. 2010;14:64–73. [Google Scholar]
- 152.King E.E., Leffler H.R. Nature and patterns of proteins during cotton seed development. Plant Physiol. 1979;63:260–263. doi: 10.1104/pp.63.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.John M.E., Keller C. Characterization of mRNA for a proline-rich protein of cotton fiber. Plant Physiol. 1995;108:669–676. doi: 10.1104/pp.108.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu W.-L., Zhang D.-J., Wu Y.-F., Qin L.-X., Huang G.-Q., Li J., Li L., Li X.-B. Cotton PRP5 gene encoding a proline-rich protein is involved in fiber development. Plant Mol. Biol. 2013;82:353–365. doi: 10.1007/s11103-013-0066-8. [DOI] [PubMed] [Google Scholar]
- 155.Zhang J., Idowu O.J., Wedegaertner T., Hughs S.E. Genetic variation and comparative analysis of thrips resistance in glandless and glanded cotton under field conditions. Euphytica. 2014;199:373–383. doi: 10.1007/s10681-014-1137-x. [DOI] [Google Scholar]
- 156.McMichael S.C. Hopi cotton, a source of cotton-seed free of gossypol pigments. Agron. J. 1959;51:630. doi: 10.2134/agronj1959.00021962005100100025x. [DOI] [Google Scholar]
- 157.Lusas E.W., Jividen G.M. Glandless cottonseed: A review of the first 25 years of processing and utilization research. J. Am. Oil Chem. Soc. 1987;64:839–854. doi: 10.1007/BF02641491. [DOI] [Google Scholar]
- 158.Romano G.B., Scheffler J.A. Lowering seed gossypol content in glanded cotton (Gossypium hirsutum L.) lines. Plant Breed. 2008;127:619–624. doi: 10.1111/j.1439-0523.2008.01545.x. [DOI] [Google Scholar]
- 159.Lawhon J.T., Cater C.M., Mattil K.F. Evaluation of the food potential of sixteen varieties of cottonseed. J. Am. Oil Chem. Soc. 1977;54:75–80. doi: 10.1007/BF02912394. [DOI] [PubMed] [Google Scholar]
- 160.Nergiz C., Yalçin H., Yildiz H. Some analytical characters of cottonseed varieties grown in Turkey. Grasasy Aceites. 1997;48:411–414. doi: 10.3989/gya.1997.v48.i6.813. [DOI] [Google Scholar]
- 161.Downes S., Mahon R. Evolution, ecology and management of resistance in Helicoverpa spp. to Bt cotton in Australia. J. Invertebr. Pathol. 2012;110:281–286. doi: 10.1016/j.jip.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 162.Héma O., Somé H.N., Traoré O., Greenplate J., Abdennadher M. Efficacy of transgenic cotton plant containing the Cry1Ac and Cry2Ab genes of Bacillus thuringiensis against Helicoverpa armigera and Syllepte derogata in cotton cultivation in Burkina Faso. Crop Protect. 2009;28:205–214. doi: 10.1016/j.cropro.2008.09.014. [DOI] [Google Scholar]
- 163.Yan F., Xu C., Bengtsson M., Witzgall P.P. Volatile compositions of transgenic Bt cotton and their electrophysiological effects on the cotton bollworm. Kun Chong Xue Bao. 2002;45:425–429. [Google Scholar]
- 164.Jaenson T.G.T., Pålsson K., Borg-Karlson A.-K. Evaluation of extracts and oils of mosquito (Diptera: Culicidae) repellent plants from Sweden and Guinea-Bissau. J. Med. Entomol. 2006;43:113–119. doi: 10.1093/jmedent/43.1.113. [DOI] [PubMed] [Google Scholar]
- 165.Nerio L.S., Olivero-Verbel J., Stashenko E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010;101:372–378. doi: 10.1016/j.biortech.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 166.Wanzala W., Hassanali A., Mukabana W.R., Takken W. Repellent activities of essential oils of some plants used traditionally to control the brown ear tick, Rhipicephalus appendiculatus. J. Parasitol. Res. 2014;2014:434506. doi: 10.1155/2014/434506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mohanta R.K., Singhal K.K., Ebrahimi S.H., Rajput Y.S., Mohini M. Comparative nutritional evaluation of transgenic cottonseeds containing Cry1C protein for ruminant feeding. Livest. Res. Rural Dev. 2011;23:14. [Google Scholar]
- 168.Khan V., Najmi A.K., Akhtar M., Aqil M., Mujeeb M., Pillai K.K. A pharmacological appraisal of medicinal plants with antidiabetic potential. J. Pharm. Bioallied Sci. 2012;4:27–42. doi: 10.4103/0975-7406.92727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ekpenyong C.E., Akpan E., Nyoh A. Ethnopharmacology, phytochemistry, and biological activities of Cymbopogon citratus (DC.) Stapf extracts. Chin. J. Nat. Med. 2015;13:321–337. doi: 10.1016/S1875-5364(15)30023-6. [DOI] [PubMed] [Google Scholar]
- 170.Maganha E.G., Halmenschlager R.D.C., Rosa R.M., Henriques J.A.P., Ramos A.L.L.D.P., Saffi J. Pharmacological evidences for the extracts and secondary metabolites from plants of the genus Hibiscus. Food Chem. 2010;118:1–10. doi: 10.1016/j.foodchem.2009.04.005. [DOI] [Google Scholar]
- 171.Olorunnisola S.K., Asiyanbi H.T., Hammed A.M., Simsek S. Biological properties of lemongrass: An overview. Int. Food Res. J. 2014;21:455–462. [Google Scholar]
- 172.Rufino A.T., Ribeiro M., Sousa C., Judas F., Salgueiro L., Cavaleiro C., Mendes A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015;750:141–150. doi: 10.1016/j.ejphar.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 173.Leite A.M., Lima E.D.O., de Souza E.L., Diniz M.D.F.M., Trajano V.N., de Medeiros I.A. Inhibitory effect of β-pinene, α-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Braz. J. Pharm. Sci. 2007;43:121–126. doi: 10.1590/S1516-93322007000100015. [DOI] [Google Scholar]
- 174.Rivas da Silva A.C., Lopes P.M., Barros de Azevedo M.M., Costa D.C., Alviano C.S., Alviano D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules. 2012;17:6305. doi: 10.3390/molecules17066305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cho J.-Y., Moon J.-H., Seong K.-Y., Park K.-H. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci. Biotechnol. Biochem. 1998;62:2273–2276. doi: 10.1271/bbb.62.2273. [DOI] [PubMed] [Google Scholar]
- 176.Manuja R., Sachdeva S., Jain A., Chaudhary J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013;22:109–115. [Google Scholar]
- 177.Chavan M.J., Wakte P.S., Shinde D.B. Analgesic and anti-inflammatory activity of caryophyllene oxide from Annona squamosa L. bark. Phytomedicine. 2010;17:149–151. doi: 10.1016/j.phymed.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 178.Krishnan K., Mathew L., Vijayalakshmi N.R., Helen A. Anti-inflammatory potential of β-amyrin, a triterpenoid isolated from Costus igneus. Inflammopharmacology. 2014;22:373–385. doi: 10.1007/s10787-014-0218-8. [DOI] [PubMed] [Google Scholar]
- 179.Çelİk K., Toğar B., Türkez H., TaŞpinar N. In vitro cytotoxic, genotoxic, and oxidative effects of acyclic sesquiterpene farnesene. Turk. J. Biol. 2014;38:253–259. doi: 10.3906/biy-1309-55. [DOI] [Google Scholar]
- 180.Rozza A.L., Moraes Tde M., Kushima H., Tanimoto A., Marques M.O.M., Bauab T.M., Hiruma-Lima C.A., Pellizzon C.H. Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and β-pinene: Involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E2. Chem. Biol. Interact. 2011;189:82–89. doi: 10.1016/j.cbi.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 181.Pastor J., García M., Steinbauer S., Setzer W.N., Scull R., Gille L., Monzote L. Combinations of ascaridole, carvacrol, and caryophyllene oxide against Leishmania. Acta Trop. 2015;145:31–38. doi: 10.1016/j.actatropica.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 182.Wu C., Jia Y., Lee J.H., Jun H.-J., Lee H.-S., Hwang K.-Y., Lee S.-J. trans-Caryophyllene is a natural agonistic ligand for peroxisome proliferator-activated receptor-α. Bioorg. Med. Chem. Lett. 2014;24:3168–3174. doi: 10.1016/j.bmcl.2014.04.112. [DOI] [PubMed] [Google Scholar]
- 183.Russell K., Jacob S.E. Bisabolol. Dermatitis. 2010;21:57–58. [PubMed] [Google Scholar]
- 184.Darra E., Abdel-Azeim S., Manara A., Shoji K., Maréchal J.-D., Mariotto S., Cavalieri E., Perbellini L., Pizza C., Perahia D., et al. Insight into the apoptosis-inducing action of α-bisabolol towards malignant tumor cells: Involvement of lipid rafts and Bid. Arch. Biochem. Biophys. 2008;476:113–123. doi: 10.1016/j.abb.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 185.Jun N.J., Mosaddik A., Moon J.Y., Jang K.-C., Lee D.-S., Ahn K.S., Cho S.K. Cytotoxic activity of beta-caryophyllene oxide isolated from jeju guava (Psidium cattleianum Sabine) leaf. Rec. Nat. Prod. 2011;5:242–246. [Google Scholar]
- 186.Essenberg M., Grover P.B., Cover E.C. Accumulation of antibacterial sesquiterpenoids in bacterially inoculated Gossypium leaves and cotyledons. Phytochemistry. 1990;29:3107–3113. doi: 10.1016/0031-9422(90)80166-E. [DOI] [Google Scholar]
- 187.Humphrey A.J., Beale M.H. Strigol: Biogenesis and physiological activity. Phytochemistry. 2006;67:636–640. doi: 10.1016/j.phytochem.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 188.Yasuda N., Sugimoto Y., Kato M., Inanaga S., Yoneyama K. (+)-Strigol, a witchweed seed germination stimulant, from Menispermum dauricum root culture. Phytochemistry. 2003;62:1115–1119. doi: 10.1016/S0031-9422(02)00679-9. [DOI] [PubMed] [Google Scholar]
- 189.Khaledi H., Alhadi A.A., Yehye W.A., Ali H.M., Abdulla M.A., Hassandarvish P. Antioxidant, cytotoxic activities, and structure–activity relationship of gallic acid-based indole derivatives. Arch. Pharm. 2011;344:703–709. doi: 10.1002/ardp.201000223. [DOI] [PubMed] [Google Scholar]
- 190.Badhani B., Sharma N., Kakkar R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015;5:27540–27557. doi: 10.1039/C5RA01911G. [DOI] [Google Scholar]
- 191.Pugazhendhi D., Pope G.S., Darbre P.D. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J. Appl. Toxicol. 2005;25:301–309. doi: 10.1002/jat.1066. [DOI] [PubMed] [Google Scholar]
- 192.Coutinho E.M. Gossypol: A contraceptive for men. Contraception. 2002;65:259–263. doi: 10.1016/S0010-7824(02)00294-9. [DOI] [PubMed] [Google Scholar]
- 193.Randel R.D., Chase C.C., Wyse S.J. Effects of gossypol and cottonseed products on reproduction of mammals. J. Anim. Sci. 1992;70:1628–1638. doi: 10.2527/1992.7051628x. [DOI] [PubMed] [Google Scholar]
- 194.Wungsintaweekul B., Umehara K., Miyase T., Noguchi H. Estrogenic and anti-estrogenic compounds from the Thai medicinal plant, Smilax corbularia (Smilacaceae) Phytochemistry. 2011;72:495–502. doi: 10.1016/j.phytochem.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 195.Rinaldo D., Batista J.M., Rodrigues J., Benfatti A.C., Rodrigues C.M., Dos Santos L.C., Furlan M., Vilegas W. Determination of catechin diastereomers from the leaves of Byrsonima species using chiral HPLC-PAD-CD. Chirality. 2010;22:726–733. doi: 10.1002/chir.20824. [DOI] [PubMed] [Google Scholar]
- 196.Razavi S., Zahri S., Zarrini G., Nazemiyeh H., Mohammadi S. Biological activity of quercetin-3-O-glucoside, a known plant flavonoid. J. Bioorg. Chem. 2009;35:376–378. doi: 10.1134/S1068162009030133. [DOI] [PubMed] [Google Scholar]
- 197.Sermakkani M., Thangapandian V. GC-MS analysis of Cassia italica leaf methanol extract. Asian J. Pharm. Clin. Res. 2012;5:90–94. [Google Scholar]
- 198.Lara-Hernández B., Hernández-León A., Villafuerte-Robles L. Effect of stearic acid on the properties of metronidazole/methocel K4M floating matrices. Braz. J. Pharm. Sci. 2009;45:497–505. doi: 10.1590/S1984-82502009000300016. [DOI] [Google Scholar]
- 199.Walters D., Raynor L., Mitchell A., Walker R., Walker K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia. 2004;157:87–90. doi: 10.1023/b:myco.0000012222.68156.2c. [DOI] [PubMed] [Google Scholar]
- 200.Tarmadi D., Himmi S.K., Yusuf S. The efficacy of the oleic acid isolated from Cerbera manghas L. seed against a subterranean termite, Coptotermes gestroi wasmann and a drywood termite, Cryptotermes cynocephalus Light. Procedia Environ. Sci. 2014;20:772–777. doi: 10.1016/j.proenv.2014.03.093. [DOI] [Google Scholar]
- 201.Dilika F., Bremner P.D., Meyer J.J.M. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia. 2000;71:450–452. doi: 10.1016/S0367-326X(00)00150-7. [DOI] [PubMed] [Google Scholar]
- 202.Burdock G.A., Carabin I.G. Safety assessment of myristic acid as a food ingredient. Food Chem. Toxicol. 2007;45:517–529. doi: 10.1016/j.fct.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 203.Thoorens G., Krier F., Leclercq B., Carlin B., Evrard B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014;473:64–72. doi: 10.1016/j.ijpharm.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 204.Viernes L.B.G., Garcia R.N., Torio M.A.O., Angelia M.R.N. Antihypertensive peptides from vicilin, the major storage protein of mung bean (Vigna radiata (L.) R. Wilczek) J. Biol. Sci. 2012;12:393–399. [Google Scholar]