Abstract
Therapies to prevent maternal Zika virus (ZIKV) infection and its subsequent fetal developmental complications are urgently required. We isolated three potent ZIKV-neutralizing monoclonal antibodies (nmAbs) from the plasmablasts of a ZIKV-infected patient—SMZAb1, SMZAb2, and SMZAb5—directed against two different domains of the virus. We engineered these nmAbs with Fc LALA mutations that abrogate Fc-gamma receptor (FcγR) binding, thus eliminating potential therapy-mediated antibody-dependent enhancement (ADE). We administered a cocktail of these three nmAbs to nonhuman primates (NHP) one day before challenge with ZIKV and demonstrated that the nmAbs completely prevented viremia in serum following challenge. Given that numerous Abs have exceptional safety profiles in humans, the cocktail described here could be rapidly developed to protect uninfected pregnant women and their fetuses.
Overline:
Emerging infections
One Sentence Summary:
Neutralizing antibodies prevent Zika infection
Introduction:
ZIKV infection is a serious global public health threat with the potential to impact millions of individuals (1-4). Several locations will remain susceptible to yearly ZIKV outbreaks and new regions of the world will likely experience epidemics similar to the one described in South America in 2016. Developing novel preventive therapies will be central to limiting complications associated with the future epidemics. Of particular concern is the link between ZIKV infection of pregnant women and abnormal fetal development (1-3). Prophylaxis using Abs constitutes a well-established class of clinical intervention, including preventative and therapeutic uses in expectant mothers and premature infants (5, 6). The administration of human nmAbs in pregnant women to prevent ZIKV infection could be especially beneficial as they are generally considered safe and maternal Abs cross the placenta and are the predominant means of fetal immunity (7). Thus, a passively administered appropriately engineered human nmAb may be able to protect a fetus from ZIKV infection. nmAb-therapy is therefore likely to be one of the most promising interventions to prevent and treat ZIKV infection during pregnancy.
Results:
To isolate candidate potent neutralizing nmAb for use in passive transfer therapies, we cloned Ab genes from blood-derived plasmablasts of a ZIKV-infected subject from Colombia in the acute phase. After individual amplification of the heavy (H) and light (L) chains from the isolated B cells, we expressed and purified 91 mAbs. These mAbs were tested for neutralization and eleven of them reduced ZIKV infection by greater than 80 % at 1 μg ml−1 (fig. S1). Since our long term goal is to prevent infection (or reduce viral replication) with nmAbs in humans in resource-limited areas, we selected the three most potent nmAbs, SMZAb1 (IGHV3-23*04, IGKV1-5*03), SMZAb2 (IGHV1-69*01, IGLV8-61*01) and SMZAb5 (IGHV3-23*01, IGKV1-5*03). The isolated nmAbs had, on average, 18% heavy chain nucleotide mutations with antibodies SMZAb1 and SMZAb5 both representing related variants of the IGHV3-23 gene family. The three nmAbs have Neut50 potencies of less than 500 ng ml−1 (Fig. 1, tables S1, S2, and S3), and were selected for in vivo studies so that antiviral effects would be achieved at low concentrations in vivo and nmAb costs would be minimized.
Fig. 1.
The plasmablast-derived human nmAbs SMZAb1, SMZAb2, and SMZAb5 neutralize ZIKV in vitro. Ninety-one mAbs generated from a ZIKV-infected patient were screened for ZIKV-neutralization potency using Vero cell infectivity assays, and the three most potent—SMZAb1, SMZAb2, and SMZAb5, neutralized ZIKV at low concentrations. The neutralization titers were determined by focus reduction neutralization test (FRNT).
The ZIKV E protein is the main target of nmAbs and contains three domains with the majority of the anti-ZIKV nmAb targeting either domain III or the fusion loop epitope in domain II (8). We screened our three most potent nmAbs for their ability to recognize different regions of the ZIKV E protein in domain binding experiments (fig. S2). We used a domain III protein binding ELISA to identify nmAbs that target this domain. We also used a mAb competition assay to evaluate mAbs which target epitopes that overlap with the fusion loop epitope targeted by the well characterized anti-flavirus mAb, 4G2 (clone D1-4G2-4-15 MAB10216, MilliporeSigma). Based on these analyses, we demonstrated that our three most potent nmAbs bind to two different regions of the virus; SMZAb1 and SMZAb5 bind domain III and SMZAb2 binds the fusion loop.
Flaviviruses are RNA viruses and have been shown to readily escape nmAb therapy (9, 10). To avoid this possible outcome, we screened our three nmAbs for their ability to select for escape mutants in vitro using previously published protocols (11).
Since our nmAbs showed some cross-reactivity with dengue virus (DENV) (figs. S3 and S4), we engineered them to prevent any potential ADE effects in future applications by incorporating the L234A and L235A (LALA) immunoglobulin (Ig)G1 mutations which reduce or prevent FcγR binding (12). We then produced large quantities of the nmAbs by transfection of mammalian cells and subsequent purification. We re-tested our three LALA-modified nmAbs before infusion in two different neutralization assays and determined that they all neutralized ZIKV with Neut50 and plaque reduction neutralization test (PRNT50) values in the nanogram ml−1 range (Table S1 and S3).
We next tested the ability of the nmAbs to protect against ZIKV infection in eight Indian rhesus macaques (Macaca mulatta) (Table S4). We recently developed a NHP ZIKV macaque challenge model using a low-passaged primary isolate (Rio U-1/2016), recovered from the urine of a pregnant woman during the 2016 outbreak in Rio de Janeiro (13). In our first experimental group (Group 1; Fig. 2A) of four macaques, we delivered a cocktail of SMZAb1, SMZAb2, and SMZAb5 at 20 mg kg−1 (of each nmAb) to achieve plasma levels exceeding 1000X the Neut50 of each nmAb. We also delivered the same dose of a human IgG1 isotype control (wild type), CB1, to four control macaques (Group 2; Fig. 2A). We then challenged the macaques subcutaneously with 1,000 plaque forming units (PFU) of our low passage primary isolate ZIKV stock one day after passive transfer. Since the actual amount of ZIKV delivered to humans during mosquito feeding is unknown (14), we chose an infectious dose similar to that which has been recently used in ZIKV vaccination and challenge experiments in NHPs (15, 16). After infusion we monitored mAb levels using an Ab ELISA. Levels of our nmAbs averaged approximately 600 μg ml−1 at day of challenge and approximately 500 μg ml−1 at day 7 post-challenge, well above 1000X the Neut50 of these potent nmAbs (Fig. 2B and C). We also monitored serum neutralizing activity on the day of challenge and demonstrated that sera from the macaques infused with our ZIKV-specific nmAbs neutralized ZIKV at a dilution of approximately 1:50,000 (Fig. 2D and E; fig. S5).
Fig. 2.
Study design and pharmacokinetics of SMZAb cocktail administration to Indian rhesus macaques. (A) We administered a cocktail containing the three nmAbs, SMZAbs 1, 2, and 5 at a dose of 20 mg kg−1 each to four rhesus macaques (Group 1). A control group (Group 2) received the human CB1 isotype control at a dose of 60 mg kg−1 total. All animals were challenged with 1,000 PFU of ZIKV Rio U-1 2016 one day post mAb administration. Serum was collected at the indicated time points for viral load, neutralization, IgG, and IgM measurements. (A) Serum levels of recombinant Abs were determined by ELISA. Antibody level values for each SMZAb cocktail (blue) or control mAb (open symbols) animal. (C) Median values for the Group 1 (blue) and Group 2 (open symbols). (D) ZIKV-neutralizing activity in serum post mAb infusion was determined by FRNT. Median ZIKV-foci-neutralization percentage values for each SMZAb cocktail (blue) or control mAb (open symbols) animal. (E) Median values for the Group 1 (blue) and Group 2 (open symbols).
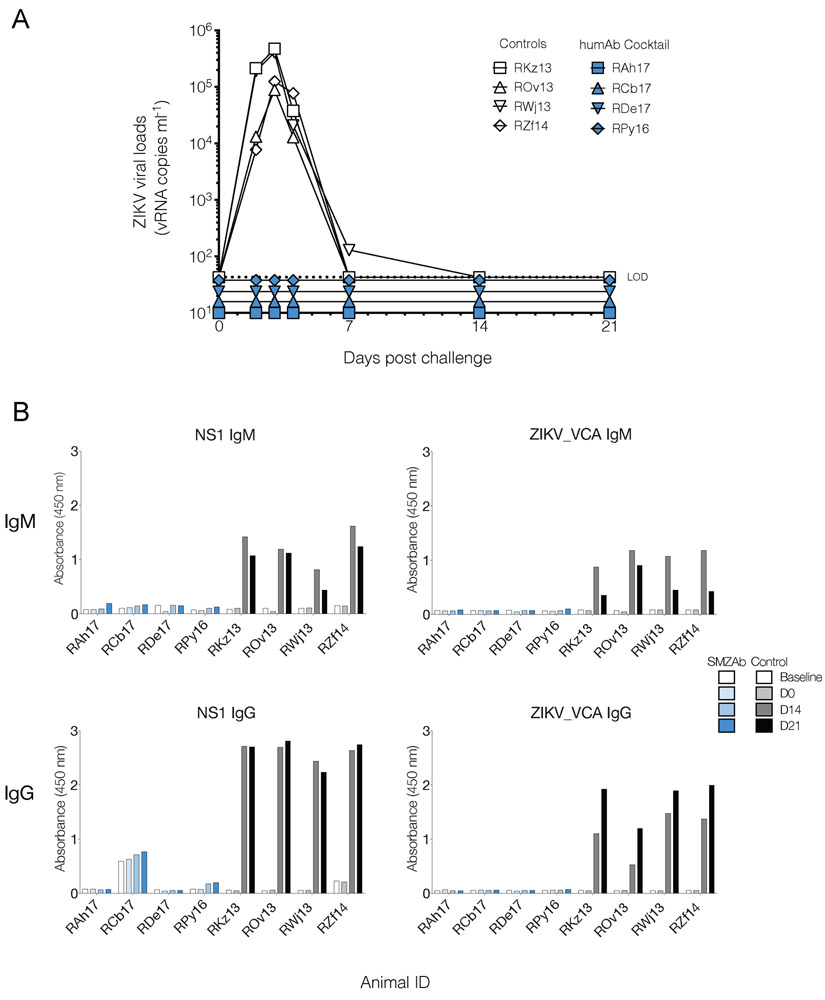
Our nmAb cocktail appeared to confer sterilizing immunity on the four treated macaques. To determine whether our nmAbs had blocked ZIKV infection in the Group 1 animals, we measured serum viral concentrations by quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) and plaque assay from day 0-21 post-challenge. Our ZIKV-specific nmAb cocktail completely prevented viral replication in all four nmAb-treated macaques in Group 1 (Fig. 3A, table S5). Since occult viral replication can occur in the absence of plasma viremia, we then tested macaque IgG and IgM responses against ZIKV in the treated and control animals. We could find no evidence for any ZIKV-specific Ab responses against either NS1 or whole virus in the nmAb-treated macaques (fig. 3B. As expected, the control macaques mounted robust ZIKV-specific IgG and IgM responses (fig. 3B).
Fig. 3.
Viral loads and humoral responses post ZIKV challenge. (A) We administered a cocktail containing the three nmAbs, SMZAbs 1, 2, and 5 at a dose of 20 mg kg−1 each to four rhesus macaques (Group 1). A control group (Group 2) received the human CB1 isotype control at a dose of 60 mg kg−1 total. All animals were challenged with 1,000 PFU of ZIKV Rio U-1 2016 one day post mAb administration. Serum viral loads for SZMAb- (blue) and control CB1 mAb- (open symbols) treated macaques. Dotted lines indicate the limit of detection (LOD) of the assay. (B) Humoral IgM and IgG responses of challenged animals against whole ZIKV or NS1. Serum binding (diluted 1:100) was measured by ELISA using rhesus-specific antibodies.
Discussion:
ADE is a serious technical hurdle to the development of vaccines against flaviviruses like ZIKV (17). Specially engineered nmAbs would avoid this unfortunate consequence of vaccination. ADE has been well described in the setting of DENV (18, 19) where subneutralizing concentrations of neutralizing antibodies via an FcγR mediated mechanism (20) bind viral particles and direct them to cells of the myeloid lineage. Indeed, ADE can transform a secondary DENV infection into potentially fatal hemorrhagic fever (21) and anti-DENV or anti-WNV sera can enhance ZIKV infection in vivo (22). It has already been established that immune responses against ZIKV and DENV cross-react (8, 23, 24) raising the possibility that a vaccine for one of these viruses could result in a poor outcome after infection with another. It is also of concern that individuals with a diminished immune response against a ZIKV vaccine run the risk of a much more severe ZIKV (or DENV) infection. For this reason, and others, it will take a number of years of testing before a ZIKV vaccine can safely be administered to women of childbearing age. Additionally, millions of individuals in an at-risk area would have to volunteer to be vaccinated to provide sufficient herd immunity to limit ZIKV-caused birth defects. Recombinant nmAbs can be engineered through well-established procedures to both avoid ADE and increase their half-lives by more than three-fold resulting in efficacious levels of mAbs for more than six months after a single injection of mAb (25). Thus, the quickest and most effective way to prevent fetal defects caused by ZIKV may be to develop human nmAbs or cocktails of these nmAbs that can be administered to pregnant women. Although other Abs have shown some degree of efficacy in preventing or limiting mouse ZIKV infections (26), they remain to be evaluated in NHP models that can recapitulate essential aspects of human ZIKV pathology. The co-formulation of three antibodies that include epitope specificity for both the fusion loop (domain II) and domain III was selected to reduce the probability of ZIKV E protein escape variants. In this NHP study, we observed no evidence of viral replication in vivo and therefore are hopeful that the delivery of these three Abs in humans as a ZIKV preventative therapy would prevent the generation of viral escape as well. It should be noted, however, that our nmAb cocktail was not tested in pregnant NHPs. Thus, the nmAb cocktail described here warrants additional pre-clinical testing in pregnant macaques as a strategy to prevent fetal defects caused by ZIKV infection.
Materials and Methods:
Study design
We have tested the hypothesis that a cocktail of three ZIKV-neutralizing antibodies is sufficient for preventing ZIKV infection in rhesus macaques. A control and a test group of four macaques each was used in this efficacy experiment. Macaques were selected based on availability and were not randomized during assignment. Because the supply of mAbs was limited, we selected the lighter macaques to receive the SMZAbs. The primary outcome measured was the presence of viral RNA in serum, performed in two independent assays. Primary data are located in table S6.
Human research
Pre-existing blood samples were obtained through Antibody Systems Inc. under The Scripps Research Institute IRB-15-6683. Research on human subjects was conducted in compliance with existing regulations relating to the protection of human subjects. All human subjects were consented and all specimens, data, and human subject research were gathered by Antibody Systems Inc.
Animal experiments
The eight Indian rhesus macaques (Macaca mulatta) utilized in this study were housed at the Yerkes National Primate Research Center (YNPRC). The YNPRC is fully accredited by AAALAC International (Association for the Assessment and Accreditation of Laboratory Animal Care), Animal Welfare Assurance No. A3180-01. Animals were cared for in accordance with the NRC Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act Animal experiments were approved by the Institutional Animal Care and Use Committee of Emory University (protocol YER-2003415). The macaques were separated in 2 groups, as follows: Group 1 (SMZAb cocktail, n = 4); Group 2 (Isotype control mAb, n = 4). The mAb cocktail was prepared in saline bags, and administered intravenously into each animal. All animals were challenged one day post mAb administration with 1 χ 103 plaque forming units (PFU) of ZIKV Rio U-1 2016 delivered subcutaneously. After the challenge, we collected serum at the indicated time points to measure viremia, mAb levels, and seroconversion.
Viruses
ZIKV strain Rio U-1 2016 was isolated in Rio de Janeiro, Brazil in 2016 (KU926309). Viral challenge stocks were prepared by propagating the virus in Vero cells for two passages post virus isolation (13). Virus concentrations in the stocks were quantitated by viral plaque assay. The viral stocks were diluted in Leibovitz’s L-15 and SPG media as described previously (27). DENV1 (West Pac74; U88535.1), DENV2 (New Guinea C; AF038403.1), DENV3 (Sleman/78; AY648961), DENV4 (Dominica/8129; AF326573.1), ZIKV (MR766; AY632535.2), ZIKV (Paraiba/2015; KX280026) were propagated in Vero cells (ATCC) and used for binding virus capture assays (VCA), and neutralization assays as described below.
Monoclonal antibody isolation and screening
Peripheral blood mononuclear cells were isolated as previously described (28). Plasmablasts were sorted into 96 well plates by flow cytometry by sorting for CD3− CD19+ CD20low CD38hi CD27hi cells. The sorts were at a density of one cell per well for Ab chain pairing. Reverse transcription and PCR amplification of heavy and light chain variable genes were performed, and the Abs assembled for each source cell.
Focus reduction neutralization test (FRNT)
Virus-specific neutralizing antibody responses were titrated essentially as previously described (29). Briefly, plasma or antibody was serially diluted in Minimal Essential Medium (Corning Cellgro) containing 5% heat-inactivated fetal bovine serum (Gibco-Invitrogen), and incubated 1 h at 37°C with virus. After incubation, the antibody-virus or plasma-virus mixture was added in triplicate to 96-well plates containing 80% confluent monolayers of Vero E6 cells. Plates were incubated for 1.5 h at 37°C. Following the incubation, wells were overlaid with 1% methylcellulose in supplemented MEM media with 2% heat-inactivated fetal bovine serum (Gibco-Invitrogen) and 1:100 HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid). Plates were incubated at 37°C, 5% CO2 for 40 h after which cells were fixed and permeabilized with Perm/Wash buffer (BD Biosciences) for 5 min. After permeabilization, cells were incubated with 1:2000 dilution of anti-flavivirus antibody (MAB10216; EMD Millipore) for 2 h then washed with PBS. Following washing, cells were incubated with anti-mouse HRP conjugated secondary Ab (115035146, Jackson ImmunoResearch Laboratories) in Perm/Wash buffer for 2 h. Following washing of cells with PBS, plates were developed with peroxidase substrate (KPL). The endpoint titer was determined to be the highest dilution with a 50% reduction (IC50) in the number of plaques compared to control wells.
Plaque reduction neutralization test (PRNT)
PRNTs were conducted as previously described (27). Briefly, serum samples were serially diluted in 199 media supplemented with 2% human serum albumin, 5% fetal bovine serum, and gentamicin. ZIKV was diluted to a final concentration of ~500-1,000 PFU ml−1 in the same diluent and added to equal volumes of the diluted sample. The virus/serum mixture was incubated at 37°C for 60 min. Cell culture medium was removed from 90% confluent monolayer cultures of Vero cells on 24-well plates and 100 μμl of the virus per mAb mixture was transferred onto duplicate cell monolayers. Cell monolayers were incubated for 60 min at 37°C and overlaid with 1% methylcellulose in OptiMEM (Thermo Fisher Scientific) supplemented with 2% FBS 2 mM glutamine plus 50 μg ml−1 gentamicin. Samples were incubated at 37°C for four days after which plaques were visualized by immunoperoxidase staining, and a 50% plaque-reduction neutralization titer (PRNT50) was calculated.
Flow cytometry-based neutralization assay
The neutralizing potency of the mAbs was measured using a flow cytometry-based assay (30, 31). In brief, recombinant mAbs were diluted and pre-incubated with ZIKV (Paraiba 2015, KX280026.1) in a final volume of 220 μl for 1 h at 37°C. The virus and mAb mixture (100 μl) was added onto wells of a 24-well plate of 100% confluent Vero cell monolayers in duplicate. The inoculum was incubated in a 37°C incubator at 5% CO2 for 1 h with agitation of the plates every 15 min. After 1 h, the virus and mAb-containing supernatants were aspirated and the wells were washed with media. Fresh media was then added and the plates were incubated for a total of 24 h. Cells were trypsinized with 0.5 % trypsin (Life Technologies), fixed (BD cytofix), and permeabilized (BD cytoperm). Viral infection was detected with the 4G2 Ab recognizing ZIKV or DENV, followed by staining with an anti-mouse IgG2a APC fluorophore-conjugated secondary reagent (Biolegend). The concentration to achieve half-maximal neutralization (Neut50) was calculated using a nonlinear regression analysis with Prism 7.0 software (GraphPad Software)The ZIKV Paraiba 2015 strain was used in our neutralization assays.
Virus Capture Assay (VCA)
Antibody binding to whole virus was determined in a side-by-side DENV1, DENV2, DENV3, DENV4, and ZIKV VCA ELISA. The ELISA plate was coated with the mouse-anti-flavivirus monoclonal antibody 4G2 diluted 1:1,000 in carbonate binding buffer and incubated overnight at 4°C. The next day, the plate was washed 5 times with PBS-Tween20 and the wells were blocked with 5% nonfat dry milk in PBS for 1 h at 37°C. Following the block, the plate was washed and each virus was added to the corresponding VCA wells, respectively, and incubated for 1 h at room temperature. Subsequently, the plate was washed with PBS and mAbs from different time points diluted in 5% nonfat dry milk with PBS were added to designated wells and incubated for 1 h at 37°C. Following sample addition, plates were washed and detection was carried out using one of the following antibodies: goat anti-human IgG HRP (SouthernBiotech, 2045-05) diluted 1:10,000, Mouse Anti-Monkey IgG-HRP (SouthernBiotech, 4700–05) diluted 1:4,000, or the Goat Anti-Human IgM-HRP diluted 1:2,000 (SouthernBiotech, 2023-05). The diluted detection antibody was added to all wells and incubated for 1 h at 37°C. The plate was then washed and the wells were developed with the tetramethylbenzidine (TMB) substrate at room temperature for 3-4 min. The reaction was then stopped with the TMB solution, and absorbance was read at 450 nm.
NS1 ELISA
ZIKV-NS1-specific antibodies in serum were detected by ELISA. To begin, the plate was coated with ZIKV-NS1 antigen (MyBioSource, MBS568704) diluted to 10 μg ml−1 in carbonate binding buffer and incubated overnight at 4°C. The following day, the plate was washed 5-times with PBS-Tween20 and all wells were blocked with 5% nonfat dry milk in PBS for 1 h at 37°C. After the block, the plate was washed, and serum samples diluted 1:100 were added to corresponding wells and incubated for 1 h at 37°C. Subsequently, the plate was washed 5-times and IgG or IgM detection was carried out. ZIKV-NS1-specific rhesus IgG and IgM were detected using the Mouse Anti-Monkey IgG-HRP (Southern Biotech, 4700-05) diluted 1:4,000 and the Goat Anti-Human IgM-HRP diluted 1:2,000 (Southern Biotech, 2023-05), respectively. The diluted detection antibodies were added to corresponding wells and incubated for 1 h at 37°C. The plate was then washed 8-times and developed with TMB substrate at room temperature for 2–3 min. The reaction was then stopped with TMB Stop Solution, and the absorbance was read at 450nm.
In vivo human antibody quantitation by ELISA
The presence of the recombinant nmAbs in sera was quantitated with an ELISA specific for human Abs. In brief, 96-well ELISA plates were coated overnight with 5 μg ml−1 of the monkey Ab-adsorbed goat anti-human IgG (Southern Biotech, 2049-01) diluted in phosphate-buffered saline (PBS). Each plate was washed with PBS-Tween20 and the wells were blocked with 5% nonfat dry milk in PBS for 1 h at 37°C. Subsequently, the plate was washed with PBS and serum samples were added to designated wells. After 1 h incubation at 37°C, the plate was washed and detection was carried out using a HRP-conjugated goat anti-human IgG (Southern Biotech, 2045-05), which was added to all wells at a dilution of 1:10,000. Following a 1 h incubation at 37°C, the plate was washed with PBS-Tween20 and developed with TMB substrate (Millipore) at room temperature for 3-4 min. Reaction was then stopped with TMB stop solution, and absorbance was read at 450 nm.
Measurement of viral RNA load (qRT-PCR)
The assay for the quantification of viral loads in sera was based on a previously validated qRT-PCR assay (32, 33). In brief, RNA was extracted from 280 μl of frozen sera using the QIAamp Viral RNA Mini Kit (Qiagen). The total nucleic acid was eluted in two centrifugation steps with 40 μl of Buffer AVE each. A qRT-PCR reaction was then carried out with 20 μl of samples and 10 μl of primer, probes and TaqMan Fast Virus 1-Step Master Mix (Applied Biosystems). We used pre-combined probe and primers (500 nM primers and 250 nM probe; IDT Technologies). The primer and probe sequences were designed to match sequences to the ZIKV isolate (KU926309) and were as follows: Primer 1 5’TTGAAGAGGCTGCCAGC3’; Primer 2 5’CCCACTGAACCCCATCTATTG3’; Probe 5’TGAGACCCAGTGATGGCTTGATTGC3’. The probe was double-quenched (ZEN/Iowa Black FQ) and labeled with the FAM dye (IDT Technologies). Ten-fold serial dilutions of in vitro transcript RNA starting at approximately 5 × 105 copies μl−1 were used as standards, and results were reported as the equivalent viral RNA genomes per ml.
Measurement of viral load (plaque)
Quantification of ZIKV PFU is serum was determined as described before for PRNT, without the antibody incubation step.
Domain III binding ELISA
The domain specificity of our recombinant nmAbs was determined using Zika domain III recombinant protein ELISA. Ninety six-well high-binding plates were coated overnight with 5 μg ml−1 Zika domain III recombinant protein, generously supplied by Daved Fremont and previously described (34), was diluted in PBS. Each plate was washed 3-times with PBS-Tween20 and the wells were blocked with 3% BSA in water for 1.5 h at room temperature. Subsequently, the plate was washed 3-times with PBS-Tween20 and our recombinant nmAbs were added to their designated wells at starting concentrations of 50 μg ml−1 with subsequent 6-fold dilutions. After a 1.5 h incubation at room temperature the plate was washed and detection was carried out using anti-human IgG-AP (109055098; Jackson ImmunoResearch Laboratories), which was added to all wells at a dilution of 1:2,000. Following a 1 h incubation at room temperature, the plate was washed and developed with phosphatase substrate (MilliporeSigma) at room temperature. The plates were immediately read at 405 nm with 5 minute incubations at room temperature between reads.
4G2 mAb competition ELISA
To further determine the domain specificity of our recombinant nmAbs, a competition ELISA was performed. Plates were coated overnight with 2 μg ml−1 ZIKV Envelope protein (ZIKV-SU-ENV100, Native Antigen) diluted in PBS. Each plate was washed 3-times with PBS-Tween20 and the wells were blocked with 3% BSA in water for 1.5 h at room temperature. Subsequently, the plate was washed 3-times with PBS-Tween20 and antibodies, 4G2 and a known domain III binding mAb characterized in our laboratory, ADI24255, were added to their designated wells at concentrations of 25 μg ml−1 with subsequent 10-fold dilutions (35). After a 1.5 h incubation at room temperature the plate was washed and our previously biotinylated nmAbs (EZ-LINK NHS-Biotin, ThermoFisher Scientific) were added to their designated wells. After a 1.5 h incubation at room temperature the plate was washed and alkaline phosphatase (AP)-conjugated streptavidin (016-059-084, Jackson ImmunoResearch Laboratories) was added to all wells at a dilution of 1:2,000. Following a 1 h incubation at room temperature, the plate was washed and developed with phosphatase substrate (MilliporeSigma) at room temperature. The plates were immediately read at 405 nm with 5 min incubations at room temperature between reads.
Statistics
The main outcome measure in this study is the viremia levels on mAb treated and control animals. We did not perform statistical comparisons, because there was not virus detected in any of the treated animals and all controls got infected.
Supplementary Material
Fig.S1. Plasmablast-derived human mAbs neutralize ZIKV in vitro.
Fig.S2. Epitope mapping. SMZAb1, SMZAb2, and SMZAb5 ZIKV E protein epitope specificity was evaluated.
Fig.S3. Binding of SMZAb1, SMZAb2, SMZAb5 to whole ZIKV or DENV was evaluated by virus-capture ELISA using 4G2.
Fig.S4. DENV-neutralization by SMZAbs. SMZAb1, SMZAb2, and SMZAb5 neutralize a subset of DENV serotypes in vitro.
Fig.S5. ZIKV-neutralizing activity post mAb infusion (FRNT).
Table S1. SMZAb amino acid sequences
Table S2. Neutralization of ZIKV strains and DENV serotypes by SMZAbs (FRNT50 μg ml−1).
Table S3. ZIKV Paraiba/2015-neutralization by SMZAbs prior to infusion.
Table S4. Study animals
Table S5. Viral titers (Log10 PFU ml−1) in serum post ZIKV-challenge.
Table S6. Viral titers (vRNA copies ml−1) in serum post ZIKV-challenge.
Acknowledgments:
We thank the veterinary and animal care staff for providing care of the rhesus macaques included in this experiment.
Funding: This work was supported by National Institutes of Health (NIH) grant 4P01AI09442005, the Wallace H. Coulter Center for Translational Research at the University of Miami, the Miami Clinical and Translational Science Institute (CTSI), and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH. This research was also supported in part by the Defense Advanced Research Projects Agency (DARPA) Autonomous Diagnostics to Enable Prevention and Therapeutics: Prophylactic Options to Environmental and Contagious Threats (ADEPT-PROTECT) program (W31P4Q-13-1-0011), as well as the NIH grant P30AI073961 to the Miami Center for AIDS Research and grant P51 OD011132 to the Yerkes National Primate Research Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing interests: Dennis Burton and Thomas Rogers are inventors on a patent application submitted by The Scripps Research Institute that covers anti-Zika monoclonal antibodies.
Data and materials availability: The antibody sequences are provided in the supplementary materials.
References and Notes:
- 1.Reynolds MR, Jones AM, Vital Signs: Update on Zika Virus–Associated Birth Defects and Evaluation of All U.S. Infants with Congenital Zika Virus Exposure — U.S. Zika Pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep 66, 366–373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasil P, Pereira JP Jr., Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, Zin AA, Horovitz D, Daltro P, Boechat M, Raja Gabaglia C, Carvalho de Sequeira P, Pilotto JH, Medialdea-Carrera R, Cotrim da Cunha D, Abreu de Carvalho LM, Pone M, Machado Siqueira A, Calvet GA, Rodrigues Baiao AE, Neves ES, Nassar de Carvalho PR, Hasue RH, Marschik PB, Einspieler C, Janzen C, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K, Zika Virus Infection in Pregnant Women in Rio de Janeiro. The New England journal of medicine 375, 2321–2334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, Avsic Zupanc T, Zika Virus Associated with Microcephaly. The New England journal of medicine 374, 951–958 (2016). [DOI] [PubMed] [Google Scholar]
- 4.WHO, Fifth meeting of the Emergency Committee under the International Health Regulations (2005) regarding microcephaly, other neurological disorders and Zika virus. http://www.who.int/mediacentre/news/statements/2016/zika-fifth-ec/en/. (Accessed on Aug 24, 2017).
- 5.The IMpact-RSV Study Group, Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics 102, 531–537 (1998). [PubMed] [Google Scholar]
- 6.Juckstock J, Rothenburger M, Friese K, Traunmuller F, Passive Immunization against Congenital Cytomegalovirus Infection: Current State of Knowledge. Pharmacology 95, 209–217 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Simister NE, Placental transport of immunoglobulin G. Vaccine 21, 3365–3369 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Loriere E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, England P, Stiasny K, Mongkolsapaya J, Heinz FX, Screaton GR, Rey FA, Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 536, 48–53 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Lai CJ, Goncalvez AP, Men R, Wernly C, Donau O, Engle RE, Purcell RH, Epitope determinants of a chimpanzee dengue virus type 4 (DENV-4)-neutralizing antibody and protection against DENV-4 challenge in mice and rhesus monkeys by passively transferred humanized antibody. Journal of virology 81, 12766–12774 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnani DM, Ricciardi MJ, Bailey VK, Gutman MJ, Pedreno-Lopez N, Silveira CGT, Maxwell HS, Domingues A, Gonzalez-Nieto L, Su Q, Newman RM, Pack M, Martins MA, Martinez-Navio JM, Fuchs SP, Rakasz EG, Allen TM, Whitehead SS, Burton DR, Gao G, Desrosiers RC, Kallas EG, Watkins DI, Dengue Virus Evades AAV-Mediated Neutralizing Antibody Prophylaxis in Rhesus Monkeys. Molecular therapy (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukupolvi-Petty S, Austin SK, Engle M, Brien JD, Dowd KA, Williams KL, Johnson S, Rico-Hesse R, Harris E, Pierson TC, Fremont DH, Diamond MS, Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. Journal of virology 84, 9227–9239 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR, Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449, 101–104 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Bonaldo MC, Ribeiro IP, Lima NS, Dos Santos AA, Menezes LS, da Cruz SO, de Mello IS, Furtado ND, de Moura EE, Damasceno L, da Silva KA, de Castro MG, Gerber AL, de Almeida LG, Lourenco-de-Oliveira R, Vasconcelos AT, Brasil P, Isolation of Infective Zika Virus from Urine and Saliva of Patients in Brazil. PLoS neglected tropical diseases 10, e0004816 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Styer LM, Kent KA, Albright RG, Bennett CJ, Kramer LD, Bernard KA, Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS pathogens 3, 1262–1270 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng’ang’a D, Kirilova M, Nityanandam R, Mercado NB, Li Z, Moseley ET, Bricault CA, Borducchi EN, Giglio PB, Jetton D, Neubauer G, Nkolola JP, Maxfield LF, De La Barrera RA, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH, Vaccine protection against Zika virus from Brazil. Nature 536, 474–478 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, Gordon DN, Gallagher JR, Chen X, Todd JP, Tsybovsky Y, Harris A, Huang YS, Higgs S, Vanlandingham DL, Andersen H, Lewis MG, De La Barrera R, Eckels KH, Jarman RG, Nason MC, Barouch DH, Roederer M, Kong WP, Mascola JR, Pierson TC, Graham BS, Rapid development of a DNA vaccine for Zika virus. Science 354, 237–240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marston HD, Lurie N, Borio LL, Fauci AS, Considerations for Developing a Zika Virus Vaccine. The New England journal of medicine 375, 1209–1212 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Wang TT, Sewatanon J, Memoli MJ, Wrammert J, Bournazos S, Bhaumik SK, Pinsky BA, Chokephaibulkit K, Onlamoon N, Pattanapanyasat K, Taubenberger JK, Ahmed R, Ravetch JV, IgG antibodies to dengue enhanced for FcgammaRIIIA binding determine disease severity. Science 355, 395–398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halstead SB, In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. The Journal of infectious diseases 140, 527–533 (1979). [DOI] [PubMed] [Google Scholar]
- 20.Dowd KA, Pierson TC, Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology 411, 306–315 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzman MG, Alvarez M, Halstead SB, Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Archives of virology 158, 1445–1459 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, Stramer SL, Garcia-Sastre A, Krammer F, Lim JK, Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 356, 175–180 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, Mongkolsapaya J, Screaton GR, Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nature immunology 17, 1102–1108 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, Ahmed R, Suthar MS, Wrammert J, Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proceedings of the National Academy of Sciences of the United States of America 113, 7852–7857 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbie GJ, Criste R, Dall’acqua WF, Jensen K, Patel NK, Losonsky GA, Griffin MP, A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrobial agents and chemotherapy 57, 6147–6153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sapparapu G, Fernandez E, Kose N, Bin C, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, Davidson E, Mysorekar IU, Fremont DH, Doranz BJ, Diamond MS, Crowe JE, Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 540, 443–447 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, Thumar B, Men R, Lai CJ, Elkins WR, Chanock RM, Murphy BR, Whitehead SS, Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3’-untranslated region. The American journal of tropical medicine and hygiene 65, 405–413 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Fuss IJ, Kanof ME, Smith PD, Zola H, Isolation of whole mononuclear cells from peripheral blood and cord blood Current protocols in immunology / edited by Coligan John E. [et al. ] 7, Unit7 1 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Brien JD, Lazear HM, Diamond MS, Propagation, quantification, detection, and storage of West Nile virus. Curr Protoc Microbiol 31, 15D 13 11–15D 13 18 (2013). [DOI] [PubMed] [Google Scholar]
- 30.de Alwis R, de Silva AM, Measuring antibody neutralization of dengue virus (DENV) using a flow cytometry-based technique. Methods in molecular biology 1138, 27–39 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Kraus AA, Messer W, Haymore LB, de Silva AM, Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. Journal of clinical microbiology 45, 3777–3780 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santiago GA, Vergne E, Quiles Y, Cosme J, Vazquez J, Medina JF, Medina F, Colon C, Margolis H, Munoz-Jordan JL, Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS neglected tropical diseases 7, e2311 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson BW, Russell BJ, Lanciotti RS, Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. Journal of clinical microbiology 43, 4977–4983 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, Govero J, Nelson CA, Pierson TC, Diamond MS, Fremont DH, Structural Basis of Zika Virus-Specific Antibody Protection. Cell 166, 1016–1027 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers TF, Goodwin EC, Briney B, Sok D, Beutler N, Strubel A, Nedellec R, Le K, Brown ME, Burton DR, Walker LM, Zika virus activates de novo and cross-reactive memory B cell responses in dengue-experienced donors. Sci Immunol 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig.S1. Plasmablast-derived human mAbs neutralize ZIKV in vitro.
Fig.S2. Epitope mapping. SMZAb1, SMZAb2, and SMZAb5 ZIKV E protein epitope specificity was evaluated.
Fig.S3. Binding of SMZAb1, SMZAb2, SMZAb5 to whole ZIKV or DENV was evaluated by virus-capture ELISA using 4G2.
Fig.S4. DENV-neutralization by SMZAbs. SMZAb1, SMZAb2, and SMZAb5 neutralize a subset of DENV serotypes in vitro.
Fig.S5. ZIKV-neutralizing activity post mAb infusion (FRNT).
Table S1. SMZAb amino acid sequences
Table S2. Neutralization of ZIKV strains and DENV serotypes by SMZAbs (FRNT50 μg ml−1).
Table S3. ZIKV Paraiba/2015-neutralization by SMZAbs prior to infusion.
Table S4. Study animals
Table S5. Viral titers (Log10 PFU ml−1) in serum post ZIKV-challenge.
Table S6. Viral titers (vRNA copies ml−1) in serum post ZIKV-challenge.