Abstract
Background:
The continuous development of drug-resistant tuberculosis in recent years has brought new attention to tuberculosis. linezolid is usually used to treat infection in patients with pulmonary tuberculosis and pneumonia, for it has good effects on Mycobacterium tuberculosis, and has strong antibacterial activity on the drug-resistant strain. This study aims to investigate the effects of linezolid on serum procalcitonin (PCT), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) in patients with pulmonary tuberculosis and pneumonia.
Methods:
Forty patients with pulmonary tuberculosis and pneumonia were divided into 2 groups: observation group (n = 20), patients treated with linezolid; control group (n = 20), patients treated with moxifloxacin. At 14 days, one month and 3 months of treatment, changes in serum PCT, ESR, CRP, and bacterial eradication rate (negative conversion rate) were compared between the 2 groups, and the incidence of adverse reactions was compared.
Results:
Serum PCT, ESR, and CRP in the 2 groups were significantly lower after 14 days of treatment than before treatment (P < .05), the decrease was more significant in the observation group, and the differences in ESR and CRP were statistically significant (t = 2.199, 2.494, P < .05). Furthermore, the negative conversion rate was higher in the observation group, but the difference was not statistically significant (P > .05). At one month of treatment, serum PCT, ESR, and CRP were lower in the observation group, and the difference in CRP was statistically significant (t = 3.274, P < .05). Furthermore, the negative conversion rate was slightly higher in the observation group, but the difference was not statistically significant (P > .05). At 3 months of treatment, differences in PCT, ESR, and CRP were not statistically significant, and the negative conversion rate was the same between the 2 groups. Furthermore, the incidence of adverse reactions was higher in the observation group, but all were mild, and the differences between these 2 groups were not statistically significant (P > .05).
Conclusion:
In the treatment of tuberculosis and pneumonia, linezolid can improve serum PCT, ESR, and CRP levels, and eradicate bacteria. However, adverse reactions should be strictly monitored to ensure its safety.
Keywords: antimicrobial resistance, linezolid, moxifloxacin, pneumonia, pulmonary tuberculosis
1. Introduction
Since the 1980s, the number of global tuberculosis patients has increased, and the mortality of tuberculosis has also deteriorated. One of the main causes of this phenomenon is the dual infection of human immunodeficiency virus (HIV) and tubercule bacillus.[1–4] Another important reason is the emergence and spread of multidrug-resistant tuberculosis (MDR-TB).[5–7] The pulmonary tuberculosis of patients are easily secondary to pulmonary infection due to structural changes in the lungs and the inhibition of its cellular immune functions induced by long-term medication, which worsens the condition and even endangers the patient's life. At the same time, it is difficult for common antibacterial therapies to play an effective and timely role in treating tuberculosis (especially MDR-TB) combined with severe pneumonia. Linezolid is an oxazolidinone involved in antibiotic synthesis. In 2000, it was approved by the United States Food and Drug Administration (FDA) for infections caused by aerobic gram-positive bacteria.[8] In particular, it has played a major role in the treatment of drug-resistant bacterial infections. It was also reported that linezolid could be used for the treatment of MDR-TB, and good results could be achieved.[9–11] In the present study, linezolid was used in the treatment of patients with pulmonary tuberculosis and pneumonia, and its clinical value was explored through changes in serum procalcitonin (PCT), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). The details are reported as follows.
2. Data and methods
2.1. General information
Forty patients with pulmonary tuberculosis and severe pneumonia, who were treated in our hospital from March 2014 to March 2016, were enrolled into the present study. All patients met the Chinese Diagnostic Criteria for Tuberculosis (WS288-2008), and had received first-line therapy treatment such as isoniazid and rifampicin. However, the effects were not good or the condition was repeated. In addition, these patients met the Diagnostic Criteria of Severe Pneumonia (2001) developed by the American Thoracic Society, with an acute physiology and chronic health evaluation II (APACHE II) score of ≥30 points. Patients with severe liver, renal insufficiency, or AIDS were excluded. These patients were divided into 2 groups according to the sequence number. The observation group (n = 20) comprised of 14 male patients and 6 female patients. The age of these patients ranged within 16 to 72 years old, with an average age of 55.39 ± 17.24 years old. The course of tuberculosis was 2 to 18 months, with an average of 11.01 ± 2.740 months. The course of complicated severe pneumonia was 4 to 40 days, with an average of 14.65 ± 2.321 days. The control group (n = 20) comprised of 15 male patients and 5 female patients. The age of these patients ranged within 13 to 76 years old, with an average of 54.54 ± 18.11 years old. The course of tuberculosis was 4 to 19 months, with an average of 11.50 ± 2.663 months. The course of complicated severe pneumonia was 3 to 50 days, with an average of 12.98 ± 2.932 days. Differences in male-to-female ratio, age, the course of tuberculosis, and severe pneumonia between the 2 groups were not statistically significant (P > .05). Therefore, these 2 groups of patients were comparable. All subjects enrolled into the present study provided a signed informed consent. This study was approved by the Ethics Committee of our hospital.
2.2. Therapeutic methods
After the 2 groups of patients were enrolled, sputum samples were obtained from the deep pharynx of all patients before treatment. The treatment began when the infection bacterial strains were determined to be linezolid- or moxifloxacin-sensitive strains by bacterial identification and drug sensitivity test. The 2 groups of patients were treated with routine regimens, including symptomatic oxygen inhalation, cough-relieving and expectorant. Nutritional support was timely given through fluid replacement. On this basis, patients in the treatment group were treated with 600 mg of linezolid (commodity name: Zyvox, Pfizer, New York) by intravenous drip, bid, and the course of treatment was 14 days. Then, the dose was adjusted to 600 mg, qd, for 3 months.[12] Patients in the control group were treated with 400 mg of moxifloxacin (commodity name: Avelox, Bayer, Leverkusen, Germany) by intravenous drip, qd, for 3 months.[13] The treatment effects were evaluated after 14 days, 1 month and 3 months of treatment, respectively.
2.3. Observation indexes
Laboratory assessment: Around 5 mL of fasting venous blood was drawn from all the enrolled patients before the treatment and after 14 days, one month and 3 months of treatment. The blood was centrifuged and the serum was obtained. A fluorescent immunoanalyzer (VIDAS, bioMerieux, shanghai, China) and PCT kit were used to detect serum PCT by electrochemiluminescence (ECL). ESR was detected using an ESR meter Monitor-100 (Vital). An automatic biochemical analyzer (7180, Hitachi, Tokyo, Japan) was used to detect CRP by particle-enhanced immunoturbidimetry. Changes in these inflammatory markers were recorded and compared before and after the treatment.
Etiological assessment: Before the treatment and after 14 days, 1 month and 3 months of treatment, sputum samples were obtained from the deep pharynx by natural expectoration method or using a disposable sterile suction catheter. Then, the identification of bacteria and drug sensitivity tests were conducted. These results were determined according to the National Committee for Clinical Laboratory Standards 2002 (NCCLS 2002), and classified into 5 levels: eradicated, tentative eradicated, uneradicated, replacement, and reinfection. Eradicated and tentative eradicated levels were regarded as negative conversion. The negative conversion rate was calculated.
Comparison of adverse reactions: Patients were closely monitored for adverse reactions during the routine treatment. Routine blood test, and liver and kidney function tests were performed after 14 days, 1 month and 3 months of treatment.
2.4. Statistics analysis
Normally distributed measurement data were expressed as mean ± standard deviation (x ± SD), and were compared between the 2 groups by independent sample t-test. Count data such as therapeutic effect and adverse reactions were expressed in percentage, and evaluated using X2-test. P < .05 was considered statistically significant.
3. Results
At 3 days of treatment, 1 patient in the control group died of massive hemoptysis. Therefore, in the following comparisons, the observation group had a sample size of 20 (n = 20), and the control group had a sample size of 19 (n = 19). After 50 days of treatment, optic neuritis was found in 1 patient in the observation group. After treatment, a good result was not achieved, and the treatment was terminated. Therefore, after 3 months of treatment, in the comparison of the negative conversion rate, the observation group had a sample size of 19 (n = 19) and the control group had a sample size of 19 (n = 19).
3.1. Comparisons of serum PCT, ESR, and CRP before and after treatment between the 2 groups
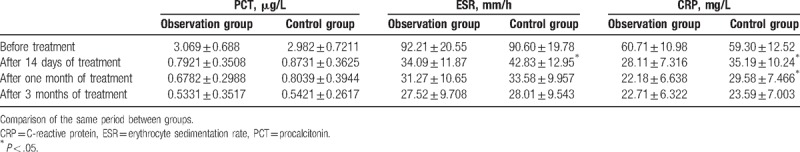
The differences in serum PCT, ESR, and CRP between these 2 groups before treatment were not statistically significant (P > .05). Furthermore, the indexes in these 2 groups were significantly lower after 14 days of treatment than before treatment (P < .05). Furthermore, the decrease was more significant in the observation group, in which the differences in ESR and CRP were statistically significant (t = 2.199, 2.494, P < .05). After 1 month of treatment, the decrease in indexes were more significant in the observation group, and the difference in CRP was statistically significant (t = 3.274, P < .05). After 3 months of treatment, differences between the 2 groups were not statistically significant (P > .05). The details are presented in Table 1.
Table 1.
Comparisons of serum PCT, ESR, and CRP before and after treatment between the 2 groups (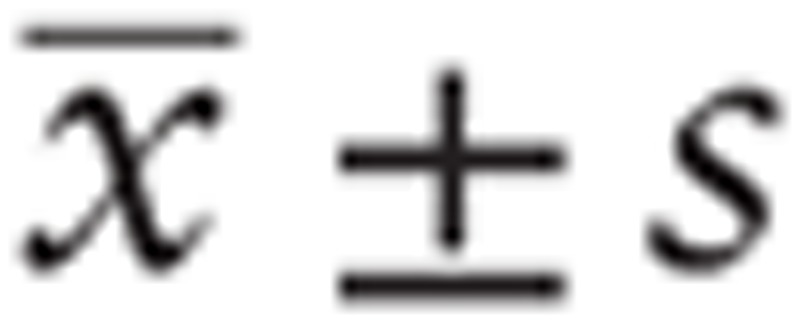 ).
).
3.2. Comparison of negative conversion rates after treatment between the 2 groups
After 14 days of treatment, the negative conversion rate was slightly higher in the observation group, but the difference was not statistically significant (P > .05). After 1 month of treatment, the negative conversion rate was basically the same between the 2 groups. After 3 months of treatment, the negative conversion rate was the same between the 2 groups (Table 2).
Table 2.
Comparison of negative conversion rates after treatment between the 2 groups.

3.3. Comparison of adverse reactions between the 2 groups
Adverse reactions were found in 7 patients in the observation group, and the total incidence was 35.00%. Among these, gastrointestinal reaction was found in 4 patients, anemia was found in 1 patient, thrombocytopenia was found in 2 patients, and optic neuritis was found in 1 patient. The first 6 patients had mild symptoms (or was only abnormal in the laboratory indexes, have no symptom). These patients improved after symptomatic treatment, and the continuous medication was not affected. Furthermore, optic neuritis was found after 50 days of treatment, which did not improve after glucocorticoid and vitamin B6 treatment. Therefore, the drugs were stopped. Adverse reactions were found in 4 patients in the control group, and the incidence was 21.05%. Among these, hyperhidrosis at night was found in 2 patients, transient blood pressure elevation was found in 1 patient who had no previous history of hypertension, and blood sugar reduction was found in one patient with diabetes. All these symptoms were mild, and spontaneously relieved without treatment. The incidence of adverse reactions was higher in the observation group than in the control group, but the difference was not statistically significant (P > .05).
4. Discussion
The continuous development of drug-resistant tuberculosis in recent years has brought new attention to tuberculosis, which is a disease with a long history. MDR-TB and extensively resistant tuberculosis (XDR-TB) caused by the abuse of antibiotics and other reasons increased the mortality of tuberculosis. Thus, a novel antibiotic is urgently needed to effectively treat this kind of tuberculosis.[5–7,14] Linezolid is an oxazolidinone synthetic antibiotic, and its antibacterial mechanism is to inhibit the synthesis of bacterial protein. Linezolid selectively acts on the 50S subunit ribosome, and works in the initial stage of translation and synthesis of protein. Due to its unique target and mechanism of action, it is not easy to develop the cross-resistance of linezolid with other antimicrobial drugs (including other antimicrobial drugs that inhibit the synthesis of bacterial proteins).[15,16] After being approved on the market, the drug was mainly used to control infection caused by gram-positive bacteria resistant to vancomycin. Recent studies have revealed that linezolid also has good effects on Mycobacterium tuberculosis, and has strong antibacterial activity on the drug-resistant strain.[8,11,17] In the present study, the moxifloxacin used in the control group is the fourth generation of quinolone antibiotics, which has higher activity, more extensive antimicrobial spectrum and higher bioavailability, when compared with the existing quinolones, which is also a very promising drug for MDR-TB treatment, at present.[13,17] Its antibacterial mechanism is the interference of topoisomerase II/IV and the inhibition of the synthesis and transcription of bacterial DNA.
Patients with pulmonary tuberculosis often impair their cellular immune function due to the long-term use of antituberculosis drugs. Furthermore, tuberculosis can also cause pulmonary structural lesions, together with some invasive diagnosis and treatment techniques, and tuberculosis patients have a greater probability to develop secondary pulmonary infections. The symptoms of tuberculosis itself are similar to those of pulmonary infections caused by other pathogens. However, early infections are difficult to determine, and it is often confirmed and identified after a period of time until the condition becomes severer, delaying treatment or even endangering the life of the patient. The present blood markers for tuberculosis and infection monitoring are mainly white blood cell count (WBC), and serum PCT, ESR, and CRP, which were used in the present study. There are certain correlations among these indexes.[18,19] WBC and ESR are often used in the diagnosis of tuberculosis. However, these indicators fluctuate to a certain extent, and do not have the significance of independent diagnosis for patients with pneumonia. Hence, these needs to be combined to improve its diagnostic value for tuberculosis complicated with infective inflammation.
Based on the results of the present study, patients in the observation group and control group achieved significant effects after 14 days of treatment, PCT, ESR, and CRP significantly decreased, and the negative conversion rate of bacterial infection increased. Regarding the degree of changes in some indexes, it was revealed that the manifestation of linezolid used in the observation group was better than that of moxifloxacin used in the control group. As the treatment was further carried out, after 1 month and 3 months of treatment, the differences between these 2 groups were not significant. These above results reveal to a certain extent that both linezolid and moxifloxacin had ideal effects in the treatment of drug-resistant tuberculosis complicated with severe pneumonia, and had equal long-term curative effects, in which linezolid worked more rapidly.[20–23] From the angle of adverse reactions, the adverse reactions of linezolid observed in the present study were basically consistent with those in other studies,[21,24] such as gastrointestinal reaction, myelosuppression (thrombocytopenia), anemia, and peripheral neuritis (optic neuritis). Most of which were milder or only manifested as laboratory results without symptoms. Compared to this, the incidence of adverse reactions of moxifloxacin was lower and milder.[13,25] Compared with linezolid, the price of moxifloxacin is lower, and its economic burden on patients is lighter. From this angle, moxifloxacin also has certain clinical advantages.
In summary, in the treatment of tuberculosis complicated with pneumonia, linezolid can improve serum PCT, ESR, and CRP levels, and eradicate bacteria. However, adverse reactions should be strictly monitored to ensure its safety. Furthermore, it is noteworthy that the present study was conducted under the guidance of the results of bacterial identification and the drug sensitivity test. That is, these patients had drug-resistant Tubercle Bacillus infection complicated with severe pneumonia. This suggests that in the treatment of patients with tuberculosis complicated with bacterial pneumonia, the pathogenic bacteria should be determined as clearly as possible, and the antibiotics should be chosen rationally according to the results of the drug sensitivity test. “broad-spectrum” and “high activity” novel antibiotics should not be blindly pursued. Thus, the occurrence of more serious bacterial drug resistance caused by the abuse of antibiotics is avoided.
Author contributions
Conceptualization: Ruidong Ding.
Data curation: Hongjun Zhang.
Formal analysis: Hongjun Zhang.
Investigation: Ruidong Ding.
Methodology: Hongjun Zhang.
Project administration: Ruidong Ding.
Resources: Ruidong Ding.
Software: Hongjun Zhang.
Supervision: Ruidong Ding.
Validation: Ruidong Ding.
Writing – original draft: Ruidong Ding.
Writing – review & editing: Ruidong Ding.
Footnotes
Abbreviations: APACHE II = acute physiology and chronic health evaluation II, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, FDA = Food and Drug Administration, HIV = human immunodeficiency virus, MDR-TB = multidrug-resistant tuberculosis, NCCLS 2002 = National Committee for Clinical Laboratory Standards 2002, PCT = procalcitonin, XDR-TB = extensively resistant tuberculosis.
The authors have no conflicts of interest to disclose.
References
- [1].Sinha S, Gupta K, Tripathy S, et al. Nevirapine-versus Efavirenz-based antiretroviral therapy regimens in antiretroviral-naive patients with HIV and tuberculosis infections in India: a multi-centre study. BMC Infect Dis 2017;17:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Turaka VP, Varghese GM. Risk Factors for development of active tuberculosis (TB) in HIV infected individuals. J Assoc Physicians India 2016;64:112. [Google Scholar]
- [3].Henostroza G, Harris JB, Chitambi R, et al. High prevalence of tuberculosis in newly enrolled HIV patients in Zambia: need for enhanced screening approach. Int J Tuberc Lung Dis 2016;20:1033–9. [DOI] [PubMed] [Google Scholar]
- [4].Pawlowski A, Jansson M, Sköld M, et al. Tuberculosis and HIV co-infection. Plos Pathogens 2013;34:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burki T. Multidrug resistant tuberculosis: a continuing crisis. Lancet Infect Dis 2016;16:1337–8. [DOI] [PubMed] [Google Scholar]
- [6].Kumar K, Abubakar I. Clinical implications of the global multidrug-resistant tuberculosis epidemic. Clin Med (Lond) 2016;16:565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Glaziou P, Falzon D, Floyd K, et al. Global epidemiology of tuberculosis. Tuberculosis 2014;367:938–40. [Google Scholar]
- [8].Li Y, Lv Y, Xue F, et al. Antimicrobial susceptibility surveillance of gram - positive bacterial from Ministry of Health National Antimicrobial Resistant Investigation Net (Mohnarin) 2011–2012. Chin J Clin Pharmacol 2014;30:251–9. [Google Scholar]
- [9].Yi L, Yoshiyama T, Okumura M, et al. Linezolid as a potentially effective drug for the treatment of multidrug-resistant tuberculosis in Japan. Jpn J Infect Dis 2017;70:96–9. [DOI] [PubMed] [Google Scholar]
- [10].Santin M. Linezolid for multidrug-resistant tuberculosis: how should we approach it? Enferm Infecc Microbiol Clin 2016;34:83–4. [DOI] [PubMed] [Google Scholar]
- [11].Zhang XF, Zhang FH, Zhang Y, et al. In vitro bactericidal assay of linezolid on multidrug-resistant tuberculosis and nontuberculous mycobacteria. Chin J Lab Med 2014;37:535–8. [Google Scholar]
- [12].Nie WJ, Chu NH. Progress of linezolid in the treatment of drug resistant tuberculosis. Chin J Tubere Respir Dis 2013;36:601–3. [PubMed] [Google Scholar]
- [13].Gillespie SH, Crook AM, McHugh TD, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014;371:1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rodrigues M, Brito M, Villar M, et al. Treatment of multidrug-resistant and extensively drug-resistant tuberculosis in adolescent patients. Pediatr Infect Dis J 2014;33:657–9. [DOI] [PubMed] [Google Scholar]
- [15].Flamm RK, Mendes RE, Ross JE, et al. An international activity and spectrum analysis of linezolid: ZAAPS Program results for 2011. Diagn Microbiol Infect Dis 2013;76:206–13. [DOI] [PubMed] [Google Scholar]
- [16].Biedenbach DJ, Farrell DJ, Mendes RE, et al. Stability of linezolid activity in an era of mobile oxazolidinone resistance determinants: results from the 2009 Zyvox® Annual Appraisal of Potency and Spectrum program. Diagn Microbiol Infect Dis 2010;68:459–67. [DOI] [PubMed] [Google Scholar]
- [17].Riccardi G, Pasca MR, Buroni S. Mycobacterium tuberculosis: drug resistance and future perspectives. Future Microbiol 2009;4:597–614. [DOI] [PubMed] [Google Scholar]
- [18].Chen ZH, Tan YH. Comparative study among procalcitonin. C-reactive protein, erythrocyte sedimentation rate and white blood cell in diagnosing pulmonary tuberculosis complicated with pulmonary infection. J Clin Pulm Med 2014;19:2009–11. [Google Scholar]
- [19].Lu NH, Wang YL, Yang R. Clinical significance of serum procalcitonin in differentiation of bacterial pneumonia and smear-negative pulmonary tuberculosis. Infect Dis Info 2015;28:96–8. [Google Scholar]
- [20].Sun XL, Zhang TH, Zhang XY, et al. Clinical efficacy of linezolid in the treatment of pulmonary gram positive cocci infection in patients with pulmonary tuberculosis. Shaanxi Med J 2016;45:958–9. [Google Scholar]
- [21].Lao SH, Yu CX, Chen H, et al. The clinical efficacy and safety of pulmonary tuberculosis complicated with severe pneumonia treated by linezolid. Chin J Antituberculosis 2013;35:578–80. [Google Scholar]
- [22].Lee JY, Kim DK, Lee JK, et al. Substitution of ethambutol with linezolid during the intensive phase of treatment of pulmonary tuberculosis: study protocol for a prospective, multicenter, randomized, open-label, phase II trial. Trials 2017;18:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McGee B, Dietze R, Hadad DJ, et al. Population pharmacokinetics of linezolid in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 2009;53:3981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang YX, Li G, Qian ZG. Analysis of adverse drug reaction reports of linezolid. Pract Pharm Clin Remedies 2013;7:16621–3. [Google Scholar]
- [25].Lu LH, He YD, Gao M, et al. A variety of adverse reactions induced by moxifloxacin hydrochloride. Chin J Nosocomiol 2009;19:2479. [Google Scholar]


