Abstract
Cardiac myxoma (CM) is the most common benign cardiac tumor. We retrospectively reviewed our single center experience in 153 patients with CM over a period 25 years.
From November 1993 to May 2017, 153 patients were operated in our institution with diagnosis of a CM. In all patients preoperative, perioperative, and postoperative data were recorded including the long-term follow-up. All patients followed up in the outpatient's clinics and echocardiography at regular intervals.
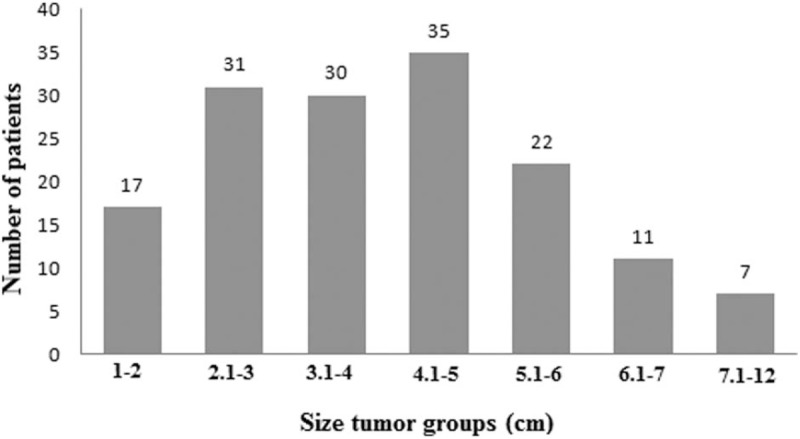
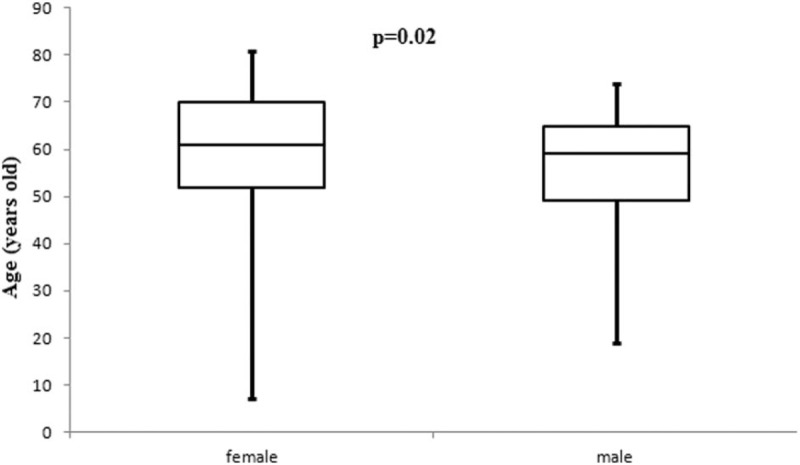
Mean age 59 ± 12 years old. There were 104 women and 49 men. Preoperative clinical manifestations of the patients were hemodynamic consequences (47.7%), asymptomatic (46.4%), systemic embolism (4.5%), systemic manifestations-fever (0.7%), and hemoptysis (0.7%). The most common location of CM was in the left atrium in 82.4% patients. Mean tumors diameter was 4.5 ± 1.9 cm. In addition, we were observed that the age of the patients have differences between sex groups women versus men, 60.3 and 54.8 years old respectively (P = .02). On the other hand the tumor size have not differences between the sex groups (P = .56). Combine operations were performed in 24 (15.7%) patients. New cerebrovascular accident was observed in 2 patients post-op. Mean in-hospital stay was 8.02 ± 2.8 days. In-hospital mortality was 1 patient (0.7%) (from sepsis). During median follow-up 3.7 ± 4.3 years CM recurrence was identified in 5 (3.3%) patients.
Surgical resection of CMs contributes in an excellent prognosis and associated with low complications and recurrences rate. Regular long-term follow-up is recommended in all patients with CM.
Keywords: cardiac myxoma, outcomes, recurrence, surgery, symptoms
1. Introduction
Cardiac myxoma (CM) is the most common tumor accounting for about 50% of the primary cardiac neoplasms. Diagnosis of CM has been established by transthoracic echocardiography (TTE) or transesophageal echocardiography (TEE) with an accuracy approaching 100%. Currently surgical excision of these tumors remains the only successful treatment with the low complications and recurrence rate.
We retrospectively reviewed our single center experience in 153 consecutive patients with CM treatment.
2. Patients and methods
2.1. Study population
From November 1993 to May 2017, 153 consecutive patients were operated in our institution with diagnosis-CM. All patients preoperative underwent TTE, computed tomography (CT) chest scan, and coronary angiography or CT coronary angiography (CT coronary angiography preferably in patients <40 years old and after 2010).
The study is retrospective analysis and it was approved by the institutional review board and hospitals ethic committee (No 466/31-10-2011).
2.2. Surgical technique
CM approach and excision was depending from the tumor location. Standard sternotomy and cardiopulmonary bypass (CPB) were established in all patients. In addition, superior and inferior vena cava cannulation and cold blood cardioplegic heart arrest was applied in all cases. In cases with other cardiac pathologies, cardiac myxoma was resected firstly. After 2005, the patients who underwent of CM resection perioperative TEE had used in all cases.
2.3. Data presentation and follow-up methods
Continuous variables are expressed as mean ± standard deviation (SD). Categorical variables are shown as counts (n) and percentages (%). Comparisons of means were conducted with both parametric (t test) and nonparametric methods (sign, Wilcoxon) when appropriate. Comparisons of continuous variables between levels of categorical variables were performed using Student t test or analysis of cariance (ANOVA) for the normally distributed or Mann–Whitney U test for the rest of the variables. Subsequently, logistic regression analysis was applied to evaluate the association between baseline predictors and the likelihood of developing worse postoperative outcomes such as the postoperative cerebrovascular accident (CVA), in-hospital mortality, and recurrence. P-value <.05 was considered statistically significant. SPSS (SPSS Inc., Chicago, IL) version 18.0 software was used for all the analyses
All patients’ preoperative, perioperative, and postoperative characteristics were recorded including the long-term follow-up. All patients followed up in the outpatient's clinics and echocardiography at regular intervals (first and sixth months and thereafter annually).
3. Results
All preoperative characteristics of the patients were recorded and mean age was 59 ± 12 years old. Figure 1 presents the age groups with the most frequent CM appearances and it was, between 51 and 70 years old, 56.9% (87/153 patients). Study population was consisted from 49 men and 104 women. The most common preoperative clinical presentation was hemodynamic consequences in 47.7% patients. Furthermore the most frequent symptom due the hemodynamic consequences was dyspnoe in 32.7%. Other preoperative data such as a maximal diameter of tumors, body surface area (BSA), locations and symptoms of tumor present in Table 1. In addition, the tumor size between 2.1 and 5 cm was represented in 96 (62.7%) patients (Fig. 2). In addition, we were observed that patients age have differences between sex groups (women vs men, P = .02) (Fig. 3) and on the other hand the tumor size have not differences between sex groups (P = .56) (Fig. 4).
Figure 1.
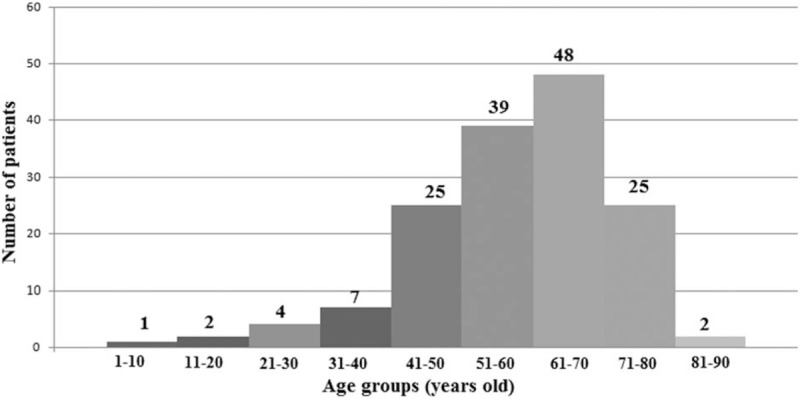
Age groups of the patients with cardiac myxoma.
Table 1.
Preoperative details.
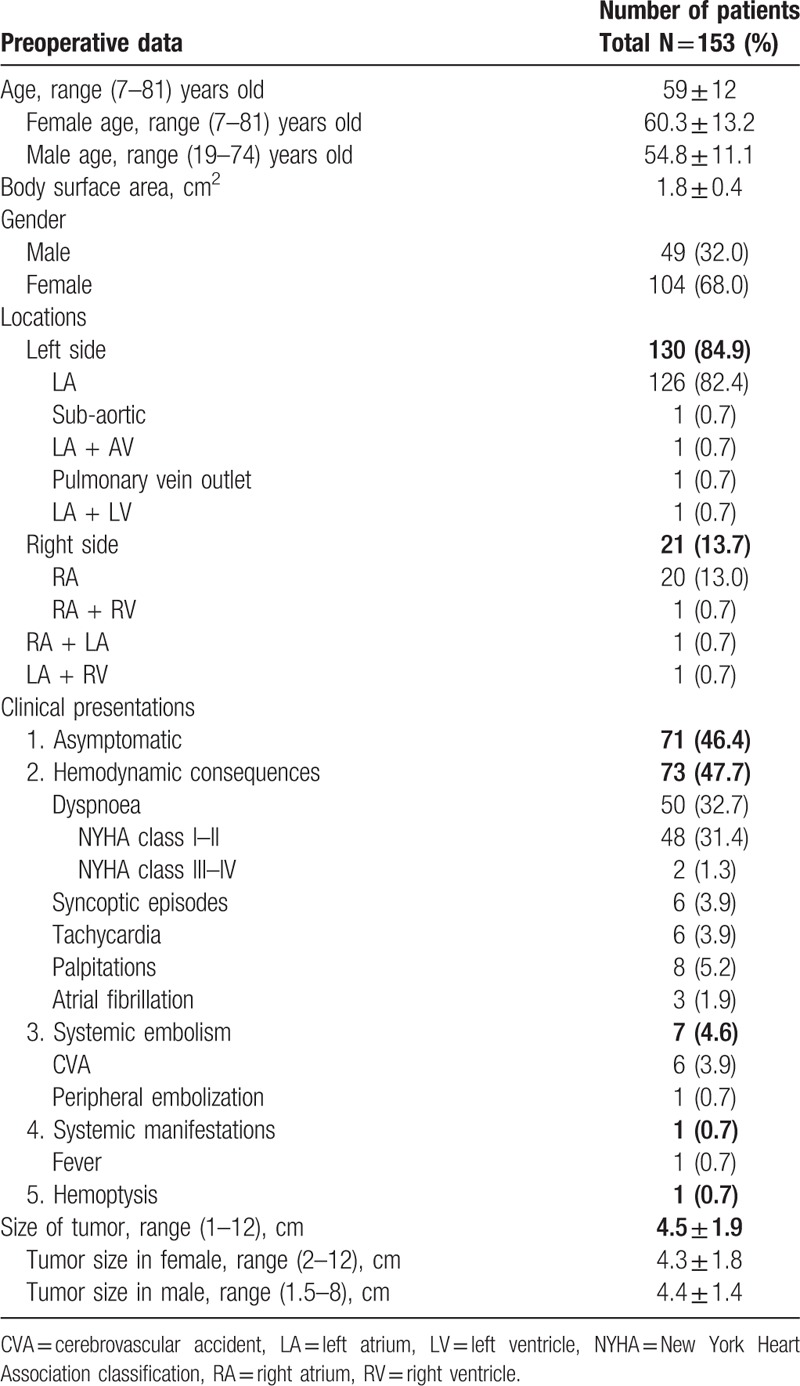
Figure 2.
Tumors size groups of the patients with cardiac myxoma.
Figure 3.
Patients age have differences between sex groups (P = .02).
Figure 4.
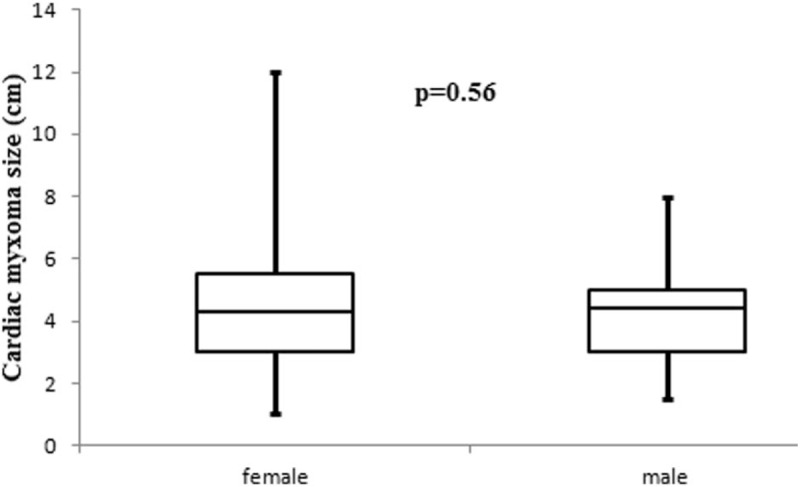
Tumor size have not differences between sex groups (P = .56).
Surgical excision of tumor was achieved with biatrial, left or right atrial, right ventricule, or aortic approach. The most common approach was biatrial for inspection of all cardiac chambers. Mean CPB time, mean cross clamp (XC) time, and concomitant procedures which were performed shown in Table 2.
Table 2.
Postoperative and follow-up details.
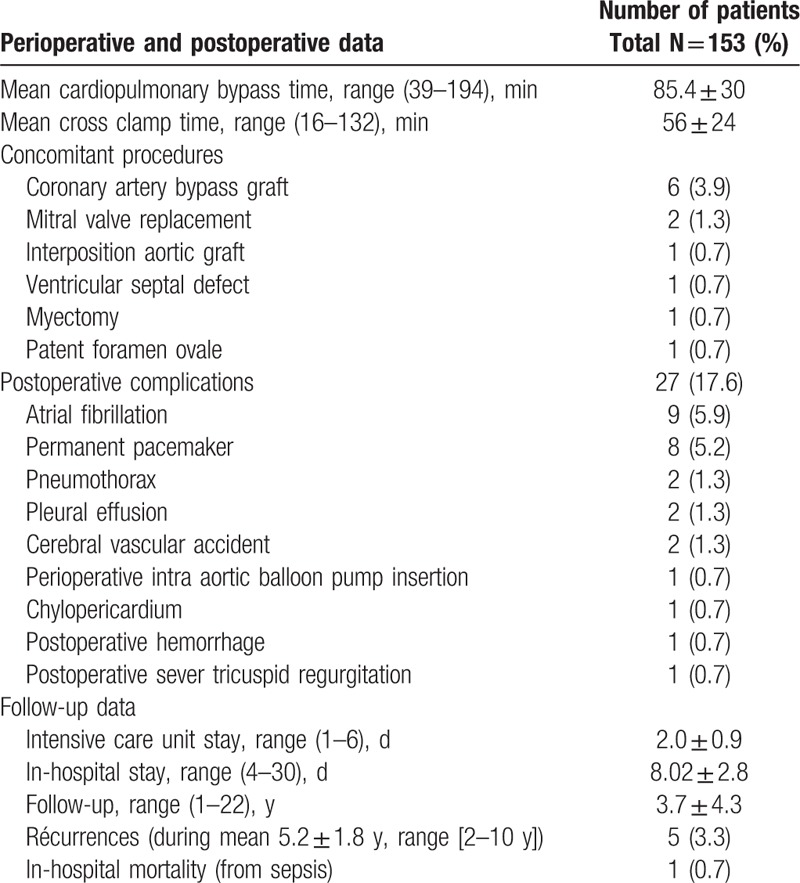
Furthermore all postoperative complications and postoperative follow up data (intensive care unit [ICU] stay, in-hospital stay, recurrence and mean follow up) present in Table 2. The most common complications after operation were atrial fibrillation (AF) and the overall postoperative complications were observed in 27 (17.6%) patients and it shows that a CM resection in our study associated with low postoperative complications rate. In all patients tumors’ pathological diagnosis after resection was primary CM. In-hospital mortality was 1 (0.7%) patient from sepsis. Complete follow-up was achieved in all patients. During mean follow-up 3.7 ± 4.3 years CM recurrence was revealed (by TTE and TEE) in 5 (3.3%) patients (mean time to recurrence 5.2 ± 1.8 years, range [2–10 years]). Mean recurrence CM size was 4.56 ± 1.5 cm with range between 2 and 5.5 cm. Recurrence location was same with original location (left atrium) in 4 patients and the time from the first operation to recurrence in these patients was 2, 3, 3, and 10 years. Furthermore, in one patient (man) was observed 4 time recurrences (familial type CM) after first operation (3, 6, 7, and 9 years). In this case the first CM origin was in the right ventricle (closed intra-ventricle septum) and a recurrence location was different from the first origin: right atrium, right ventricle (closed to pulmonary valve), left atrium, and left ventricule. When fourth recurrence was revealed the patient declined to fifth surgical intervention.
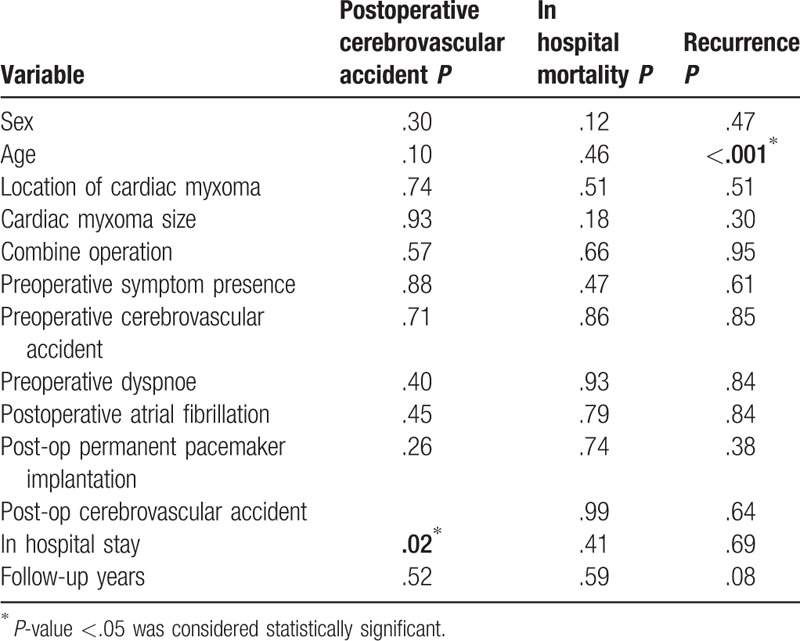
Subsequently regression analysis was evaluated the association between variables and the likelihood of developing worse prognosis such as postoperative CVA, in-hospital mortality, and CM recurrence. Analysis shows that only predictor factors associated with worse prognosis for postoperative CVA was in-hospital stay (P = .02) and for CM recurrence was patient age (P < .001) (Table 3).
Table 3.
Logistic regression analysis evaluates the association between baseline predictors and the likelihood of developing postoperative cerebrovascular accident, in-hospital mortality, and cardiac myxoma recurrence.
4. Discussion
CM is a rare cardiac disease with an overall incidence of about 0.5/million/y and accounts for approximately 70% of all cardiac tumors.[1] Locations of tumor in the general population are: 75% in the left atrium (LA), 23% in the right atrium (RA), and only 2% in the ventricles.[2–4] The most common site of attachment is the fossa ovalis. Other rare arising of CM are the hearts valves.[5,6] Multiple locations present in 50% in familial forms.[7]
The clinical signs, manifestations, and symptoms produced are non-specific and determined by the location, size, and mobility of tumor. There are 3 main patterns of clinical presentation for patients with CM: hemodynamic consequences (dyspnoea, syncoptic episodes, arrhythmia, palpitations, congestive heart failure, and sudden death), systemic embolism (embolization in the peripheral arterial tree and transient ischemic attack [TIA] or cerebral vascular accident [CVA]), and constitutional or systemic manifestations (fever, weight loss, arthralgia, and fatigue). In our study one patient had presented hemoptysis (as a first symptom of tumor) which was not due to the mitral valve obstruction or congestive heart failure. On the other hand we had only 1 patient with systemic manifestations (fever) which retreated 1 month after operation.
Hemodynamic consequences may be intermittent and this is associated with changes in position of the patient because of the tumor floating or prolapsing through mitral or tricuspid valve into the right or left ventricule. Younger and male patients have more specific neurologic symptoms, while female patients have more systemic symptoms.[8] Patients with myxoma have neurological complications in 20% to 25% cases and systemic embolism is not related to the size of myxoma, but it depends from friability and mobility of the tumor.[9,10] Temporary or permanent neurological defects arise when the myxoma gets infected and the friable tumor tissue embolizes into systemic circulation. Rare complication with the abdominal aorta occlusion from friable CM was described in the literature.[11–14] In addition upper and lower extremities embolization was described in other study.[15] The incidence of these complications is small, but they are affected the quality of life in the patients with this tumor. On the other hand, Wang et al[16] in the recent study and after clinicopathologic analysis of the 61 patients were concluded that pathological profiles of cardiac myxoma not related with the clinical presentation. Fever, malaise, and weight loss are the most symptoms due in the possible elaboration of the cytokine interleukin-6 (IL-6).[17]
Sometimes CM is described in the context of Carney syndrome or complex, in which cardiac myxoma, cutaneous lesions, endocrine disorders, testicular, thyroid, and hypophysis tumors. Carney complex is an autosomal dominant condition. Molecular genetic studies show that mutations in the PRKAR1A gene, encoding the R1a regulatory subunit of cyclic-AMP-dependent protein kinase A (PKA) cause inherited myxomas in setting of the Carney complex tumor syndrome. Further genetic studies in human beings have highlighted novel variant phenotypes, such as congenital contractures, which potentially associated with Carney complex, and have identified alternative genetic pathways to cardiac tumorgenesis including mutation of the MYH8 gene that encodes perinatal myosin.[18]
Screening for occult myxoma and follow-up after surgical excision of CM should involve medical history, physical examination, and TTE and/or TEE. TTE have approximately 95% sensitivity for detection of the cardiac myxomas while TEE has 100% sensitivity.[19,20] CT and magnetic resonance imaging (MRI) may also be helpful. Coronary angiography or CT coronary angiography in patients over 40 years of age is generally required to rule out concomitant coronary artery disease. Surgical excision of the tumor should be performed after diagnosis because of risk of sudden death and neurological complications is high.
When diagnosis has been established the treatment of choice for CM is surgical resection and in most cases it is curative. The median sternotomy, cardiopulmonary bypass, aorta, and bicaval cannulation with or without cardioplegic heart arrest is the standard approach to resection of these tumor. Novel minimally invasive surgical techniques such as video-assisted or totally robotic resection were described in the literature.[21–24] Surgical approach (atrial or ventricular or aortic) depends from location and size of the mass. While the access of the CM may be different in most cases, however the general rule to resection of these tumor should be remain. Manipulations of the tumor during resection must be minimizing to prevention peripheral embolization. Complete excision of the mass with the attachment area in the cardiac structures (atrial septum, ventricular septum, atrium or ventricles free wall, and cardiac valves) should be performed. The atrial septal defect and free wall of the atrium repaired with autologous pericardium or synthetic patch. Ventricular septal defect and free wall of the ventricule closed with prosthetic patch. If the CM invaded cardiac valves then valve repair (if possible) or replacement should be performed. Concomitant procedures such as a coronary bypass grafting, valve replacement, and other cardiac pathology must be making during operation and after CM excision.
Recurrence may occur within a few months to several years after the initial surgical excision.[20] The recurrent frequency of myxoma is about 1% to 3% in sporadic forms, 12% in familial forms, and 22% in complex forms. Regular follow-up with clinical examinations and TTE study is recommended in patients with familial CM because of these patients have a significantly higher risk of recurrence.[25] Causes of multiple recurrences include familial forms of the tumor, incomplete excision, intra-cardiac implantation from the original tumor, and malignant transformation. Genetic screening of patients with recurrent cardiac myxomas might help to identify patients at risk for additional recurrence.
5. Conclusion
Surgical resection of cardiac myxomas contributes in an excellent prognosis and associated with low complications and recurrences rate. Regular long-term follow-up is recommended in all patients with cardiac myxoma particularly in patients with familial form of CM. Familial CM affects the patient's treatment, follow-up, and his family screening (minimum) with TTE.
Author contributions
Conceptualization: George Samanidis.
Data curation: George Samanidis.
Investigation: George Samanidis, Mazen Khoury, George Stavridis, Konstantinos Perreas.
Methodology: George Samanidis.
Supervision: Andreas Karabinis.
Writing – original draft: George Samanidis.
Writing – review & editing: George Samanidis.
Footnotes
Abbreviations: AF = atrial fibrillation, ANOVA = analysis of variance, BSA = body surface area, CM = cardiac myxoma, CPB = cardiopulmonary bypass, CT = computed tomography, CVA = cerebrovascular accident, ICU = intensive care unit, IL-6 = Interleukin-6, LA = left atrium, MRI = magnetic resonance imaging, PKA = protein kinase A, RA = right atrium, SD = standard deviation, TEE = transesophageal echocardiography, TIA = transient ischemic attack, TTE = transthoracic echocardiography, XC = cross clamp.
The authors have no conflict of interest to declare.
References
- [1].Reynen K. Cardiac myxomas. N Engl J Med 1995;333:1610–917. [DOI] [PubMed] [Google Scholar]
- [2].Markel ML, Walker BF, Armstrong WE. Cardiac myxoma: a review. Medicine (Baltimore) 1987;66:114–25. [DOI] [PubMed] [Google Scholar]
- [3].Mkalaluh S, Szczechowicz M, Torabi S, et al. Surgical treatment of cardiac tumors: insights from an 18-year single-center analysis. Med Sci Monit 2017;23:6201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Garcia-Carretero R, Vela BB, Martínez-Quesada G, et al. Demographic and clinical features of atrial myxomas: a case series analysis. Acute Card Care 2016;18:65–9. [DOI] [PubMed] [Google Scholar]
- [5].Fernández AL, Vega M, El-Diasty MM, et al. Myxoma of the aortic valve. Interact Cardiovasc Thorac Surg 2012;15:560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yoon JH, Kim JH, Sung YJ, et al. Cardiac myxoma originating from the anterior mitral valve leaflet. J Cardiovasc Ultrasound 2011;19:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. Medicine (Baltimore) 2001;80:159–72. [DOI] [PubMed] [Google Scholar]
- [8].Ekinci EJ, Donnan GA. Neurological manifestations of cardiac: a review of the literature and report of cases. Intern Med J 2004;34:243–9. [DOI] [PubMed] [Google Scholar]
- [9].Hirose H, Youdelman B, Entwistle JW. Stroke from a large left atrial myxoma. Open Cardiovasc Med J 2008;2:115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang CY, Chang YY, Hsieh MY, et al. Atrial myxoma presenting as total occlusion of the abdominal aorta and its major four branches. J Chin Med Assoc 2012;75:349–52. [DOI] [PubMed] [Google Scholar]
- [11].Hong S, Park KT, Choe H. Total occlusion of the abdominal aorta caused by detachment of cardiac myxoma. Korean J Thorac Cardiovasc Surg 2012;45:183–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fang BR, Chang CP, Cheng CW, et al. Total detachment of cardiac myxoma causing saddle embolization and mimicking aortic dissection. Jpn Heart J 2004;45:359–63. [DOI] [PubMed] [Google Scholar]
- [13].Veroux P, Mignosa C, Veroux M, et al. Acute occlusion of abdominal aorta: unusual embolization site for a cardiac tumor mass. Tumori 2002;88:417–9. [DOI] [PubMed] [Google Scholar]
- [14].Horn KD, Becich MJ, Rhee RY, et al. Left atrial myxoma with embolization presenting as an acute infrarenal aortic occlusion. J Vasc Surg 1997;26:341–5. [DOI] [PubMed] [Google Scholar]
- [15].Abu Abeeleh M, Saleh S, Alhaddad E, et al. Cardiac myxoma: clinical characteristics, surgical intervention, intra-operative challenges and outcome. Perfusion 2017;32:686–90. [DOI] [PubMed] [Google Scholar]
- [16].Wang JG, Li YJ, Liu H, et al. Clinicopathologic analysis of cardiac myxomas: seven years’ experience with 61 patients. J Thorac Dis 2012;4:272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yokomuro H, Yoshihara K, Watanabe Y, et al. The variations in the immunologic features and interleukin-6 levels for the surgical treatment of cardiac myxomas. Surg Today 2007;37:750–3. [DOI] [PubMed] [Google Scholar]
- [18].Wilkes D, McDermott DA, Basson CT. Clinical phenotypes and molecular genetics mechanisms of Carney complex. Lancet Oncol 2005;6:501–8. [DOI] [PubMed] [Google Scholar]
- [19].Percell RL, Jr, Henning RJ, Siddique Patel M. Atrial myxoma: case report and a review of the literature. Heart Dis 2003;5:224–30. [DOI] [PubMed] [Google Scholar]
- [20].Vohra HA, Vohra H, Patel RL. Cardiac myxoma with three recurrences. J R Soc Med 2002;95:252–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gao C, Yang M, Wang G, et al. Totally robotic resection of myxoma and atrial septal defect repair. Interact Cardiovasc Thorac Surg 2008;7:947–50. [DOI] [PubMed] [Google Scholar]
- [22].Vistarini N, Alloni A, Aiello M, et al. Minimally invasive video-assisted approach for left atrial myxoma resection. Interact Cardiovasc Thorac Surg 2010;10:9–11. [DOI] [PubMed] [Google Scholar]
- [23].Gao C, Yang M, Wang G, et al. Excision of atrial myxoma using robotic technology. J Thorac Cardiovasc Surg 2010;139:1282–5. [DOI] [PubMed] [Google Scholar]
- [24].Panos A, Myers PO. Video-assisted cardiac myxoma resection: basket technique for complete and safe removal from the heart. Ann Thorac Surg 2012;93:e109–10. [DOI] [PubMed] [Google Scholar]
- [25].Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab 2001;86:4041–6. [DOI] [PubMed] [Google Scholar]


