Abstract
A new schiartane-type nortriterpenoid, micrandilactone H was isolated from Kadsura longipedunculata Finet et Gagnep. Its 2D (two dimension) structure was elucidated by NMR spectroscopic analysis, and it is similar to that of Kadnanolactones H and the absolute configuration was established through X-ray diffraction and ECD data analysis. This represents the first complete assignment of the absolute configuration of a schiartane-type nortriterpenoid by X-ray diffraction and the ECD method. Micrandilactone H showed moderate hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced toxicity in HepG2 cells with cell survival rates of 56.84% at 10 μM.
Keywords: schiartane-type nortriterpenoid, X-ray, absolute configuration, hepatoprotective
1. Introduction
The roots and stems of plants of the genus Kadsura, have been used in traditional Chinese medicines (TCM) to treat blood disease, remove stasis and relieve pain. These plants are widely distributed in the southwest of China. Phytochemical studies showed that the principal bioactive constituents of the genus Kadsura are lignans and triterpenoids. The lignans include dibenzocyclooctadiene lignans, spiro-benzofuran-type dibenzocyclooctadiene lignans, cyclolignans, neolignans and simple lignans. The triterpenoids include lanostane-type, cycloartane-type and nortriterpenoids. Nortriterpenoids are a structurally intriguing group of highly oxygenated, polycyclic, fused heterocyclic natural products produced by the plants of the genera Schisandra and Kadsura. Some of these compounds showed promising anti-HIV (human immunodeficiency virus) bioactivities. The C29 type is the most common and can be divided into five classes including schiartane, 18(13→14)-abeo-schiartane, schisanartane, preschisanartane and wuweiziartane [1]. Their planar structures and relative configurations have been successfully elucidated by modern NMR techniques. Thus far, the side chain of only one member of this class has been successfully assigned unambiguously using X-ray diffraction combined with the modified Mosher method. [2] Assignment of the absolute configuration is critical since it influences the bioactivity and is essential for future efforts focused on synthesis and medicinal chemistry. Owing to the unique structures and activities there has been an escalating interest in their discovery, total synthesis, biological activities and biosynthesis studies [1].
Kadsura longipedunculata belongs to the family Schisandraceae [2]. The roots have been used in Chinese folk medicine to treat irregular menstruation, gastroenteritis, fractures and rheumatoid arthritis [2]. In order to identify new natural compounds with interesting bioactivities, we performed a phytochemical study on the roots of K. longipedunculata that lead to the identification of a new C29 triterpenoid dilactone, micrandilactone H. Micrandilactone H showed moderate hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced toxicity in HepG2 (human hepatocellular liver carcinoma cell line) cells with cell survival rates of 56.84% at 10 μM. This is the first report of the full stereochemistry assignment of a schiartane-type nortriterpenoid via X-ray diffraction and it is supported by ECD and NMR spectroscopic data.
2. Results
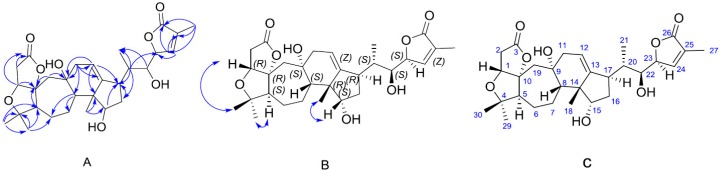
Micrandilactone H was isolated as colorless crystals, and gave an [M + Na]+ ion at m/z 539.2637 in the HRESIMS, consistent with a molecular formula of C29H40NaO8 (calcd 539.2615), indicating nine indices of hydrogen deficiency. The 13C and HSQC NMR spectra (Table 1 and Table S7) revealed two lactone carbonyls resonating at δC 175.9 and 175.1; two olefinic functionalities with four carbon resonances at δC 147.7, 120.9, 148.9 and 130.5; four sp3 non-protonated carbons (three oxygenated) resonances at δC 99.7, 85.2, 70.8 and 52.8; eight sp3 methine (three oxygenated) resonances at δC 82.5, 59.6, 58.8, 77.1, 43.3, 44.3, 74.9 and 83.0 ppm; six sp3 methylene resonances at δC 46.0, 42.7, 40.4, 36.9, 28.7, and 25.3; and five methyl resonances at δC 29.9, 26.6, 23.5, 16.1, and 10.9. The 1H-NMR spectra revealed an ABX spin system, with δH 4.27 (d, J = 4.5 Hz), 2.94 (dd, J = 18.0, 4.5 Hz) and 2.72 (d, J = 18.0 Hz), which were assigned to H-1, H-2α and H-2β, respectively, together with four methyl singlets at δH 1.83 (3H, s), 1.27 (3H, s), 1.14 (3H, s), 1.12 (3H, s); one methyl doublet at δH 1.34 (3H, d, J = 7.0 Hz); two olefinic protons (δH 7.23, 5.76); four methine protons (δH 5.27, 4.00 and 3.31); and several methylene protons between δH 1.22 and 2.80. According to these data, micrandilactone H is a schiartane-type nortriterpenoid whose structure is similar to micrandilactone C, except for an extra double bond, one less hydroxy group and the different location of the C-18 methyl group [3]. The HMBC cross-peaks of H-18 with C-8, C-13, C-14 and C-15, H-11 with the C-8, C-9, C-12, C-13 and C-19, and H-12 with C-9, C-14 and C-17 (Figure S8) indicated that Me-18 was connected at C-14 and the double bond was located at C-12 and C-13. Thus, the 2D (two dimension) structure of micrandilactone H was established as shown in Figure 1.
Table 1.
1H- and 13C-NMR data for micrandilactone H in pyridine-d5 using an INOVA-500 MHz spectrometer.
| Position | δH | δC |
|---|---|---|
| 1 | 4.27 (d, 4.5) | 82.5 |
| 2α | 2.72 (d, 18.0) | 36.9 |
| 2β | 2.94 (dd, 18.0, 4.5) | |
| 3 | 175.9 | |
| 4 | 85.2 | |
| 5 | 2.57 (dd, 13.0, 4.0) | 59.6 |
| 6α | 1.69 (overlapped) | 28.7 |
| 6β | 1.31 (overlapped) | |
| 7α | 2.37 (m) | 25.3 |
| 7β | 2.07 (overlapped) | |
| 8 | 1.72 (overlapped) | 58.8 |
| 9 | 70.8 | |
| 10 | 99.7 | |
| 11α | 2.35 (m) | 42.7 |
| 11β | 2.26 (m) | |
| 12α | 5.76 (d, 7.0) | 120.9 |
| 12β | ||
| 13 | 147.7 | |
| 14 | 52.8 | |
| 15 | 4.00 (s) | 77.1 |
| 16α | 2.30 (m) | 40.4 |
| 16β | 2.11 (dd, 8.5, 3.0) | |
| 17 | 3.31 (m) | 43.3 |
| 18 | 1.12 (s) | 26.6 |
| 19α | 2.15 (d, 15.0) | 46.0 |
| 19β | 2.02 (d, 15.0) | |
| 20 | 2.21 (m) | 44.3 |
| 21 | 1.34 (d, 7.0) | 16.1 |
| 22 | 4.00 (s) | 74.9 |
| 23 | 5.27 (br, s) | 83.0 |
| 24 | 7.23 (s) | 148.9 |
| 25 | 130.5 | |
| 26 | 175.1 | |
| 27 | 1.83(s) | 10.9 |
| 29 | 1.27(s) | 29.9 |
| 30 | 23.5 |
Figure 1.
Key HMBC (A), NOESY (B) correlations of micrandilactone H (C).
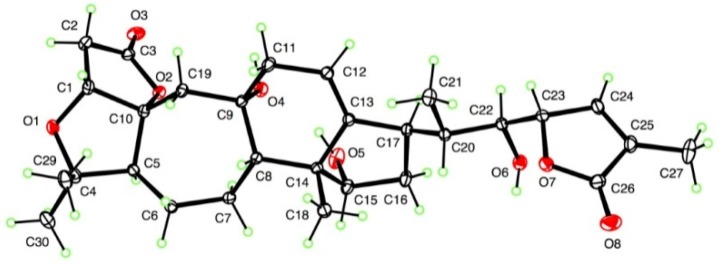
The absolute configuration was unambiguously determined as (1R, 5S, 8S, 9S, 10R, 14R, 15S, 17R, 20S, 22S, 23S) by X-ray crystallographic analysis (Figure 2) based on resonant scattering of only light atoms in both Mo and Cu radiations. These results matched the assignment of the (23S) absolute configuration based on the negative Cotton effect at 214 nm in the ECD spectrum [4]. The molecular structure features an intramolecular hydrogen bond between the 15- and 9-OH groups. However, the 22-OH group does not form an intramolecular hydrogen bond with the adjacent lactone carbonyl, instead forming an intermolecular hydrogen bond with the C-3 lactone carbonyl.
Figure 2.
ORTEP drawing of micrandilactone H.
Micrandilactone H was evaluated in vitro for its hepatoprotective activity against APAP-induced toxicity in HepG2 cells, using the hepatoprotective activity drug bicyclol as the positive control. Micrandilactone H affected a cell survival rate of 56.84% (bicyclol, 49.00%) at 10 μM when added into the resuscitated HepG2 cells incubated with APAP for 48 h.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were measured on a JASCO P-2000 polarimeter (Jasco Inc., Easton, MD, USA) and UV spectra with a JASCO V-650 spectrophotometer (Jasco Inc., Easton, MD, USA). ECD spectra were recorded on a JASCO J-815 spectrometer (Jasco Inc., Easton, MD, USA). IR spectra were recorded on a Nicolet 5700 spectrometer (Thermo Electron Corporation, Madison, WI, USA) using an FT-IR microscope transmission method. The 1H- and 13C-NMR spectra were recorded on INOVA-500 (Varian, Inc., Palo Alto, CA, USA) and BRUKER AV500-III spectrometers (Bruker, Billerica, MA, USA). The standard pulse sequences were used for each 2D measurement. HRESIMS data were acquired an Agilent 6520 Accurate-Mass Q-Tof LC/MS spectrometer (Agilent Technologies, Waldbronn, Germany). Analytical reversed-phase HPLC was performed on a COSMOSIL 5C18-PAQ Waters column (4.6 i.d. × 250 mm, Waters, Nacalai, San Diego, CA, USA) eluted with water/methanol (flow rate, 1 mL/min; 220 nm UV detection) at room temperature. Preparative reversed-phase HPLC was performed on a COSMOSIL 5C18-PAQ Waters column (250 × 10 mm, 5 μm) at room temperature. Column chromatography was performed on silica gel (40–63 μm; Silicyle, Quebec City, QC, Canada), C18 120 Å reversed-phase silica gel (RP-18; 50μm; Silicycle) and Sephadex LH-20 (GE Healthcare Bio-Science AB, Uppsala, Sweden). Fractions were monitored by TLC and spots were visualized by heating silica gel plates sprayed with 10% H2SO4 in EtOH.
3.2. Plant Material
The roots of K. longipedunculata were collected in the Jiujiang County of Jiangxi Province, China, in March 2010, and identified by Ce-Ming Lin, Institute of Biology Resources, Jiangxi Academy of Science. A voucher specimen (ID-S-2428) is deposited in the herbarium of the Institute of Materia Medica (IMM), Chinese Academy of Medical Science (CAMS) and Peking Union Medical College (PUMC).
3.3. Extraction and Isolation
The air-dried and powered roots (34.0 kg) were percolated with 95% aqueous ethanol (4 × 12 L) at room temperature and the solvent removed in vacuo to yield a residue (2400 g). The residue was subjected to silica gel (200–300 mesh, 2400 g) column chromatography, eluting with a petroleum ether/acetone gradient system (100:1, 50:1, 10:1, 5:1, 3:1, 1:1), acetone and 80% aqueous ethanol, to give fractions 1–15. Fraction 14 was partitioned between water and EtOAc. The EtOAc extract (60 g) was chromatographed on an ODS column eluted with gradient water/methanol (4:1, 3:2, 2:3, 1:4) to yield five fractions A–E. Fraction D was chromatographed on Sephadex LH-20 (eluted with chloroform/methanol) and gave five subfractions 1–5. Fraction D1 (1.7 g) was subjected to silica gel (200–300 mesh, 3 g) column chromatography, eluting with chloroform/methanol gradient system (4:1 to 1:4) and give nine subfractions, D1.1–D1.9. Fraction D1.3 (365 mg) purified by semi-preparative HPLC (46% methanol/water), to give micrandilactone H (3 mg). Micrandilactone H (3 mg) was dissolved in 1mL of methanol, 0.5 mL of water was added, the sample was kept at room temperature, and the single crystal was generated.
Micrandilactone H: Colorless crystals; [α] 3 (c 0.4, methanol); UV (methanol) λmax (log ε) 211 (2.29) nm; ECD (methanol) [θ] (nm) −12.0 × 103 (214). IR (KBr) νmax 3525, 3468, 3386, 2973, 2935, 1747, 1066, 923, 867 cm−1. ESIMS (m/z): 539 [M + Na]+;HRESIMS (m/z): 539.2637 [M + Na]+ (calcd 539.2615 for C29H40NaO8).
3.4. X-Ray Crystallographic Analysis of Micrandilactone H
C29H40O8, M = 516.61, monoclinic, space group P21, a = 10.8666 (16) Å, b = 10.3174 (16) Å, c = 12.6133 (19) Å, β = 113.828 (7)o, V = 1293.6 (3) Å3, Z = 2, d = 1.326 g cm−3. Intensity data were measured at T = 90 K on a Bruker Kappa APEX-II DUO diffractometer, including both Mo Kα and Cu Kα datasets. The total number of reflections measured with Mo radiation to θmax = 36.4o was 21,401, yielding 10,250 independent data, of which 9425 were observed with F2 > 2σ(F2), Rint = 0.027. The structure was solved using SHELXS-97 and refined [5] by full-matrix least-squares using SHELXL-2014/7. R = 0.036, wR(F2) = 0.095 for all data and 348 refined parameters. The Flack parameter based on 3290 quotients [6] was 0.0(2), indicating the absolute configuration shown in Figure 2, CCDC 1520861. To confirm the absolute configuration, Cu data from a microfocus source was measured using the same crystal to a maximum θ value of 69.1°, yielding 20,583 total data and 4561 independent data, Rint = 0.041. Refinement yielded R = 0.026, wR(F2) = 0.066, and Flack parameter 0.01(4) for 2044 quotients, giving a much stronger indication of an absolute configuration of (1R, 5S, 8S, 9S, 10R, 14R, 15S, 17R, 20S, 22S, 23S), CCDC 1520862.
3.5. Hepatoprotective Activity Assay
Micrandilactone H was tested for hepatoprotective activity against APAP-induced toxicity in HepG2 cells by means of the published MTT method [7].
Acknowledgments
This study was supported financially by the CAMS Initiative for Innovative Medicine (CAMS-I2M-1-010). X.W. and M.T.H. Thank NCCIH, CSC, The Cooper Family and The Abney Foundation for Financial Support.
Supplementary Materials
1H-NMR, 13C-NMR, HMBC, HSQC, ROESY, ECD and HRESIMS data for micrandilactone H and the detailed experimental conditions for hepatoprotective assay are available free of charge via the Internet at http://www.mdpi.com/1420-3049/22/1/65/s1. CCDC 1520861-1520862 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk.
Author Contributions
S.L. and M.T.H. conceived and designed the experiments; X.W., F.R.F. and J.C. performed the experiments; X.W., D.F., J.L., M.T.H. and F.R.F. analyzed the data; S.L. contributed reagents/materials/analysis tools; X.W. and M.T.H. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Sample of micrandilactone H is not available.
References
- 1.Lei C., Huang S.-X., Chen J.-J., Yang L.-B., Xiao W.-L., Chang Y., Lu Y., Huang H., Pu J.-X., Sun H.-D. Propindilactones E−J, schiartane nortriterpenoids from Schisandra propinqua var. propinqua. J. Nat. Prod. 2008;71:1228–1232. doi: 10.1021/np8001699. [DOI] [PubMed] [Google Scholar]
- 2.Xiao W.-L., Li R.-T., Huang S.-X., Pu J.-X., Sun H.-D. Triterpenoids from the Schisandraceae family. Nat. Prod. Rep. 2008;25:871–891. doi: 10.1039/b719905h. [DOI] [PubMed] [Google Scholar]
- 3.Li R.-T., Han Q.-B., Zheng Y.-T., Wang R.-R., Yang L.-M., Lu Y., Sang S.-Q., Zheng Q.-T., Zhao Q.-S., Sun H.-D. Structure and anti-HIV activity of micrandilactones B and C, new nortriterpenoids possessing a unique skeleton from Schisandra micrantha. Chem. Commun. 2005;23:2936–2938. doi: 10.1039/b501932j. [DOI] [PubMed] [Google Scholar]
- 4.Gawronski J.K., van Oeveren A., van der Deen H., Leung C.W., Feringa B.L. Simple circular dichroic method for the determination of absolute configuration of 5-substituted 2(5H)-furanones. J. Org. Chem. 1996;61:1513–1515. doi: 10.1021/jo951400l. [DOI] [Google Scholar]
- 5.Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015;C71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons S., Flack H.D., Wagner T. Use of intensity quotients and differences in absolute structure refinement. Acta Cryst. 2013;B69:249–259. doi: 10.1107/S2052519213010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C.-J., Ma J., Sun H., Zhang D., Zhang D.-M. Guajavadimer A, a dimeric caryophyllene-derived meroterpenoid with a new carbon skeleton from the leaves of Psidium guajava. Org. Lett. 2015;18:168–171. doi: 10.1021/acs.orglett.5b03117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.