Supplemental Digital Content is available in the text
Keywords: hemodialysis, meta-analysis, residual renal function
Abstract
Background:
Residual renal function (RRF) is an important determinant of mortality and morbidity in patients undergoing hemodialysis. Different dialysis types may have different effects on RRF. We therefore conducted this meta-analysis to examine the RRF protective effect of different dialysis types for hemodialysis patients.
Methods:
A systematic search was performed on PubMed, EMbase, Web of Science, Chinese Biomedical Literature Database, Wanfang database, and China National Knowledge Infrastructure for randomized controlled trials and cohort studies. Dialysis types included low-flux hemodialysis (LFHD), high-flux hemodialysis (HFHD), hemodiafiltration (HDF), and hemodialysis and hemoperfusion (HD+HP). The mean of endogenous creatinine clearance rate (CCR) and urea clearance rate (Curea), or urine volume was used to estimate RRF [95% confidence interval (95% CI), 6.05–16.80].
Results:
There were 12 articles involving 1224 patients, including 11 random controlled trials and 1 cohort study. Meta-analysis showed that the RRF protective effect of HFHD [mean difference (MD) = 1.48, 95% CI (2.11 to 0.86), P < .01] and HD+HP [MD = 0.41, 95% CI (0.69 to 0.12), P = .005] was better than that of LFHD, and the RRF decline rate was the lowest in HFHD group [MD = 0.13, 95% CI (0.17 to 0.09), P < .01]. Descriptive analysis showed that HDF could better protect RRF when compared with LFHD. However, there was no consistency among other interventions when removing LFHD due to limited data.
Conclusion:
For patients undergoing maintenance hemodialysis, the HFHD, HD+HP and HDF may better protect RRF, compared with LFHD.
1. Introduction
Kidney function of some patients with chronic kidney disease (CKD) gradually declines and eventually progresses to end-stage renal disease (ESRD), which needs hemodialysis or peritoneal dialysis to remove toxins and excess fluid.[1,2] For patients on maintenance hemodialysis (MHD), the incidence of cardiovascular or noncardiovascular-related death is 30 to 50 folds higher than that of healthy people.[3] Recently, studies show that mortality and hospitalization rate of CKD patients can be reduced by use of statins and angiotensin II receptor antagonists.[4,5]
Of all indicators, residual renal function (RRF) is of great importance to the survival and quality of life of CKD patients.[6–9] With the commencement of hemodialysis, RRF decreases exponentially due to systemic hemodynamic changes, vascular calcification, and drug use during the hemodialysis.[10,11] The study by Brener et al[9] showed that the mortality rate and hospitalization length was significantly lower for patients with RRF than patients without RRF. The study by Rhee et al[12] showed that RRF could effectively control serum phosphate levels. In the meantime, series of studies have been conducted to explore further RRF protection.[13] For example, hemodiafiltration (HDF) uses high permeable dialysis membrane filtration to increase ultrafiltration and convective solute transport that can better clear toxins than hemodialysis.[12]
Different dialysis types may have different effects on RRF. It has been shown that high-flux hemodialysis (HFHD) may better protect RRF than low-flux hemodialysis (LFHD), and HFHD could better protect patients with parenchymal nephropathy when the primary kidney disease is further classified.[14] The study by Penne et al[15] showed no significant difference in RRF protection between HDF and LFHD after 6 months of follow-up. Except for LFHD, comparisons between other dialysis types are unclear. In this study, a systematic review and meta-analysis was performed to further examine the RRF protective effect of different dialysis types for MHD patients.
2. Materials and methods
2.1. Search strategy
A systematic search was performed on PubMed, EMbase, Web of Science, Chinese Biomedical Literature Database, Wanfang database, and China National Knowledge Infrastructure. The search terms, including high-flux, high flow, low-flux, membrane, HDF, hemoperfusion, and hemodialysis were used for the keywords and abstract of articles, and RRF for the full text until August 2017.
Detailed searching strategy is shown in Figure 1 and in Appendix 1, http://links.lww.com/MD/C484. Briefly, primary search on Cochrane Library and Joanna Briggs Institute Library for systematic reviews and clinical practice guidelines; systematic search on the above databases to extract information from eligible articles, including title, abstract, and keywords; the full text was further analyzed if the abstract met inclusion criteria; and review the references of included articles.
Figure 1.
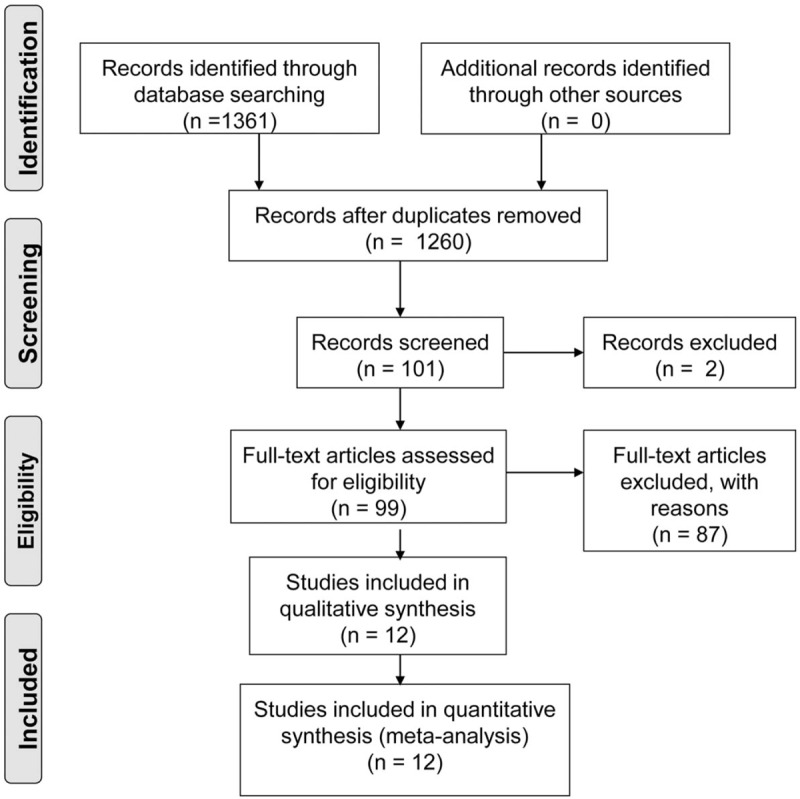
Searching strategies.
2.2. Inclusion and exclusion criteria
Articles were included if they were randomized controlled trials (RCTs) or cohort studies; patient age > 18 years; with RRF; and receiving HFHD, HDF, hemodialysis, and hemoperfusion (HD+HP) as maintenance dialysis. Articles were excluded if they were having significant proteinuria, serious infections, heart failure, cancer, or other major underlying diseases; or using nephrotoxic drugs.
The mean of endogenous creatinine clearance rate (Ccr) and urea clearance rate (Curea), or urine volume was used to estimate RRF. The outcome measures included RRF estimated by endogenous Ccr and Curea, monthly RRF decline rate, and RRF estimated by urine volume.
2.3. Information extraction and evaluation
All articles were reviewed by 2 reviewers to independently extract information, including study design, sample size, intervention, follow-up, intervention, control, outcomes, and conclusions. Studies were evaluated by 2 authors (WWL and CR) for methodological quality based on Australian Joanna Briggs Institute Evidence-Based Health Care Center Evaluation Manual (2008).[16]
2.4. Statistical analysis
Mean net change of RRF was calculated as the difference (intervention group-control group) of the change (baseline-end-point) in mean values. Standard deviations (SDs) [SD = (SDbaseline2 + SDendpoint2 − SDbaselineSDendpoint)1/2] of RRF before and after intervention were used to calculate the differences in the individual studies using the method described by Whitehead.[17] A meta-analysis was performed using RevMan5.3 (Cochrane Collaboration). The mean difference (MD) with 95% confidence interval (95% CI) was chosen to calculate the magnitude of the experimental effect. χ2 test was used for heterogeneity analysis, and heterogeneity was assessed by I2. P = .1 was used as significance level. If P > .1 and I2 < 50%, the fixed effects model was used; otherwise, the heterogeneity was assessed to determine whether random effects model can be used. If data cannot be used for meta-analysis or P < .1 with no source of heterogeneity, descriptive analysis was used. Article with the largest sample size was excluded for sensitivity analysis.
3. Results
3.1. General description of included studies
There were 1361 articles identified by systematic search, including 585 articles in English and 776 articles in Chinese. Totally, 1260 articles were excluded by screening of titles and abstracts. Upon further analysis, 12 articles were included for quality evaluation, including 7 articles in English and 5 articles in Chinese, as shown in Figure 1. General characteristics of included studies are summarized in Table 1.[14,15,18–27] The study by Zhao et al[18] was a 2∗2 RCT and thus was analyzed individually. The LFHD was used as control group in 11 articles and HFHD as control in 1 article. The intervention methods included HDF, HD + HP, or HFHD.
Table 1.
General characteristics of included studies.
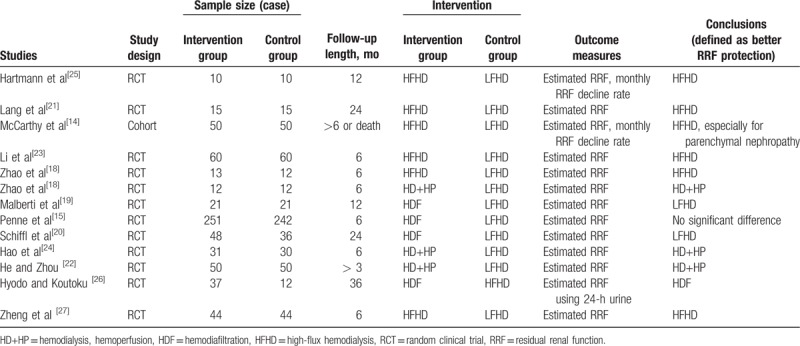
Patients were from Asia, Europe, and North America, and the research centers were mainly dialysis centers. Patients were followed up until the end of study or endpoint events, including death, kidney transplantation, or dialyzer replacement.
3.2. Quality evaluation and results of the systematic review
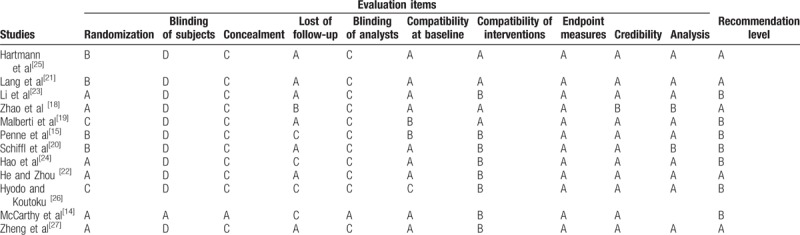
The 12 articles [14,15,18–27] were included for quality evaluation, including 11 RCTs and 1 cohort study. The overall quality was good with 5 articles of A level and 7 articles of B level, as in Table 2.
Table 2.
Quality evaluation of included studies.
3.3. The results of the meta-analysis
3.3.1. RRF protection evaluation using Ccr and Curea-estimated RRF
To determine RRF protection effect of different dialysis types, Ccr and Curea-estimated RRF were compared. First, the protective effect of HDF and LFHD on RRF was compared. Because 3 articles [15,19,20] were not eligible for meta-analysis due to either no standard value presentations or pre-existing statistical differences, statistical description was used. Two of them [19,20] showed that HDF could protect RRF. In the study by Malberti et al,[19] the RRF only significantly decreased in LFHD group (P < .05) and no significant decrease was detected in HDF group after 12 months of follow-up. In the study by Penne et al,[15] P25-P75 percentile was used to determine RRF change, and no difference was detected between HDF and LFHD group after 6 months of follow-up. In the study by Schiffl et al,[20] it showed that RRF decreased in both groups, but the RRF decrease was significantly faster in LFHD group (P < .05); the anuria (urine output < 100 mL/day) ratio was 32% in control group, significantly higher than that of HDF group (9%). The above showed that HDF had better protective effect on RRF, compared with LFHD.
The comparison between HFHD and LFHD is shown in Figure 2. The 5 articles were of high heterogeneity (P < .05, I2 = 66%); therefore, random effects model was used.[14,18,21,23,27] The results showed that the 5 articles with HFHD as intervention group with total sample size of 305 and MD of the 5 articles was −1.48 [95% CI (−2.11 to −0.86), P < .01].
Figure 2.
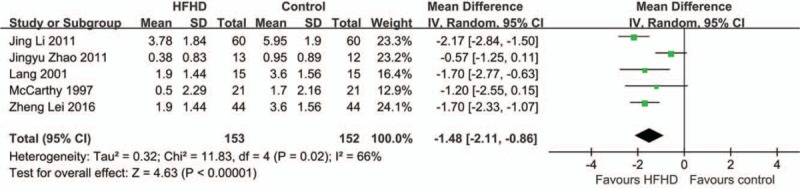
Random effect model on RRF protection for HFHD group. LFLD was considered as control group. It showed HFHD could significantly better protect RRF (P ≤ .002).
In the study by McCarthy et al,[14] the primary diseases were classified and compared. No significant differences were found between experiment and control group among polycystic kidney disease, diabetic kidney disease, and interstitial disease. HFHD could better protect RRF in primary diseases such as glomerulonephritis or renal sclerosis. The Kaplan–Meier survival analysis also showed that the mean RRF retention time was higher in experimental group (23 months) than that of control group (11 months) (P < .001).
There were 3 articles[18,22,24] with HD + HP as the intervention group with a total sample size of 185 (Fig. 3). Fixed effects model was used. The results showed that Chi-square = 2.94, P = .23, I2 = 32%, and MD was −0.41 (95% CI −0.69 to −0.12, P = .005). Sensitivity analysis showed significant differences even if the study with the largest sample size was excluded, indicating the low sensitivity and robust result. These results indicate that compared with LFHD, HFHD and HD + HP could better protect RRF.
Figure 3.
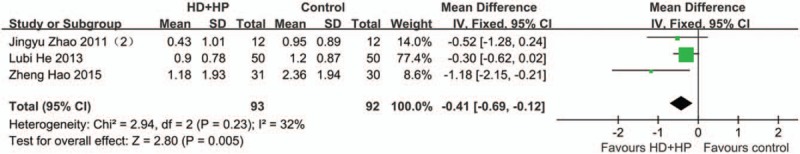
Fixed effect model on RRF protection for HD+HP group. LFLD was considered as control group. It showed HD+HP could significantly better protect RRF (P = .005).
3.3.2. RRF protection evaluation using monthly RRF decline rate
To determine RRF protection effect of different dialysis types, their protection comparisons using monthly RRF decline rate were performed. There were 2 articles included in Fig. 4 with Chi-square = 0.00, P = 1.00, I2 = 0%.[14,25] Therefore, fixed effects model was used for meta-analysis. The combined sample size was 62 and the MD was −0.13 with 95% CI −0.17 to −0.09 (P < .01). Therefore, compared with HFHD, the RRF decline rate of LFHD was higher, indicating that HFHD may better protect RRF.
Figure 4.
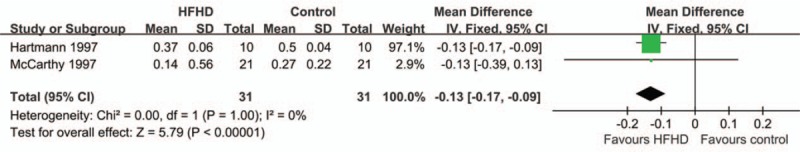
Meta-analysis of monthly RRF decline rate for HFHD group. LFLD was considered as control group with small heterogeneity. It showed the RRF decline in LFLD group was higher than that of HFHD, indicating HFHD could better protect RRF.
3.3.3. RRF protection evaluation using urine-estimated RRF
To determine RRF protection effect of different dialysis types, their protection comparisons using urine-estimated RRF were performed. The study by Hyodo and Koutoku[26] showed that the urine-estimated RRF significantly decreased in both HDF and HFHD groups with a different rate (P = .024). It indicated that HDF could better protect RRF than HFHD.
4. Discussion
Currently, ESRD treatment includes hemodialysis, peritoneal dialysis, and renal transplant, and hemodialysis is the main option.[1,2,28] Most ESRD patients still retain part of renal function before dialysis, namely RRF; however, it would gradually decrease and may be completely lost during the dialysis process.[21,25] RRF is of high protection for peritoneal dialysis patients, and its effect for hemodialysis patients is drawing increasing attention.[28] RRF would promote phosphorus control, improve nutrition status, and reduce mortality.[9,12,29] The current RRF protection measures include diet, medications, and change of hemodialysis types.[24,30,31] Dialysis could mostly contribute to RRF decline, and the dialysis dose (such as Kt/V) is of high controversy.[21] For example, the ultrafiltration during hemodialysis causes decreased effective blood volume of kidney, and the repetitive renal hemodynamic instability may lead to RRF decline and activation of complement system by interaction of dialysis membrane and dialysates.[21] In this meta-analysis, due to the inconsistent protection effect of different dialysis types, LFHD was used as control group to compare with other types of hemodialysis.
RRF is the retained filtration and endocrine function of damaged renal tissue, and is commonly expressed as glomerular filtration rate, leaving endocrine functions unanalyzed.[32] The exogenous marker measured renal clearance is considered golden standard for glomerular filtration rate, such as Inulin and iohexol.[33] However, it may not be clinically feasible due to the limited resources and various types of primary kidney diseases.[33,34] The endogenous markers, namely Ccr and Curea, are clinically used for RRF calculation; however, it may be influenced by many factors, such as its intrinsic bias, urine sample collection, muscle mass, diet, and activities.[32] Although other RRF indicators have been exploring, Ccr and Curea are still the main indicators for RRF.[32,33] In this study, there were 11 articles with LFHD as control group using Ccr and Curea to estimate RRF, and the results were stable.
Our quantitative synthesis showed that compared with LFHD, HFHD or HD + HP could better protect RRF. This may be resulted from the higher Kt/V, use of biocompatible membranes, and higher β2 microglobulin clearance rate of HFHD and HD + HP. Schiffl et al[14,19,20,21,25] showed that the RRF decline rate was slower in dialysis patients using synthetic high permeability polysulfone membrane, compared with those using nonbiocompatible membrane, which may lead to activation of peripheral blood mononuclear cells, complement activation, inflammatory responses, and associated kidney damages. It is consistently shown that HFHD may better protect RRF when compared with LFHD. When patients were further classified based on primary kidney diseases, it showed that compared with polycystic kidney, diabetic nephropathy, and interstitial nephropathy, HFHD could better protect nondiabetic parenchymal nephropathy (P < .05).[14] This may lie in that RRF decline rate differs among different primary kidney diseases, and the RRF decline rate of polycystic kidney and diabetic nephropathy is faster than that of glomerular nephritis.[35] Strictly speaking, the results of the meta-analysis should not be combined as such, but for the limited number of RCT studies, the cohort study[14] was included in the merger discussion. HP clears middle and large molecular weight substances in blood, such as waste and medications by nonspecific adsorption.[36] The above results showed that HD + HP could better protect RRF; however, all studies were conducted in China, leading to potential bias. For the studies of HDF's protection on RRF, the study by Penne et al[15] showed no significant difference between HDF and LFHD, which was due to intervention measures, such as different amount of phosphate binders, while the other 2 studies[19,20] showed the higher protection of HDF on RRF.
One article showed that without dialysis water removal, HDF could better protect RRF than HFHD.[26] However, due to the complicated dialysis treatment and significantly deviated urine-estimated RRF, there is insufficient evidence to determine the RRF protection comparison between HFHD and HDF.
This study has some limitations. First, this study is of high heterogeneity by that all included studies were small-scale with significant differences among included samples, varied intervention and follow-up length, as well as limited information on blinding. Second, in this study, there is no consistent intervention conclusion, except for LFHD, due to the limitations of the original study. Third, the cohort study by McCarthy et al[14] may increase the heterogeneity of our study.
In summary, this study suggests that HFHD, HD + HP, and HDF may be of better RRF protection than that of LFHD. Thus, in patients with RRF, different dialysis types should be comprehensively used to better protect RRF. However, high-quality RCT is needed to provide solid evidence on the RRF protection among different dialysis types.
Acknowledgment
We thank Professor Yan Hu from Nursing School, Fudan University, for the preparation of the manuscript.
Author contributions
Conceptualization: Yanpei Cao.
Formal analysis: Chong Ren.
Funding acquisition: Bihong Huang.
Software: Xiaoli Yang.
Supervision: Yanpei Cao, Bihong Huang.
Validation: Xin Han.
Writing – review & editing: Wenwen Lu.
Supplementary Material
Footnotes
Abbreviations: Ccr = endogenous creatinine clearance rate, CI = confidence interval, CKD = chronic kidney disease, Curea = urea clearance rate, ESRD = end-stage renal disease, HD+HP = hemodialysis, hemoperfusion, HDF = hemodiafiltration, HFHD = high-flux hemodialysis, LFHD = low-flux hemodialysis, MD = mean difference, MHD = maintenance hemodialysis, RCT = randomized controlled trial, RRF = residual renal function.
Funding/support: This study was funded by Shanghai Health and Family Planning Commission Hemodialysis Task [Grant Number 201540085], Nursing Association Peritoneal Dialysis Task [Grant Number 2014M5-B09], Fudan University Peritoneal Dialysis Task [Grant Number FNF2014.30], and Fudan University Hemodialysis Task [Grant Number FNF201529].
This article does not contain any studies with human participants or animals performed by any of the authors.
All authors declare that they have no conflict of interest.
Supplemental Digital Content is available for this article.
References
- [1].Rosselli D, Rueda JD, Diaz CE. Cost-effectiveness of kidney transplantation compared with chronic dialysis in end-stage renal disease. Saudi J Kidney Dis Transpl 2015;26:733–8. [DOI] [PubMed] [Google Scholar]
- [2].Palmer SC, Palmer AR, Craig JC, et al. Home versus in-centre haemodialysis for end-stage kidney disease. Cochrane Database Syst Rev 2014;20:CD009535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009;302:1782–9. [DOI] [PubMed] [Google Scholar]
- [4].Nigwekar SU, Bhan I, Turchin A, et al. Statin use and calcific uremic arteriolopathy: a matched case-control study. Am J Nephrol 2013;37:325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tanaka M, Yamashita T, Koyama M, et al. Impact of use of angiotensin II receptor blocker on all-cause mortality in hemodialysis patients: prospective cohort study using a propensity-score analysis. Clin Exp Nephrol 2016;20:469–78. [DOI] [PubMed] [Google Scholar]
- [6].Penne EL, van der Weerd NC, Grooteman MP, et al. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol 2011;6:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guzzo I, Mancini E, Wafo SK, et al. Residual renal function and nutrition in young patients on chronic hemodialysis. Pediatr Nephrol 2009;24:1391–7. [DOI] [PubMed] [Google Scholar]
- [8].Kim SG, Kim NH. The effect of residual renal function at the initiation of dialysis on patient survival. Korean J Intern Med 2009;24:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brener ZZ, Thijssen S, Kotanko P, et al. The impact of residual renal function on hospitalization and mortality in incident hemodialysis patients. Blood Purif 2011;31:243–51. [DOI] [PubMed] [Google Scholar]
- [10].Chen HC, Chou CY, Jheng JS, et al. Loss of residual renal function is associated with vascular calcification in hemodialysis patients. Ther Apher Dial 2016;20:27–30. [DOI] [PubMed] [Google Scholar]
- [11].Xydakis D, Papadogiannakis A, Sfakianaki M, et al. Residual renal function in hemodialysis patients: the role of angiotensin-converting enzyme inhibitor in its preservation. ISRN Nephrol 2012;2013:184527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rhee H, Yang JY, Jung WJ, et al. Significance of residual renal function for phosphate control in chronic hemodialysis patients. Kidney Res Clin Pract 2014;33:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mathew AT, Fishbane S, Obi Y, et al. Preservation of residual kidney function in hemodialysis patients: reviving an old concept. Kidney Int 2016;90:262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McCarthy JT, Jenson BM, Squillace DP, et al. Improved preservation of residual renal function in chronic hemodialysis patients using polysulfone dialyzers. Am J Kidney Dis 1997;29:576–83. [DOI] [PubMed] [Google Scholar]
- [15].Penne EL, van der Weerd NC, van den Dorpel MA, et al. Short-term effects of online hemodiafiltration on phosphate control: a result from the randomized controlled Convective Transport Study (CONTRAST). Am J Kidney Dis 2010;55:77–87. [DOI] [PubMed] [Google Scholar]
- [16].Luctkar-Flude M, Groll D. A systematic review of the safety and effect of neurofeedback on fatigue and cognition. Integr Cancer Ther 2015;14:318–40. [DOI] [PubMed] [Google Scholar]
- [17].Anne whitehead. Meta-Analysis of controlled clinical Trials[M]. England: John Wiley & Sons Ltd, 2002:82–88. [Google Scholar]
- [18].Zhao JY, Wu J, Wang NP. The impact of different blood purification methods on residual renal function among maintenance hemodialysis patients. Chin J Blood Purif 2011;2:71–3. [Google Scholar]
- [19].Malberti F, Surian M, Farina M, et al. Effect of hemodialysis and hemodiafiltration on uremic neuropathy. Blood Purif 1991;9:285–95. [DOI] [PubMed] [Google Scholar]
- [20].Schiffl H, Lang SM, Fischer R. Effects of high efficiency post-dilution on-line hemodiafiltration or conventional hemodialysis on residual renal function and left ventricular hypertrophy. Int Urol Nephrol 2013;45:1389–96. [DOI] [PubMed] [Google Scholar]
- [21].Lang SM, Bergner A, Topfer M, et al. Preservation of residual renal function in dialysis patients: effects of dialysis-technique-related factors. Perit Dial Int 2001;21:52–7. [PubMed] [Google Scholar]
- [22].He LB, Zhou L. Efficacy analysis of hemodialysis joint hemoperfusion for diabetic nephropathy in patients with multiple organ dysfunction. Med Recapit 2013;15:2851–3. [Google Scholar]
- [23].Li J, Xiao Q, Han ZW, et al. The influence of high flux hemodialysis on residual renal function of maintenance hemodialysis patients. J Clin Nephrol 2011;11:554–6. [Google Scholar]
- [24].Hao Z, Ma YL, Tang ZY. The effect of hemoperfusion combined with hemodialysis on residual renal function for patients with maintenance hemodialysis. Chin J Integr Trad West Nephrol 2015;7:626–7. [Google Scholar]
- [25].Hartmann J, Fricke H, Schiffl H. Biocompatible membranes preserve residual renal function in patients undergoing regular hemodialysis. Am J Kidney Dis 1997;30:366–73. [DOI] [PubMed] [Google Scholar]
- [26].Hyodo T, Koutoku N. Preservation of residual renal function with HDF. Contrib Nephrol 2011;168:204–12. [DOI] [PubMed] [Google Scholar]
- [27].Zheng L, Lei J, Wei L. Protective effects of high and low flux hemodialysis on residual renal function in uremic patients with maintenance hemodialysis. Hainan Med J 2016;12:1912–5. [Google Scholar]
- [28].Yohanna S, Alkatheeri AM, Brimble SK, et al. Effect of neutral-pH, low-glucose degradation product peritoneal dialysis solutions on residual renal function, urine volume, and ultrafiltration: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2015;10:1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Suda T, Hiroshige K, Ohta T, et al. The contribution of residual renal function to overall nutritional status in chronic haemodialysis patients. Nephrol Dial Transplant 2000;15:396–401. [DOI] [PubMed] [Google Scholar]
- [30].Moist LM, Port FK, Orzol SM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 2000;11:556–64. [DOI] [PubMed] [Google Scholar]
- [31].Caria S, Cupisti A, Sau G, et al. The incremental treatment of ESRD: a low-protein diet combined with weekly hemodialysis may be beneficial for selected patients. BMC Nephrol 2014;15:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Davenport A. Measuring residual renal function in dialysis patients: can we dispense with 24-hour urine collections? Kidney Int 2016;89:978–80. [DOI] [PubMed] [Google Scholar]
- [33].Davenport A. Will incremental hemodialysis preserve residual function and improve patient survival? Semin Dial 2015;28:16–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lew SQ. How to measure residual renal function in patients on maintenance hemodialysis. Adv Ren Replace Ther 1994;1:185–93. [DOI] [PubMed] [Google Scholar]
- [35].Haynes R, Staplin N, Emberson J, et al. Evaluating the contribution of the cause of kidney disease to prognosis in CKD: results from the Study of Heart and Renal Protection (SHARP). Am J Kidney Dis 2014;64:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cai W, Miao S, Wang P, et al. [Efficacy and mechanism of hemoperfusion plus hemodialysis for peripheral neuropathy of uremic patients on maintenance hemodialysis]. Zhonghua Yi Xue Za Zhi 2015;95:1319–22. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


