Supplemental Digital Content is available in the text
Keywords: anemia, nephroureterectomy, prognosis, upper urinary tract, urothelial carcinoma
Abstract
The aim of this study was to investigate the effect of preoperative anemia on the prognosis of patients who underwent radical nephroureterectomy (RNU) for upper tract urothelial carcinoma (UTUC).
A total of 620 patients with UTUC were retrospectively analyzed. Anemia was decided by preoperatively measured hemoglobin values based on the World Health Organization (WHO) classification. Kaplan–Meier method and Cox proportional hazards regression models were used to analyze the relationship between anemia and survival outcomes. The meta-analysis part was performed according to PRISMA guidelines.
The median follow-up was 51 (range: 1–168) months. A total of 246 patients had preoperative anemia in our cohort. Anemia was found to be related to high-grade (P < .001), sessile architecture (P = .001), advanced T stage (P < .001), lymphovascular invasion (LVI) (P = .006), and worse chronic kidney disease (CKD) stage (P = .012). Kaplan–Meier curves revealed that patients with preoperative anemia had worse overall survival (OS), cancer-specific survival (CSS), and disease recurrence-free survival (RFS) (all P < .001). Multivariable Cox analyses found that anemia was an independent predictor of CSS [hazard ratio (HR) 1.719, 95% confidence interval (95% CI): 1.285–2.300], RFS (HR 1.427, 95% CI: 1.114–1.829) and OS (HR 1.756, 95% CI: 1.353–2.279). Among patients without end-stage renal disease (ESRD, n = 614), the anemia was also proved to be associated with worse outcomes in multivariable Cox analysis (OS, HR 1.759, 95% CI: 1.353–2.287; CSS, HR 1.726, 95% CI: 1.289–2.311, and RFS, HR 1.431, 95% CI: 1.117–1.837). Seven studies were included in the meta-analysis, and the pooled results showed that anemia was also related to worse CSS (HR 2.05, 95% CI: 1.73–2.44), RFS (HR 1.57, 95% CI: 1.30–1.90), and OS (HR 1.53, 95% CI: 1.10–2.13), but not related to intravesical recurrence (HR 1.17, 95% CI: 0.75–1.82).
Preoperative anemia was proved to be significantly associated with worse oncologic outcomes in patients with UTUC following RNU.
1. Introduction
Upper tract urothelial carcinomas (UTUCs) are tumors derived from urothelium along the pyelocaliceal cavities and ureter, accounting for approximately 5% to 10% of urinary tract carcinomas.[1] While, its incidence rate is higher in China, especially in Taiwan district, due to the use of traditional herbs which contain aristolochic acid.[2] Radical nephroureterectomy (RNU) with bladder cuff excision is the gold standard treatment for UTUC and has provided durable local tumor control and better long-term survival.[1,3] Although surgical and medical management has improved, the 5-year cancer-specific mortality rates remain relatively high, ranging from 20% to 30%.[4]
Studies have reported some traditional prognostic predictors for UTUC after RNU, such as pathological tumor stage and grade, tumor location and architecture, lymphovascular invasion status, concomitant carcinoma in situ, and age.[5] Recently, an increasing interest in biomolecular predictors for UTUC has been noted, including impaired renal function,[6] and elevated C-reactive protein,[7] which have been investigated as prognostic factors for urothelial carcinoma.[8] Although several studies have reported that preoperative anemia may also have a potential to be an independent predictor of oncologic outcomes for UTUC after RNU, its role has not been fully investigated and their results are still controversial.[2,9–15] As it is easily available from preoperative routine examinations in clinical practice, a better understanding of preoperative hemoglobin level may improve prognostication for urothelial carcinoma after RNU.
Therefore, in this study, we aimed to further investigate the effect of preoperative anemia on oncologic outcomes of patients who underwent RNU for UTUC in our center and perform a literature review.
2. Patients and methods
2.1. Patients
The data of 688 patients with UTUC received RNU treatment between October 2003 and December 2015 were collected from our center with approval from the institutional review board. Six patients with the previous cystectomy for invasive bladder cancer and 5 patients underwent RNU and radical cystectomy were excluded; Fifty-seven patients were excluded due to missing data on clinicopathological variables or follow-up, and preoperative hemoglobin. No single patient received neoadjuvant chemotherapy before surgery. Therefore, a total of 620 patients were retrospectively reviewed. Lymph node dissection was not routinely performed.
All RNU specimens were respectively processed according to standard procedures. Tumor stage and grade were evaluated according to the 2002 American Joint Committee of Cancer TNM classification and the WHO International Society of Urological Pathology consensus classification, respectively. The preoperatively hemoglobin values of all patients were used for analysis and obtained from blood tests within 30 days before surgery. Anemia is defined as 13 mg/dL or less in males and 12 mg/dL or less in females according to the WHO guideline.[16] Patients were categorized into 2 groups (normal or anemia group) based on hemoglobin levels. The clinicopathological data, including patients’ age, gender, smoking history, surgical approach and renal function, preoperative hydronephrosis, and tumor side as well as tumor size were also collected. End-stage renal disease (ESRD) was defined as patients with stage 5 chronic kidney disease (CKD).
2.2. Follow-up
Patients were followed every 3 to 4 months for the first year after surgery according to the guideline, semiannually for the second and third year, and annually thereafter, or as clinically indicated with urinary cytology and excretory urography of the contralateral upper urinary tract, and routine check-ups that included history, physical examination, blood laboratory tests, and chest radiography. If clinically indicated, selective bone scan and chest/abdomen computed tomography (CT)/magnetic resonance imaging (MRI) were elevated.[17] Disease recurrence was defined as local recurrence in the operating field, lymph node spread, and/or distant metastasis that had not been found in the preoperative examination. Specifically, the tumor found in the urinary bladder or contralateral upper urinary tract after surgery was not regarded as tumor relapse.
2.3. Systematic review
The systematic literature review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) flowchart and followed the PRISMA guidelines for meta-analysis.
2.4. Statistical analysis
Continuous variables were analyzed using Student t test and categorical variables were elevated using the Chi-squared test or Fisher exact test. Univariable and multivariable logistic regression analysis were used to evaluate the anemia with clinicopathological features. The Kaplan–Meier method was used to calculate survival outcomes, including overall survival (OS), cancer-specific survival (CSS), and disease recurrence-free survival (RFS), and the log-rank test was used to assess differences. Univariable and multivariable Cox proportional hazards regression models were used to evaluate the relationship between variables and OS, CSS and RFS. Risk factors with a P value <.1 in the univariable analysis were included in the multivariable analysis model. Especially, CKD stage was also included in multivariable models due to its potential covariant effect with anemia. Hazard ratios (HRs) with their 95% confidence intervals (95% CIs) were used to assess the strength of the individual variables. All reported P values were 2-sided with statistical significance set at P < .05. Statistical analyses were performed using IBM SPSS Statistics version 22.0 (IBM Corp., Armonk, NY).
In terms of meta-analysis, Stata v. 12.0 (StataCorp, College Station, TX) was used to perform all statistical analyses. Statistic heterogeneity scores were assessed by using both the standard Cochrane Q test and I2 statistic to quantify inconsistency across studies and to assess the impact of meta-analysis heterogeneity. A value of P < .05 for Cochrane Q test or an I2 statistic > 50% indicates a considerable level of heterogeneity, resulting in the use of the random-effects model. Publication bias was investigated by visual inspection of Begg funnel plots.
3. Results
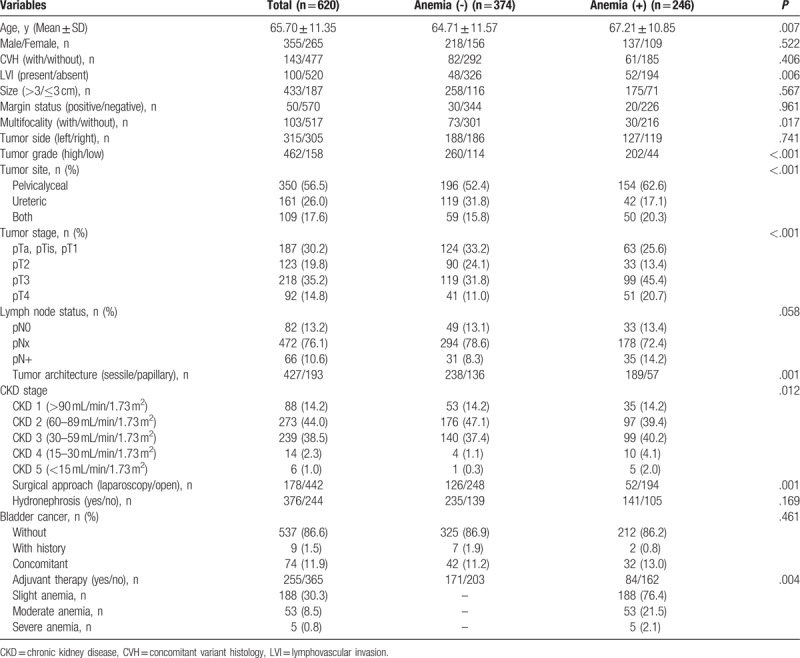
The baselines of patients included in the present study are summarized in Table 1. The median follow-up duration was 51 (range: 1–168) months. The mean age of the whole cohort was 65.70 ± 11.35 years. Only 6 patients had ESRD in this cohort. Four hundred sixty-two patients had high-grade tumors and 310 patients were diagnosed with the ≥T3 stage. A total of 246 patients had preoperative anemia. Among them, 188 patients had slight level anemia, 53 patients had moderate anemia, and only 5 cases had severe anemia. Anemia was found to be related to high-grade (P < .001), sessile architecture (P = .001), advanced T stage (P < .001), lymphovascular invasion (LVI; P = .006), and worse CKD stage (P = .012). No relationship was found between anemia and tumor size or CVH.
Table 1.
Baseline characteristics of 620 patients with upper tract urothelial carcinoma included in the present study.
The receiver operating characteristic (ROC) curve showed that the area under the curve (AUC) of anemia was 0.598. Kaplan–Meier curves revealed that patients with preoperative anemia had worse OS, CSS, and RFS (all P < .001) (Fig. 1A–C). Univariable Cox analysis found that anemia was related to poor survival outcomes of patients with UTUC (CSS: HR 2.112, 95% CI: 1.597–2.794; RFS: HR 1.724, 95% CI: 1.361–2.185; and OS: HR 2.088, 95% CI: 1.627–2.681, respectively) (Table 2).
Figure 1.
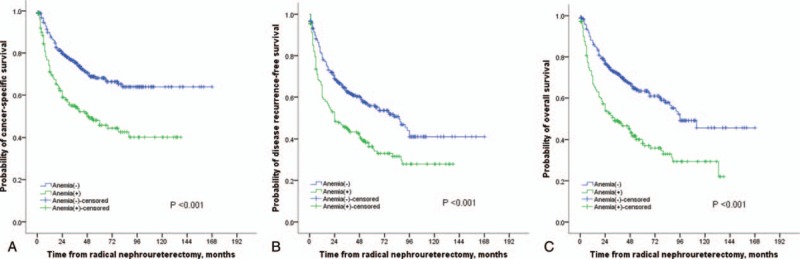
Kaplan–Meier curves for CSS (A), RFS (B), and OS (C) stratified according to preoperative hemoglobin values in patients undergoing RNU of UTUC.
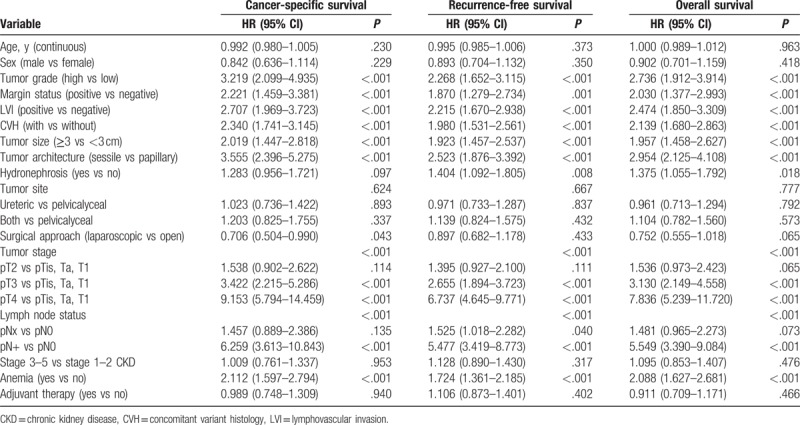
Table 2.
Cox proportional hazard univariate analysis to predict survival outcomes of patients with upper tract urothelial carcinoma.
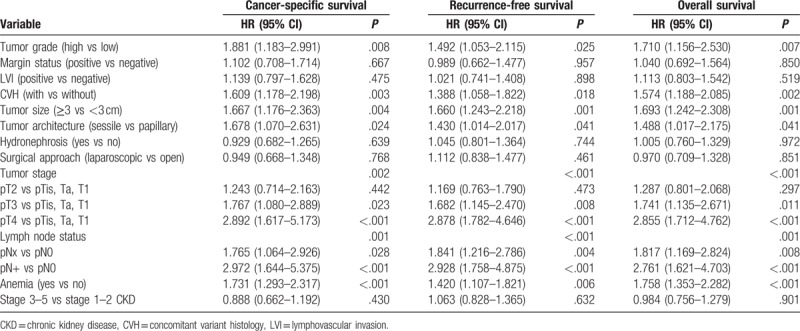
Multivariable Cox analysis further confirmed that anemia was an independent predictor of worse CSS (HR 1.719, 95% CI: 1.285–2.300; P < .001), RFS (HR 1.427, 95% CI: 1.114–1.829; P = .005), and OS (HR 1.756, 95% CI: 1.353–2.279; P < .001) (Table 3). But anemia was not associated with intravesical recurrence (IVR) (HR 1.086, 95% CI: 0.755–1.564; P = .656). The study also confirmed that tumor stage, tumor grade, LNM, CVH, tumor size, and tumor architecture were independent predictors of survival outcomes in UTUC patients (Table 3).
Table 3.
Cox proportional hazard multivariate analysis to predict survival outcomes of patients with upper tract urothelial carcinoma.
Subgroup analysis based on the level of anemia was performed and the results found that both slight and moderate anemia contributed to worse OS (HR 1.689, 95% CI: 1.278–2.233 and HR 2.101, 95% CI: 1.379–3.200, respectively), CSS (HR 1.595, 95% CI: 1.163–2.188 and HR 2.331, 95% CI: 1.491–3.644, respectively), and RFS (HR 1.339, 95% CI: 1.024–1.751 and HR 1.842, 95% CI: 1.229–2.761, respectively) in multivariable model, but severe anemia was not related to survival outcomes (OS, HR 1.206, 95% CI: 0.292–4.988; CSS, HR 1.316, 95% CI: 0.316–5.482, and RFS, HR 0.831, 95% CI: 0.197–3.360, respectively).
Among patients without ESRD (n = 614), the anemia was proved to be associated with worse outcomes in both univariable and multivariable Cox analysis (OS, HR 1.759, 95% CI: 1.353–2.287; CSS, HR 1.726, 95% CI: 1.289–2.311, and RFS, HR 1.431, 95% CI: 1.117–1.837 in multivariable model) (Table S1 and S2). However, the impact of anemia on survival in patients with ESRD was unavailable in our cohort due to a small population (n = 6).
Six studies with a total of 1776 participants and our present cohort were included in the systematic review and meta-analysis.[2,10–14,18] The characteristics of studies included are summarized in Table 4. The pooled results showed that anemia was associated with worse CSS (HR 2.05, 95% CI: 1.73–2.44), RFS (HR 1.57, 95% CI: 1.30–1.90), and OS (HR 1.53, 95% CI: 1.10–2.13), but not related to IVR (HR 1.10, 95% CI: 0.83–1.46) in UTUC patients (Fig. 2). No publication bias was observed among studies analyzing the impact of anemia on CSS (Begg P = .133) (Figure S1). The pooled results also found anemia could lead to inferior CSS (HR 1.82, 95% CI: 1.44–2.31) and MFS (HR 2.21, 95% CI: 1.54–3.18) in those without ESRD (Fig. 3).
Table 4.
Overall characteristics of studies included in meta-analysis.
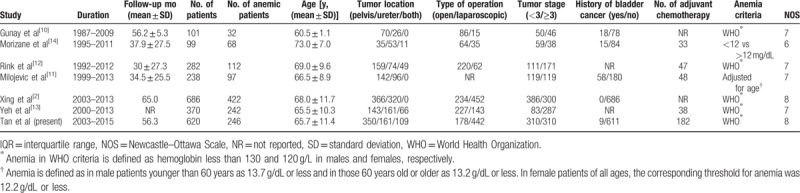
Figure 2.
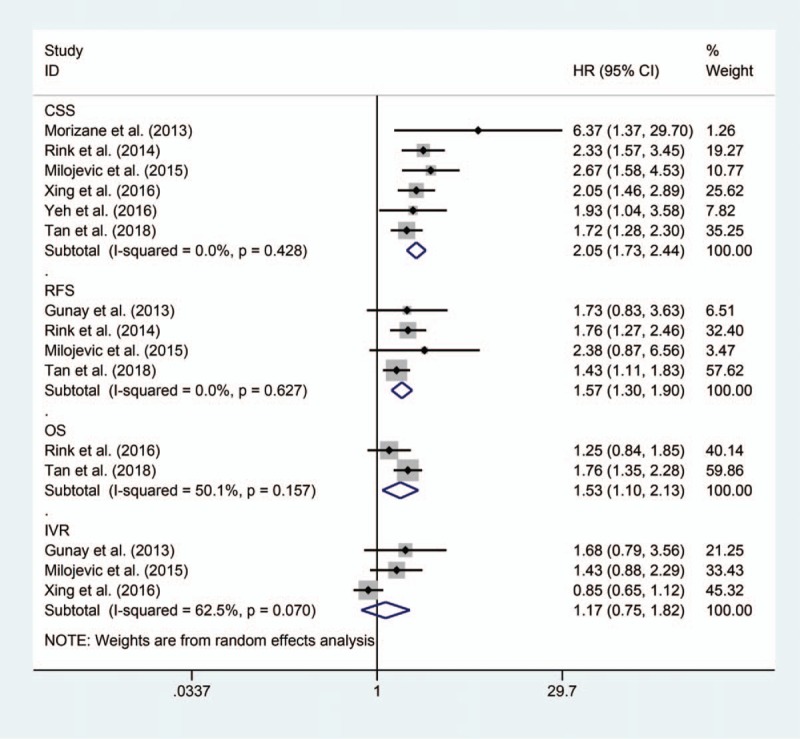
Forrest plots of meta-analyses of the effect of preoperative anemia on oncologic outcomes in patients with UTUC after RNU.
Figure 3.
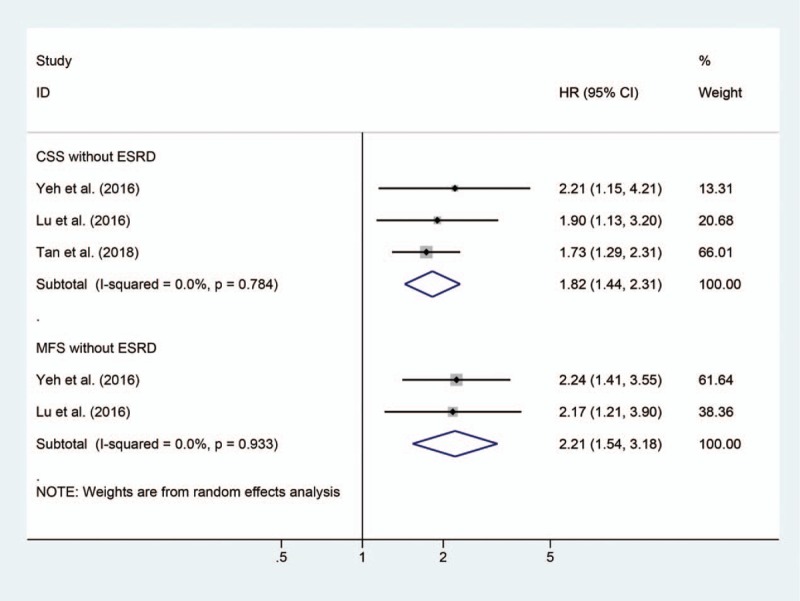
Forrest plots of meta-analyses of the effect of preoperative anemia on oncologic outcomes in UTUC patients without end-stage renal disease.
4. Discussion
Anemia can result from many causes, such as blood loss, renal insufficiency, bone marrow disorders, and hypothyroidism.[19] Anemia is also common in cancer patients and significantly influenced by the type of malignancy, tumor stage and grade, lymph node metastasis, and the choice of treatment.[11] Caro et al[20] conducted a comprehensive analysis to estimate the effect of anemia on survival in patients with malignant disease. Their result suggested that the relative risk of death increased by 65% in anemic cancer patients; however, the impact of preoperative anemia on patients with UTUC has seldom been assessed. In our cohort, the results found that anemia was an independent predictor of survival outcomes in UTUC patients. Anemia was also associated with high grade, sessile architecture, advanced T stage, LVI, and high stage CKD. But anemia had no association with tumor size and CVH. The pooled results from meta-analysis also suggested that preoperative anemia was associated with worse CSS, RFS, and OS in patients with UTUC after RNU regardless their renal function, but it did not affect IVR.
As we known, renal insufficiency could not only contribute to the poor outcomes of urothelial carcinoma but also related to causing anemia due to erythropoietin deficiency, uremic-induced inhibitors of erythropoiesis, shortened erythrocyte survival, and iron deficiency.[6,21] Therefore, it is noteworthy to understand the association of anemia with UTUC oncologic outcome at different stages of renal function. Our study revealed that anemia contributed to worse CSS, RFS, and OS in patients without ESRD, while its impact on patients with ESRD was unavailable in this study due to a small population of ESRD patients. The pooled results from meta-analysis also suggested that anemia was related to worse CSS and MFS in patients without ESRD. Moreover, Yeh et al[13] found that there was no difference in CSS or MFS in patients with renal insufficiency ranging from stage 1 to 4 CKD. And they also found in patients with ESRD, preoperative anemia was not related to CSS or MFS (P = .590 and P = .379, respectively).[13] Anemia occurred more commonly in patients with ESRD, over 90%.[13,15] Consequently, anemia in uremic patients was generally attributed to the process of renal function deterioration rather than the carcinogenesis. Thus, the ESRD may play the main role in causing the worse outcomes in urothelial cancer and anemia is a mere result of ESRD in patients with ESRD, which means anemia was not a predictor of adverse prognosis in this population. Also, Yeh et al[13] thought that the cancer-related anemia was the actual prognostic factor for patients with UTUC.
The relationship between anemia and cancer pathophysiology is complex and multifactorial. In UTUC patients, the impact of adjuvant chemotherapy on anemia by bone marrow suppression was controversial, even in opposite directions.[11–13] Gross hematuria is an important but common symptom for the diagnosis of UTUC, which also contribute to the anemia due to blood loss. However, no data on gross hematuria were available currently. Blood loss might also be attributed to the preoperative manipulation, but the only evidence reported that there was no association between interventions and hemoglobin level.[12]
In patients with malignancy, cancer-related anemia is a cytokine-mediated disorder resulting from complex interactions between tumor cells and the immune system. It usually occurs long before the diagnosis of the underlying disease and often a sign of potentially decreased function of organs and systems. Overexpressed certain inflammatory cytokines could shorten survival of red blood cells, suppress erythroid progenitor cells, impair iron utilization, and result in inadequate erythropoietin production.[22] Moreover, low hemoglobin levels correlated with poor performance status and clinical outcome.[22] Therefore, preoperative anemia could be considered as a strong prognostic predictor of worse outcome in patients with UTUC.
Regardless, it is now clear that preoperative anemia is a significant prognostic factor in UTUC following RNU, but whether correcting the preoperative anemia could improve the survival after RNU remains unknown. Currently, managements such as transfusions, erythropoiesis-stimulating agents (ESAs), and iron supplements were normally used to correct the anemia.[23] Because transfusions could quickly correct anemia, doctors often suggested the blood transfusion for anemic patients with cancer who need confine operation, radiotherapy, or chemotherapy. Around 15% anemic patients with cancer were treated with blood transfusions, but its safety and impact on prognosis remain as an issue of debate.[24] Several reports have shown that transfusion contributes to poor prognosis in some kinds of cancers.[24] However, limited evidence suggested that perioperative blood transfusion was associated with inferior OS (HR 1.6, P = .027), but not with disease recurrence or cancer-specific mortality in anemic patients with UTUC after RNU.[18] Treatment of ESAs might increase the risk of thrombosis and mortality, although it could improve patients’ quality of life.[25] Thus, transfusions and ESAs should be carefully used to treat anemic patients with UTUC.[24,25]
Some limitations of this study should be mentioned; first, its retrospective nature, which may have led to a selection bias. In addition, the surgery methods and adjuvant therapy treatments were a little different between the 2 groups, although we found that these 2 factors did not affect the survival outcomes. Meanwhile, lymphadenectomy was not routinely performed; only 148 patients received lymphadenectomy in our cohort, which may decrease the accuracy of our results even though there was no difference between the 2 groups. Compared with previous studies, we analyzed the impact of different anemia level on survival outcomes and found both the slight and moderate anemia cause worse survival status, while severe anemia had no impact on UTUC patients. Moreover, renal function was fully considered in the present study and was included in multivariable model. Due to the small population of ESRD (n = 6), the impact of anemia on patients with ESRD was unavailable in the present study. Future studies are needed to investigate this topic in population with ESRD.
5. Conclusion
Preoperative anemia was significantly associated with worse oncologic outcomes in patients with UTUC following RNU. At the moment, no evidence investigated whether correcting the preoperative anemia could improve the survival after RNU. Thus, more prospectively designed studies are needed to validate our results.
Acknowledgment
The authors convey thanks to Zhiwei Fan (Department of Statistics, West China Medicine School, Sichuan University) for giving us statistical advices.
Author contributions
Q.W. carried out project design, participated in data explanation and manuscript revision. P.T. and N.X. participated in project design, performed data collection and statistical analysis. HT. L. and LQ.Z. helped data collection and provided statistical advice. H.X. reviewed all specimens and made pathology classification. L.Y. and LR.L. checked for statistical inconsistency and interpreted data. L.Y. contributed to data interpretation. P.T. and N.X. drafted the manuscript and all other authors critically reviewed the article.
Conceptualization: Qiang Wei.
Data curation: Ping Tan, Nan Xie, Huan Xu.
Formal analysis: Ping Tan, Nan Xie.
Funding acquisition: Qiang Wei.
Methodology: Ping Tan, Nan Xie, Huan Xu, Liangren Liu.
Resources: Qiang Wei.
Supervision: Haotian Liao, Liqun Zou, Lu Yang, Liangren Liu, Qiang Wei.
Validation: Qiang Wei.
Visualization: Lu Yang, Liangren Liu.
Writing – original draft: Ping Tan, Nan Xie.
Writing – review & editing: Haotian Liao, Liqun Zou, Huan Xu, Lu Yang, Liangren Liu, Qiang Wei.
Supplementary Material
Footnotes
Abbreviations: CKD = chronic kidney disease, CSS = cancer-specific survival, ESAs = erythropoiesis-stimulating agents, ESRD = end-stage renal disease, IVR = intravesical recurrence, MFS = metastasis-free survival, OS = overall survival, RFS = recurrence-free survival, RNU = radical nephroureterectomy, UTUC = upper tract urothelial carcinoma.
PT and NX contributed equally to this work.
Funding/support: This study was funded by the National key research and development program of China (Grant No. SQ2017YFSF090096), the Prostate Cancer Foundation Young Investigator Award 2013, the National Natural Science Foundation of China (Grant No. 81300627, 81370855, 81702536, 81770756), Fundings from Science and Technology Department of Sichuan Province (Grant No. 2014JY0219 and 2017HH0063), and Young Investigator Award of Sichuan University 2017. The funders had no role in study selection, data extraction, analysis or interpretation, writing of this article, or the decision to publish.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
The authors declare that they have no conflict of interest.
Supplemental Digital Content is available for this article.
References
- [1].Roupret M, Babjuk M, Comperat E, et al. European Association of Urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol 2015;68:868–79. [DOI] [PubMed] [Google Scholar]
- [2].Xing YC, Xiong GY, Fang D, et al. [Preoperative prognostic factors and preoperative risk stratification of upper tract urothelial carcinoma]. Beijing Da Xue Xue Bao Yi Xue Ban 2016;48:1032–7. [PubMed] [Google Scholar]
- [3].Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009;115:1224–33. [DOI] [PubMed] [Google Scholar]
- [4].Ploussard G, Xylinas E, Lotan Y, et al. Conditional survival after radical nephroureterectomy for upper tract carcinoma. Eur Urol 2015;67:803–12. [DOI] [PubMed] [Google Scholar]
- [5].Roupret M, Hupertan V, Seisen T, et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol 2013;189:1662–9. [DOI] [PubMed] [Google Scholar]
- [6].Cao J, Zhao X, Zhong Z, et al. Prognostic value of pre-operative renal insufficiency in urothelial carcinoma: a systematic review and meta-analysis. Sci Rep 2016;6:35214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhou L, Cai X, Liu Q, et al. Prognostic role of C-reactive protein in urological cancers: a meta-analysis. Sci Rep 2015;5:12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rink M, Cha EK, Green D, et al. Biomolecular predictors of urothelial cancer behavior and treatment outcomes. Curr Urol Rep 2012;13:122–35. [DOI] [PubMed] [Google Scholar]
- [9].Akdogan B, Dogan HS, Eskicorapci SY, et al. Prognostic significance of bladder tumor history and tumor location in upper tract transitional cell carcinoma. J Urol 2006;176:48–52. [DOI] [PubMed] [Google Scholar]
- [10].Gunay LM, Akdogan B, Koni A, et al. Upper urinary tract transitional cell carcinoma: is there a best? Clin Genitourin Cancer 2013;11:39–44. [DOI] [PubMed] [Google Scholar]
- [11].Milojevic B, Dzamic Z, Kajmakovic B, et al. Prognostic impact of preoperative anemia on urothelial and extraurothelial recurrence in patients with upper tract urothelial carcinoma. Clin Genitourin Cancer 2015;13:485–91. [DOI] [PubMed] [Google Scholar]
- [12].Rink M, Sharifi N, Fritsche HM, et al. Impact of preoperative anemia on oncologic outcomes of upper tract urothelial carcinoma treated with radical nephroureterectomy. J Urol 2014;191:316–22. [DOI] [PubMed] [Google Scholar]
- [13].Yeh HC, Chien TM, Wu WJ, et al. Is preoperative anemia a risk factor for upper tract urothelial carcinoma following radical nephroureterectomy? Urol Oncol 2016;34:337.e1-e9. [DOI] [PubMed] [Google Scholar]
- [14].Morizane S, Iwamoto H, Masago T, et al. Preoperative prognostic factors after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Int Urol Nephrol 2013;45:99–106. [DOI] [PubMed] [Google Scholar]
- [15].Lu YM, Li CC, Wu WJ, et al. Patients’ renal function is important when evaluating preoperative anemia in upper tract urothelial carcinoma. Clin Genitourin Cancer 2016;14:e241–243. [DOI] [PubMed] [Google Scholar]
- [16].WHO Group of Experts on Nutritional Anaemias & World Health Organization. Nutritional anaemias. Report of a WHO group of experts. World Health Organ Tech Rep Ser 1972;503:1–29. [PubMed] [Google Scholar]
- [17].Roupret M, Babjuk M, Comperat E, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol 2018;73:111–22. [DOI] [PubMed] [Google Scholar]
- [18].Rink M, Soave A, Dahlem R, et al. Impact of perioperative allogenic blood transfusion on survival after radical nephroureterectomy for upper tract urothelial carcinoma. Clin Genitourin Cancer 2016;14:96–104. [DOI] [PubMed] [Google Scholar]
- [19].Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc 2005;80:923–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Caro JJ, Salas M, Ward A, et al. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 2001;91:2214–21. [PubMed] [Google Scholar]
- [21].Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol 2012;23:1631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Birgegard G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology 2005;68(suppl 1):3–11. [DOI] [PubMed] [Google Scholar]
- [23].Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004;40:2293–306. [DOI] [PubMed] [Google Scholar]
- [24].Schrijvers D. Management of anemia in cancer patients: transfusions. Oncologist 2011;16(suppl 3):12–8. [DOI] [PubMed] [Google Scholar]
- [25].Tonia T, Mettler A, Robert N, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012;12:Cd003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


