Abstract
This study investigated the anti-inflammatory effects of omega 3 fatty acids (O3FAs) for patients with advanced nonsmall cell lung cancer (ANSCLC).
A total of 137 patients with ANSCLC were included in this study. Of those, 77 patients underwent O3FA and were assigned to a treatment group, while 60 patients did not receive it, and were assigned to a control group. C-reactive protein (CRP), and interleukin (IL)-6 levels, as well as the levels of tumor necrosis factor-alpha (TNFα) and prostaglandin E2 (PGE2) were checked. In addition, nutritional status and quality of life were also evaluated. All patients in the treatment group received a total of 6 weeks treatment.
After 6 weeks treatment, patients in the treatment group exerted better outcomes in CRP and IL-6, although no significant differences were found in nutritional status, as well as the quality, compared with patients in the control group.
The results of this retrospective study found that O3FA may change levels of CRP and IL-6, except the nutritional status and quality of life.
Keywords: advanced nonsmall cell lung cancer, effect, omega 3 fatty acids
1. Introduction
Lung cancer is the most leading cause of cancer mortality around the world.[1–4] It has been reported that the 5-year survival rate of patients with lung cancer is about 15% for all stages, although patients at an earlier stage have a longer survival.[5,6] Of all types of lung cancers, nonsmall cell lung cancer (NSCLC) is the most common type, and accounts for 80% of all lung cancers.[4,7]
Presently, chemoradiotherapy is used as a first-line treatment for patients with NSCLC. It has promising efficacy, and also helps for the rehabilitation and recovery process in patients with this condition.[8–10] Unfortunately, it is also accompanied with lots of toxicities, such as nausea, vomiting, poor appetite, and also anorexia, anemia, and weight loss.[8,11–13] In addition, such kinds of toxicities often lead to a poor nutritional status and quality of life.[8] Moreover, it can also result in treatment-related morbidity and mortality.[14] If this condition cannot be treated very well, it may result in cancer cachexia.[15,16]
Several studies have reported that cancer cachexia is associated with anti-inflammatory factors, such as the levels of C-reactive protein (C-RP), interleukin-6 (IL-6), tumor necrosis factor-a (TNF-a), and prostaglandin E2 (PGE2).[17,18] It is also reported that omega 3 fatty acids (O3FAs) can treat such condition effectively.[19–24] However, limited data are still available for O3FA in patients with NSCLC among Chinese population. In this retrospective study, we analyzed the effect and adverse events of O3FA in patients with NSCLC among Chinese population.
2. Materials and methods
2.1. Ethics
This study was approved by the Medical Ethical Committee of The First People's Hospital of Xiaochang. The written informed consent was obtained from all patients.
2.2. Design
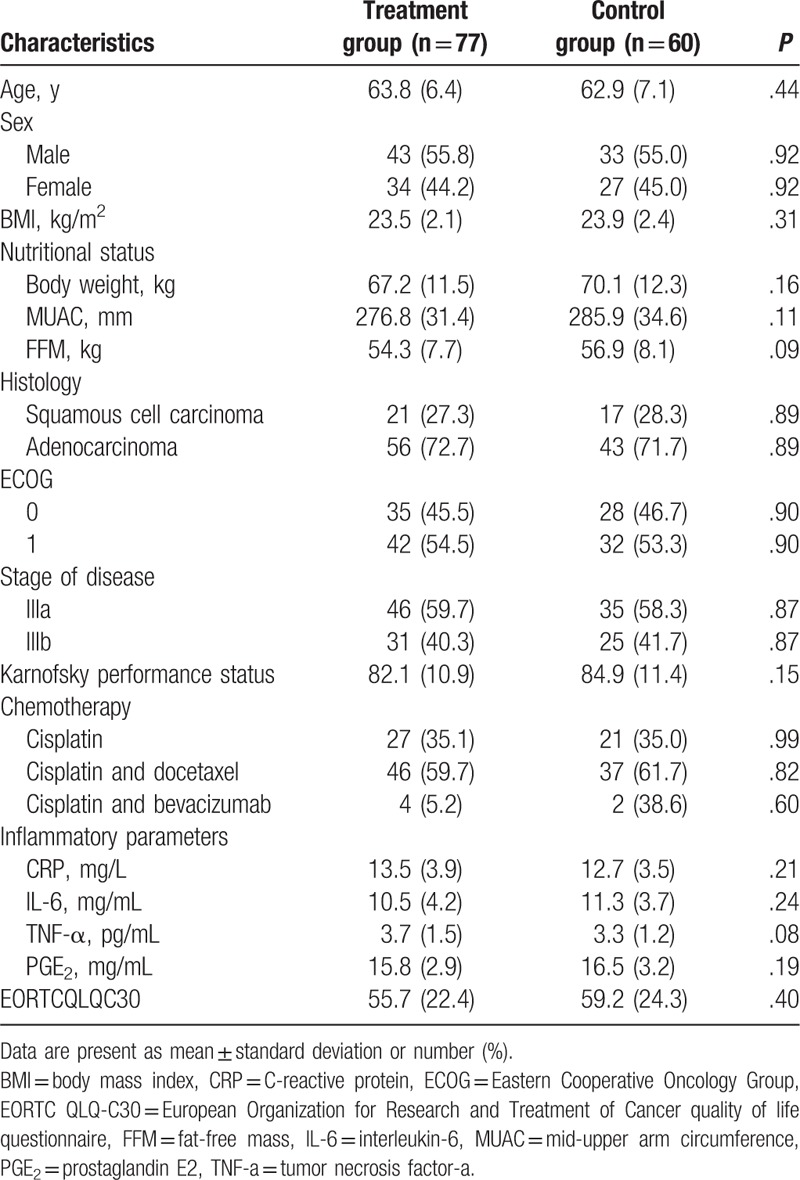
This study was conducted at The First People's Hospital of Xiaochang between January 2015 and November 2016. A total of 137 patients with stage IIIA and IIIB NSCLC were included in this study. Of those, 77 patients received O3FA, and were assigned to a treatment group, while 60 patients did not receive O3FA, and were assigned to a control group. The clinical characteristics of all included patients are listed in Table 1. All patients in the treatment group received O3FA for a total of 6 weeks. All outcome measurements were analyzed before and after 6 weeks treatment.
Table 1.
Characteristics of all included patients in both groups.
2.3. Patients
All patients were between 20 and 75 years of age. All of them were eligible for chemoradiotherapy, and had adequate hematologic, renal, and liver function. Patients were excluded if they received surgery; had severe comorbidities; and were pregnancy or breastfeeding. In addition, the subjects were excluded if the cases had insufficient information, such as characteristics and outcome data.
2.4. Treatments
Patients in a treatment group received orally O3FA capsules [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] for a total of 6 weeks. The EPA 510 mg and DHA 200 mg were taken once daily throughout the period of whole chemoradiotherapy treatment. Patients in a control group did not receive O3FA intervention.
2.5. Outcomes
The outcome measurements included the plasma levels of CRP, IL-6, TNF-a, and PGE2. We obtained the plasma and erythrocytes by centrifuging venous blood at 3000g for 5 minutes at 4°C. It was performed by centrifuge J6M (Beckman, Palo Alto, CA). The samples were stored at −80°C until use. The levels of CRP, IL-6, TNF-a, and PGE2 were measured by ELISA kits (R&D systems, Minneapolis, MN).
In addition, nutritional status and quality of life were also evaluated in this study. The nutritional status was measured by the body weight, mid-upper arm circumference (MUAC), and fat-free mass (FFM). The quality of life was also assessed by the European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ-C30). This scale varies from 0 to 100, with a higher score indicating better quality of life. All outcomes were measured before and after 6 weeks treatment. Furthermore, any kinds of adverse events related to the treatment were also recorded in this study.
2.6. Statistical analysis
In this retrospective study, SPSS software (SPSS V.18.0; IBM Corp., Armonk, NY) was used to analyze all the outcome data. t test was used to analyze the continuous data between 2 groups. Chi-square test was used to analyze the dichotomous data between 2 groups. All P values were 2-sided at a significance of P < .05.
3. Results
The characteristics of all included patients in both groups are summarized in Table 1. The comparisons of all characteristic values did not significantly differ between the 2 groups (Table 1).
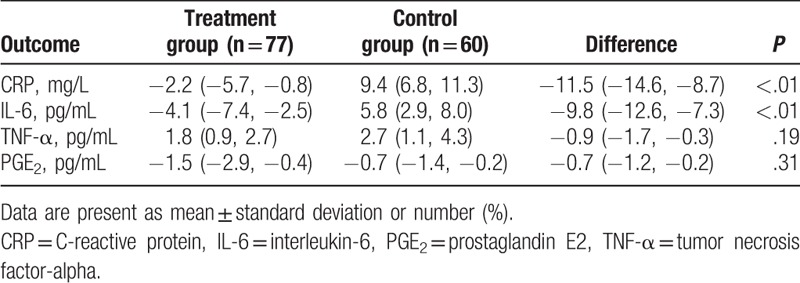
After 6 weeks intervention, patients who underwent O3FA found significant changes in CRP (P < .01, Table 2) and IL-6 (P < .01, Table 2), except the TNF-a (P = .19, Table 2) and PGE2 (P = .31, Table 2), when compared with patients who did not receive it.
Table 2.
Inflammatory parameters after 6 weeks treatment (change from baseline).
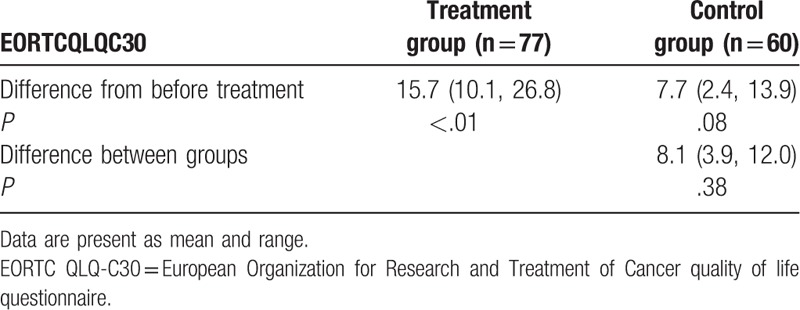
After 6 weeks treatment, patients who received O3FA neither enhanced the nutritious status (body weight, P = .44; MUAC, P = .13; and FFM, P = .19, Table 3), nor improved quality of life, measured by EORTC QLQ-C30 (P = .38, Table 4), compared with patients who did not receive it.
Table 3.
Nutritional status after 6 weeks treatment (change from baseline).
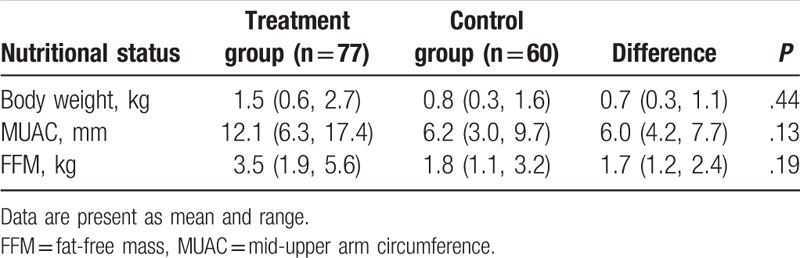
Table 4.
Comparison of quality of life after 6 weeks treatment.
As for adverse events, no treatment-related adverse events occurred in the treatment group. No death-related therapy occurred in the treatment group.
4. Discussion
Patients with lung cancer often suffer from cancer cachexia.[25] It has been found that this condition (often manifested with weight loss, decreased lean body mass, and hypermetabolism) is often associated with some inflammatory factors. Furthermore, these factors often consisted of CRP, IL-6, TNF-a, and PGE2.[17]
Although several studies have investigated the anti-inflammatory effects of O3FA for patients with lung cancer, the results are still inconsistent.[21,26,27] One study found that continual assumption of EPA and DHA can benefit the cachetic therapy in patients with lung cancer.[21] The results of other 2 studies demonstrated that O3FA may beneficially impact nutritional status, quality of life, as well as the physical activity in patients with NSCLC receiving multimodality treatment.[26,27]
The results of this study are partly consistent with the previous studies.[21,26] In this study, we found that O3FA can significantly change the serum levels of CRP and IL-6, which is consistent with the previous study. However, the results of this study did not show better outcomes in the improvement of nutritional status and quality of life.
This study has following limitations. First of all, this study collected the patient cases from 1 center only, which may affect the generalization of these results to other hospitals. Second, the outcome measurements may be not comprehensive in this study, especially for the nutritional status, and quality of life assessment, because the data analysis of the results was only based on the current available data. Third, the duration of the treatment period may be insufficient, which may also affect the results of this study. Future studies should avoid these limitations.
5. Conclusion
The results of this study demonstrated that the O3FA may impact the serum levels of CRP and IL-6. However, it may be not help to improve nutritional status and quality of life in patients with ANSCLC.
Author contributions
Conceptualization: Ren-gang Chen, Yan Lu, San-zou Wei, Han-guo Hu, Fei Sun, Chun-hui Yu.
Data curation: Ren-gang Chen, Yan Lu, Fei Sun, Chun-hui Yu.
Formal analysis: Ren-gang Chen.
Investigation: Han-guo Hu.
Methodology: Ren-gang Chen.
Project administration: Yan Lu.
Resources: Yan Lu, Chun-hui Yu.
Software: Ren-gang Chen, Han-guo Hu.
Supervision: Yan Lu, Han-guo Hu.
Validation: Yan Lu, San-zou Wei, Fei Sun, Chun-hui Yu.
Visualization: Yan Lu, San-zou Wei, Fei Sun, Chun-hui Yu.
Writing – original draft: Ren-gang Chen, Yan Lu, San-zou Wei, Han-guo Hu, Fei Sun, Chun-hui Yu.
Writing – review & editing: Ren-gang Chen, Yan Lu, San-zou Wei, Han-guo Hu, Fei Sun, Chun-hui Yu.
Footnotes
Abbreviations: ANSCLC = advanced non-small cell lung cancer, BMI = body mass index, CRP = C-reactive protein, ECOG = Eastern Cooperative Oncology Group, EORTC QLQ-C30 = European Organization for Research and Treatment of Cancer quality of life questionnaire, FFM = fat-free mass, IL-6 = interleukin-6, MUAC = mid-upper arm circumference, O3FA = omega 3 fatty acids, PGE2 = prostaglandin E2, TNF-a = tumor necrosis factor-a.
YL and R-GC contributed equally to this work and should be considered co-first authors.
The author (s) of this work have nothing to disclose and have no conflicts of interest.
References
- [1].Wu Y, Zhu Z, Chen Y, et al. Tonsillar metastasis of non small cell lung cancer with G719S mutation in exon 18: a case report. Medicine (Baltimore) 2017;96:e9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu X, Zhang W, Yin W, et al. The prognostic value of the serum neuron specific enolase and lactate dehydrogenase in small cell lung cancer patients receiving first-line platinum-based chemotherapy. Medicine (Baltimore) 2017;96:e8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Feng Q, Zhang H, Dong Z, et al. Circulating 25-hydroxyvitamin D and lung cancer risk and survival: a dose-response meta-analysis of prospective cohort studies. Medicine (Baltimore) 2017;96:e8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu Y, Qi M, Hou S, et al. Risk of rash associated with vandetanib treatment in non-small-cell lung cancer patients: a meta-analysis of 9 randomized controlled trials. Medicine (Baltimore) 2017;96:e8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- [6].Ding X, Wang L, Liu X, et al. Genetic characterization drives personalized therapy for early-stage non-small-cell lung cancer (NSCLC) patients and survivors with metachronous second primary tumor (MST): a case report. Medicine (Baltimore) 2017;96:e6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hong D, Zhang G, Zhang X, et al. Pulmonary toxicities of gefitinib in patients with advanced non-small-cell lung cancer: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Price KA, Azzoli CG, Gaspar LE. Chemoradiation for unresectable stage III non-small cell lung cancer. Semin Thorac Cardiovasc Surg 2008;20:204–9. [DOI] [PubMed] [Google Scholar]
- [9].van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442–50. [DOI] [PubMed] [Google Scholar]
- [10].Sen F, Tambas M, Ozkaya K, et al. Concomitant etoposide and cisplatin provided improved survival compared with docetaxel and cisplatin in patients with locally advanced non-small cell lung cancer treated with chemoradiotherapy. Medicine (Baltimore) 2016;95:e4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ross PJ, Ashley S, Norton A, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers. Br J Cancer 2004;90:1905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ravasco P, Monteiro-Grillo I, Marques VP, et al. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005;27:659–68. [DOI] [PubMed] [Google Scholar]
- [13].Paccagnella A, Morassutti I, Rosti G. Nutritional intervention for improving treatment tolerance in cancer patients. Curr Opin Oncol 2011;23:322–30. [DOI] [PubMed] [Google Scholar]
- [14].Zhao J, Xia Y, Kaminski J, et al. Treatment-related death during concurrent chemoradiotherapy for locally advanced non-small cell lung cancer: a meta-analysis of randomized studies. PLoS One 2016;11:e0157455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen W, Han L, Pang H, et al. Primary malignant myopericytoma with cancer cachexia: report of the first case and review of literature. Medicine (Baltimore) 2017;96:e9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Parmar MP, Vanderbyl BL, Kanbalian M, et al. A multidisciplinary rehabilitation programme for cancer cachexia improves quality of life. BMJ Support Palliat Care 2017;7:441–9. [DOI] [PubMed] [Google Scholar]
- [17].Martin F, Santolaria F, Batista N, et al. Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine 1999;11:80–6. [DOI] [PubMed] [Google Scholar]
- [18].Staal-van den Brekel AJ, Dentener MA, Schols AM, et al. Increased resting energy expenditure and weight loss are related to a systemic inflammatory response in lung cancer patients. J Clin Oncol 1995;13:2600–5. [DOI] [PubMed] [Google Scholar]
- [19].Sánchez-Lara K, Turcott JG, Juárez-Hernández E, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr 2014;33:1017–23. [DOI] [PubMed] [Google Scholar]
- [20].Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309:2005–15. [DOI] [PubMed] [Google Scholar]
- [21].Finocchiaro C, Segre O, Fadda M, et al. Effect of n-3 fatty acids on patients with advanced lung cancer: a double-blind, placebo-controlled study. Br J Nutr 2012;108:327–33. [DOI] [PubMed] [Google Scholar]
- [22].Murphy RA, Mourtzakis M, Chu QS, et al. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 2011;117:1775–82. [DOI] [PubMed] [Google Scholar]
- [23].Murphy RA, Mourtzakis M, Chu QS, et al. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer 2011;117:3774–80. [DOI] [PubMed] [Google Scholar]
- [24].Cerchietti LC, Navigante AH, Castro MA. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr Cancer 2007;59:14–20. [DOI] [PubMed] [Google Scholar]
- [25].Jankowska R, Kosacka M. Cancer cachexia syndrome in patients with lung cancer. Wiad Lek 2003;56:308–12. [PubMed] [Google Scholar]
- [26].van der Meij BS, Langius JA, Smit EF, et al. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J Nutr 2010;140:1774–80. [DOI] [PubMed] [Google Scholar]
- [27].van der Meij BS, Langius JA, Spreeuwenberg MD, et al. Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: an RCT. Eur J Clin Nutr 2012;66:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]


