Abstract
Aging-associated cognitive decline is closely linked to illness, dementia, increased mortality, and is a major health and social issue. The purpose of this study was to determine modifiable factors associated with cognitive performance.
We analyzed data from a random sample of participants of the Third National Health and Nutrition Examination Survey, which is a cross-sectional survey, of the US population, aged 20 to 59 years, who underwent computer-based neurocognitive testing. There were 5 outcome measures in 3 neurocognitive tests: the mean of simple reaction time test, the mean total latency of the symbol digit substitution test (SDST), the average number of errors of the SDST, the average trials to criterion of the serial digit learning test (SDLT), and the average total score of the SDLT.
Socioeconomic status, including older age, black ethnicity, lower income ratio, and lower education level, were associated with poorer neurocognitive function in all analyzed tests. In addition, participants with poor health, nonsmokers, and nondrinkers performed worse in all administered tests compared with individuals with good health, smokers, and participants consuming alcoholic beverages. Dietary and biochemical characteristics of the blood were not consistently associated with neurocognitive performance.
Our results indicate that socioeconomic factors, health-related and dietary habits, biochemical parameters of the blood, and job category were associated with neurocognitive performance in visual attention, learning, and concentration in a large, nationally representative sample of healthy, ethnically diverse 20 to 59-year-olds. Future studies are needed to understand the mechanisms of cognitive aging and the factors that contribute to its individual differences.
Keywords: neurocognitive function, serial digit learning test, simple reaction time test, symbol digit substitution test, the Third National Health and Nutrition Examination Survey (NHANES III)
1. Introduction
It is projected that by 2050, the number of people aged 60 and over will more than double compared with similar statistics in 2015 (www.un.org). Low birth rates along with improved survival at all ages have contributed to population aging, which has become a growing global socioeconomic concern, as it puts a strain on medical care systems and social services. Cognitive decline, poor memory, and other neurological complications often arise as individuals age. Up to 30% of people aged 85 and over experience mild cognitive impairment,[1] and up to 15% of these patients develop dementia[2]—a condition characterized by severe cognitive decline, disability, and high mortality. Assessment of modifiable factors influencing the neurocognitive outcome is essential for developing of nondrug interventions.
In addition to advanced age, multiple factors might contribute to the development of dementia such as low education level, high blood pressure, diabetes, and smoking.[3] Poor smelling ability, higher homocysteine level,[3] history of depression, low health status, coronary artery disease, stroke, and arthritis were reported as potential risk factors associated with cognitive decline.[4] Interestingly, according to the same study, antidepressant consumption along with kidney disease and married status had inverse correlation with cognitive decline. Several reports supported the idea that exposure to any type of systemic chronic illness over time jeopardizes the integrity of cognitive function.[5] Examples of such chronic illnesses include asthma, chronic obstructive pulmonary disease, liver cirrhosis, renal, autoimmune diseases, sleep disorders, multiple types of cancer, and AIDS.[5] Identified risk factors also include neurotoxicity due to environmental or occupational conditions, such as contact with solvents and lead.[6] Genetic factors may also affect cognitive performance. The apolipoprotein E ε4 allele, for instance, is predictive of poorer cognitive function, and the condition could become exacerbated by additional chronic illness.[7] Cognitive function is profoundly affected by unbalanced levels of hormones such as estrogen, thyroid and pituitary hormones, and cortisol.[5] Finally, lifestyle choices and behaviors have a significant impact on health status in general and cognitive performance in particular. Smoking,[8] drug (cocaine, opiates, etc) and alcohol abuse,[9] and physical inactivity[2] along with several dietary insufficiencies such as zinc, thiamine, folate, and vitamins B6 and B12[3] have been associated with compromised cognitive abilities. Neurocognitive performance is affected by nutritional factors obtained through diet: glucose, omega-3 fatty acids, and iron.[10,11] Inconsistent results have been observed when investigating effects of such nutrients as vitamin D,[12] carotenoids, folate, polyunsaturated fatty acids, and curcumin.[1] High calorie diets, and also increased consumption of refined carbohydrates predict poorer cognitive outcome; on the contrary, calorie restriction and adherence to the Mediterranean diet have generated mixed and even conflicting results possibly due to differences in methodology of assessment.[1,13,14] Lack of definitive conclusions on the effects of nutrition on neurocognitive function in aged individuals warrants additional research efforts such as this study to identify modifiable factors linked to cognitive performance.
2. Materials and methods
2.1. Study population
The study analyzed data from the Third National Health and Nutrition Examination Survey (NHANES III)—an assessment by the National Center for Health Statistics (NCHS) of the health and nutrition status of a nationally representative sample of noninstitutionalized US civilians 2 months and older. The goal behind NHANES III was to collect nationally representative data on the nutritional status and health of the US population.[15,16] Cognitive function evaluation was performed on a random half-sample of NHANES III participants 20 to 59 years old (n = 5662). NHANES received approval from the National Center for Health Statistics Research Ethics Review Board, and every participant in the database provided written consent.[17] Also, NHANES III database has been de-identified.
2.2. Testing of cognitive function and definition of outcomes
The primary outcome of the present study was the level of the neurocognitive function, as measured by the following 3 neurobehavioral computerized tests: the simple reaction time test (SRTT), the symbol digit substitution test (SDST), and the serial digit learning test (SDLT).[18,19]
The SRTT is designed to evaluate simple reaction time, general alertness, and motor speed measured in milliseconds. The subjects were instructed to select the button to register their response as soon as they saw the square on the screen. The interval between trials varied randomly according to a uniform distribution ranging from 2.5 to 5.0 seconds to limit anticipatory responses. Participants were given a total of 50 trials. The SRTT was scored as the average reaction time, excluding the first 10 test trials.[18,19]
The SDST is designed to measure coding ability and visual attention. The subject is presented with an array of nine numbers matched with a symbol. Next, a set of symbols is given and the participant must type the correct digit for each symbol as quickly as possible. Four trials are conducted, with a different pairing of digits and symbols on each trial. The SDST was scored as the average total time, in seconds, for completion of the 4 trials.[18,19]
The SDLT evaluates learning and recall. It involves repeated presentation of a sequence of 8 digits displayed 1 at a time on the computer screen. After the sequence of digits is displayed, the subject is required to enter the sequence of numbers in the order in which they were presented. Testing continues until the subject correctly entered 2 consecutive trials or until the subject attempted 8 trials. The total score on the SDLT equals the sum of the errors committed during the trials.[18,19]
There were 5 measures out of 3 neurocognitive tests as dependent variables in this study, including the mean of SRTT, the mean total latency of the SDST, the average number of errors of the SDST, the average trials to criterion of the SDLT, and the average total score of the SDLT.
2.3. Study variables
The variables obtained for each case included patient demographics (age, sex, race/ethnicity, poverty income ratio, education, marital status, occupation), health status/comorbidities (health status, diabetes, hypertension, anemia, major depression, dysthymic disorder, overweight), health behaviors (smoking history, alcohol use, breakfast consumption, walking activity, social support, caring for/living with pets), dietary and nutritional intake (total protein intake, total unsaturated fatty acid intake, total carbohydrate intake, intakes of ascorbic acid, vitamin E, vitamin B12, vitamin B6, riboflavin, thiamin, and folacin), environmental and laboratory variables (room temperature, presence of cigarette smoking, blood lead level, urinary cadmium level, serum C-reactive protein, serum glucose level, glycated hemoglobin level, serum vitamin D level). Marital status was categorized as married, never married, and divorced. Classification of occupations was based on the study by Hnizdo et al,[20] and was re-categorized as office building services, rubber and chemical, transportation and trucking, metal, repair service, construction, and other industries
2.4. Statistical analysis
Data were represented by mean ± standard error for continuous variables, or unweighted counts (weighted %) for categorical variables. Univariate and multivariate linear regression analyses were performed using the Complex Samples General Linear Model (CSGLM) to explore the association of the study variables with the level of central nervous system (CNS) function. Variables that showed a tendency of association with the level of cognitive function (P < .05) in univariate analysis were evaluated using a multivariate logistic regression model.
All analyses included special sample weight (WTPFCNS6, used only in conjunction with the CNS subsample and with items collected as part of the CNS component of the examination); stratum, and primary sampling units (PSU) per recommendations from NCHS; complex sample analysis to address oversampling; nonresponse; and non-coverage to provide nationally representative estimates.
All statistic assessments were 2-sided and were evaluated at the 0.05 level of significance. Statistical analyses were performed by IBM SPSS statistical software version 22 for Windows (IBM Corp., Armonk, NY).
2.5. Ethics
This study used data from NHANES III database and therefore we did not have to obtain informed consent from the participants or obtain approval from an institutional review board.
3. Results
3.1. Subject characteristics
The NHANES III database (1988–1994) included information on a total of 31,311 participants. Out of 11,306 participants aged between 20 and 59 years, 5662 participants underwent CNS function evaluation. Using NHANES sample weight, the analytic sample size was estimated to be equivalent to a population-based sample size of 137,079,473 participants. The average age of participants was 37 years with 74.4% individuals of white race; the proportion of male subjects was 49%. The subjects’ characteristics, and also unadjusted mean cognitive function test scores are summarized in Table 1. The mean of simple reaction time on the SRTT was 233.76 milliseconds, the mean total latency of the SDST was 22.78 seconds, the average number of errors on the SDST was 1.28, the average trials to criterion on the SDLT was 4.66, and the average total score of the SDLT was 4.48 (Table 1).
Table 1.
Subject characteristics (unweighted n = 5662, weighted n = 137,079,473).

3.2. Analyses of associated factors on CNS function
3.2.1. SRTT/mean reaction time test
The result of univariate linear regression analysis revealed that demographic and socioeconomic status, including age, sex, race, income ratio, education level, marital status, and occupation category, were associated with changes in the reaction time on the SRTT. In addition, lifestyle factors and health status such as smoking, alcohol consumption, living with pets, talking on the phone with family or friends, spending time with friends or relatives, diabetes diagnosis, hypertension, and body mass index (BMI) also had an effect on the results of the SRTT. And finally, dietary habits, including total protein intake, total unsaturated fatty acid intake, total carbohydrate intake, levels of vitamin E, vitamin B6, riboflavin, thiamin, folacin, urinary cadmium, serum C-reactive protein, serum glucose, glycated hemoglobin, and serum vitamin D affected the reaction time on the SRTT (Table 2).
Table 2.
Relationships between subject characteristics and the mean reaction time on the SRTT.
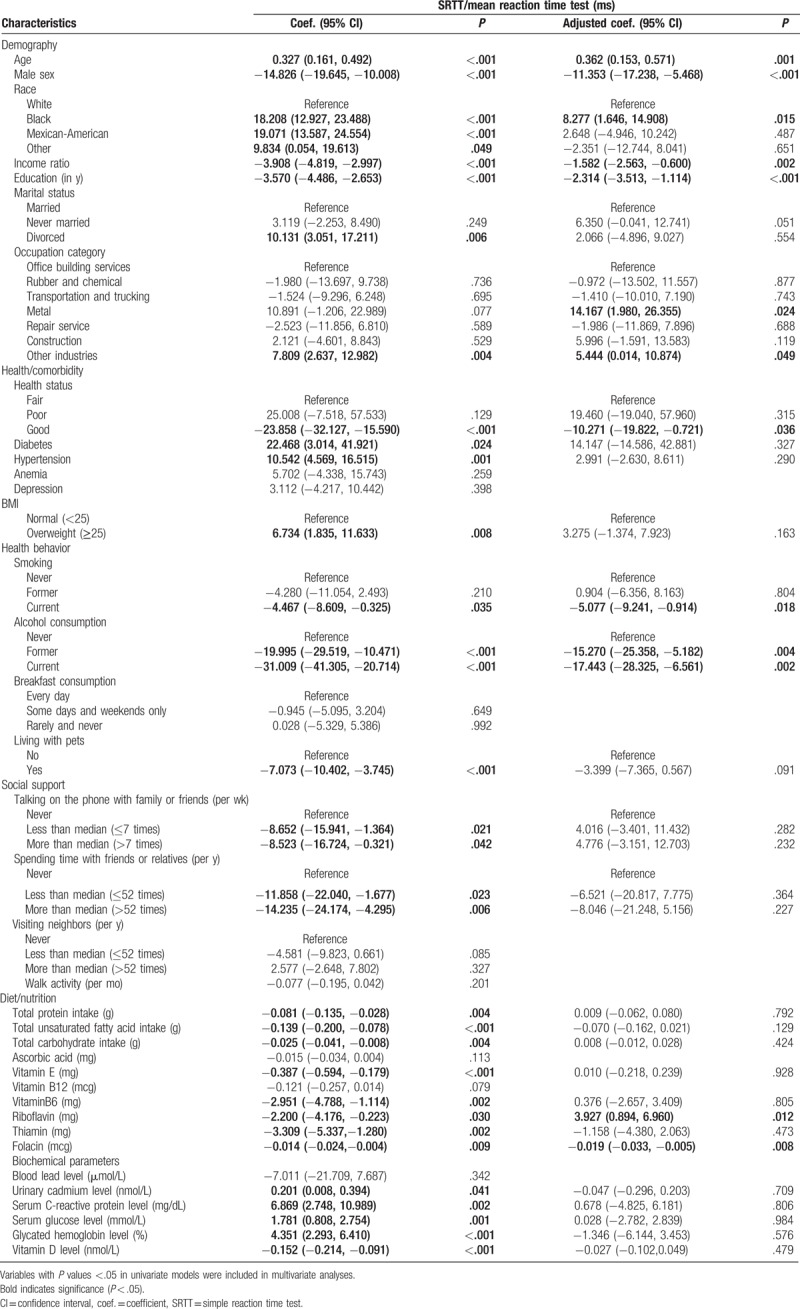
Multivariate linear regression analysis demonstrated that increased age was associated with increased reaction time on the SRTT (adjusted coefficient 0.362, 95% confidence interval [CI] 0.153, 0.571, P = .001), whereas higher income ratio and higher education level were associated with decreased reaction time on the SRTT (adjusted coefficient −1.582, 95% CI −2.563, −0.600, P = .002; and adjusted coefficient −2.314, 95% CI −3.513, −1.114, P < .001, respectively). Males had faster reaction than females (adjusted coefficient −11.353, 95% CI −17.238, −5.468, P < 0.001), and black race participants showed increased reaction time compared with white race participants (adjusted coefficient 8.277, 95% CI 1.646, 14.908, P = .015). Occupation category was also associated with variation in SRTT reaction time: metal industry workers demonstrated slower reaction compared with office building services employees (adjusted coefficient 14.167, 95% CI 1.980, 26.355, P = .024). Participants with good health status had faster reaction than participants in fair health condition (adjusted coefficient −10.271, 95% CI −19.822, −0.721, P = .036). Our analysis demonstrated that currently smoking participants had faster reaction compared to participants who never smoked (adjusted coefficient −5.077, 95% CI −9.241, −0.914, P = .018); and participants who formerly and currently consumed alcohol had faster reaction than participants who never consumed alcoholic beverages (adjusted coefficient −15.270, 95% CI −25.358, −5.182, P = .004; adjusted coefficient −17.443, 95% CI −28.325, −6.561, P = .002, respectively). Riboflavin consumption was associated with increased reaction time (adjusted coefficient 3.927, 95% CI 0.894, 6.960, P = .012), whereas intake of folacin was associated with faster reaction on the SRTT (adjusted coefficient −0.019, 95% CI −0.033, −0.005, P = .008).
3.2.2. SDST/mean total latency
The result of univariate linear regression analysis indicated that demographic and socioeconomic status, including age, sex, race, income ratio, education level, marital status, and occupation category, were associated with changes in the mean total latency in the SDST. In addition, health status and lifestyle habits, such as diagnosed diabetes, hypertension, BMI, smoking, drinking, habits to eat breakfast, living with pets, talking on phone with family or friends, and spending time with friends or relatives, also affected the results for the SDST. Nutrition habits and biochemical parameters, including total protein intake, total unsaturated fatty acid intake, total carbohydrate intake, ascorbic acid, vitamin E, vitamin B6, riboflavin, thiamin, folacin, blood lead levels, urinary cadmium, serum C-reactive protein, serum glucose, glycated hemoglobin, and serum vitamin D levels, were also strongly associated with changes in the mean total latency of SDST (Table 3).
Table 3.
Relationships between subject characteristics, mean total latency, and the number of errors on the SDST.
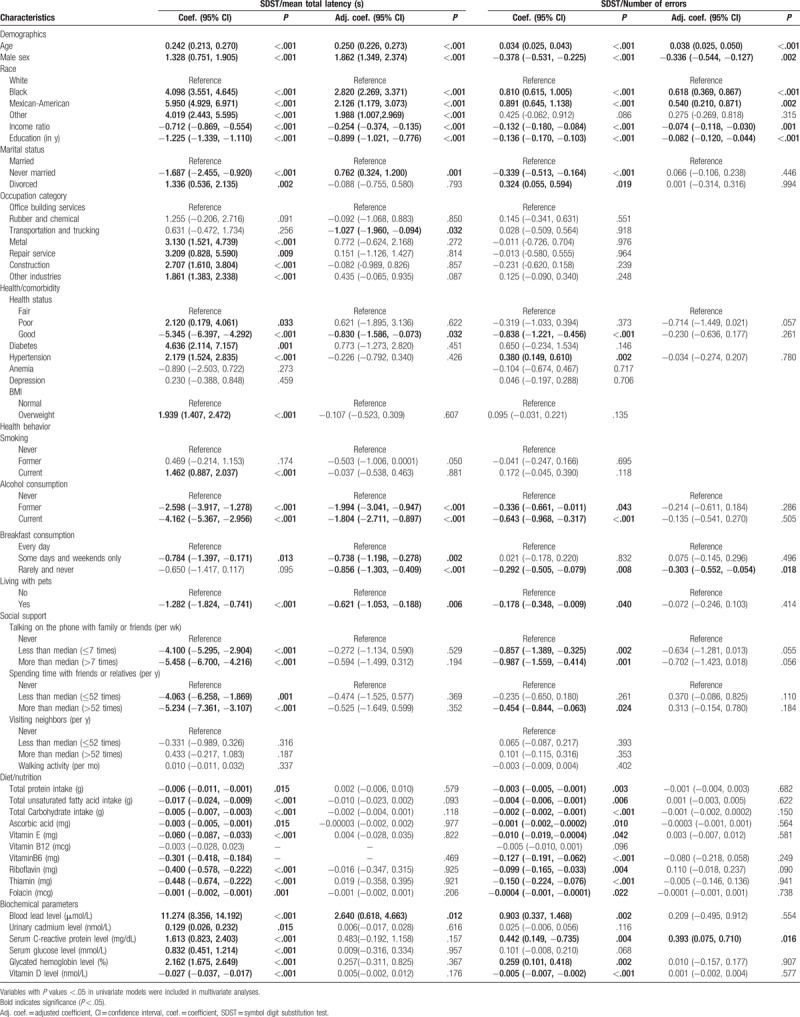
Multivariate linear regression analysis demonstrated that increased age (adjusted coefficient 0.250, 95% CI 0.226, 0.273, P < .001) was associated with increased mean total latency in the SDST, whereas higher income ratio and higher education level were associated with decreased mean total latency (adjusted coefficient −0.254, 95% CI −0.374, −0.135, P < .001; and adjusted coefficient −0.899, 95% CI −1.021, −0.776, P < .001, respectively). Moreover, sex, race, and marital status were associated with variations in the mean total latency. Specifically, males had higher latency time than females (adjusted coefficient 1.862, 95% CI 1.349, 2.374, P < .001), Black and Mexican-American participants had higher latency time compared with white participants (adjusted coefficient 2.820, 95% CI 2.269, 3.371, P < .001; and adjusted coefficient 2.126, 95% CI 1.179, 3.073, P < .001, respectively); and never married participants showed higher latency time compared with married participants (adjusted coefficient 0.762, 95% CI 0.324, 1.200, P < .001). Participants with good health status had lower latency time than participants with fair health status (adjusted coefficient −0.830, 95% CI −1.586, −0.073, P = .032). Health-related behaviors, including drinking, habit to eat breakfast, and living with pets, were also associated with the changes in the mean total latency in the SDST. Specifically, former and current alcohol consumers had lower latency time than participants who never consumed alcoholic beverages (adjusted coefficient −1.994, 95% CI −3.041, −0.947, P < .001; adjusted coefficient −1.804, 95% CI −2.711, −0.897, P < .001, respectively); participants who ate breakfast occasionally or rarely had lower latency time than those who regularly ate breakfast (adjusted coefficient −0.738, 95% CI −1.198, −0.278, P = .002; adjusted coefficient −0.856, 95% CI −1.303, −0.409, P < .001); participants who lived with pets also had lower latency time than those who did not live with pets (adjusted coefficient −0.621, 95% CI −1.053, −0.188, P = .006). Higher levels of lead in the blood were associated with increase in the mean total latency of SDST (adjusted coefficient 2.640, 95% CI 0.618, 4.663, P = .012).
3.2.3. SDST/number of errors
The result of univariate linear regression analysis indicated that demographic and socioeconomic status, including age, sex, race, income ratio, education level, and marital status, was significantly associated with number of errors in the SDST. In addition, health status and lifestyle habits, such as hypertension, drinking, habit to eat breakfast, living with pets, habit to talk on the phone with family or friends, and spending time with friends or relatives, had an effect on the errors in the SDST. Dietary habits and blood biochemical parameters, including total protein intake, total unsaturated fatty acid intake, total carbohydrate intake, ascorbic acid, levels of vitamin E, vitamin B6, riboflavin, thiamin, folacin, blood lead, serum C-reactive protein, glycated hemoglobin, and serum vitamin D, were also associated with the number of errors in the SDST (Table 3).
Multivariate linear regression analysis demonstrated that increased age was associated with increased number of errors on the SDST (adjusted coefficient 0.038, 95% CI 0.025, 0.050, P < .001), whereas higher income ratio and higher education level were associated with decreased number of errors on the SDST (adjusted coefficient −0.074, 95% CI −0.118, −0.030, P = .001; and adjusted coefficient −0.082, 95% CI −0.120, −0.044, P < .001, respectively). We found that sex and race had a strong association with the number of errors of SDST, with males having a lower number of errors than females (adjusted coefficient −0.336, 95% CI −0.544, −0.127, P = .002), and black and Mexican-American participants having a higher number of errors than white participants (adjusted coefficient 0.618, 95% CI 0.369, 0.867, P < .001; adjusted coefficient 0.540, 95% CI 0.210, 0.871, P = .002, respectively). A habit to eat breakfast was associated with the number of errors on the SDST, with participants who rarely eat breakfast having a lower number of errors than participants who eat breakfast every day (adjusted coefficient −0.303, 95% CI −0.552, −0.054, P = .018). Higher levels of serum C-reactive protein was associated with increased number of errors on the SDST (coefficient 0.393, 95% CI 0.075, 0.710, P = .016).
3.2.4. SDLT/trials to criterion
The result of univariate linear regression analysis revealed that demographic and socioeconomic status, including age, race, income ratio, education level, marital status, and occupation category, was significantly associated with the number of trials to reach criterion in the SDLT. Health status and lifestyle habits, such as diabetes, hypertension, BMI, smoking, drinking, living with pets, talking on the phone with family or friends, and spending time with friends or relatives, also effected the number of trials to reach criterion in the SDLT. In addition, dietary habits and blood biochemical parameters, including total protein intake, total unsaturated fatty acid intake, total carbohydrate intake, ascorbic acid, vitamin E, vitamin B12, vitamin B6, riboflavin, thiamin, folacin, blood lead, urinary cadmium, serum C-reactive protein, serum glucose, glycated hemoglobin, and serum vitamin D, were significantly associated with the number of trials needed to reach criterion in the SDLT (Table 4).
Table 4.
Relationships between subject characteristics, number of trials needed to reach criterion, and the total score on the SDLT.
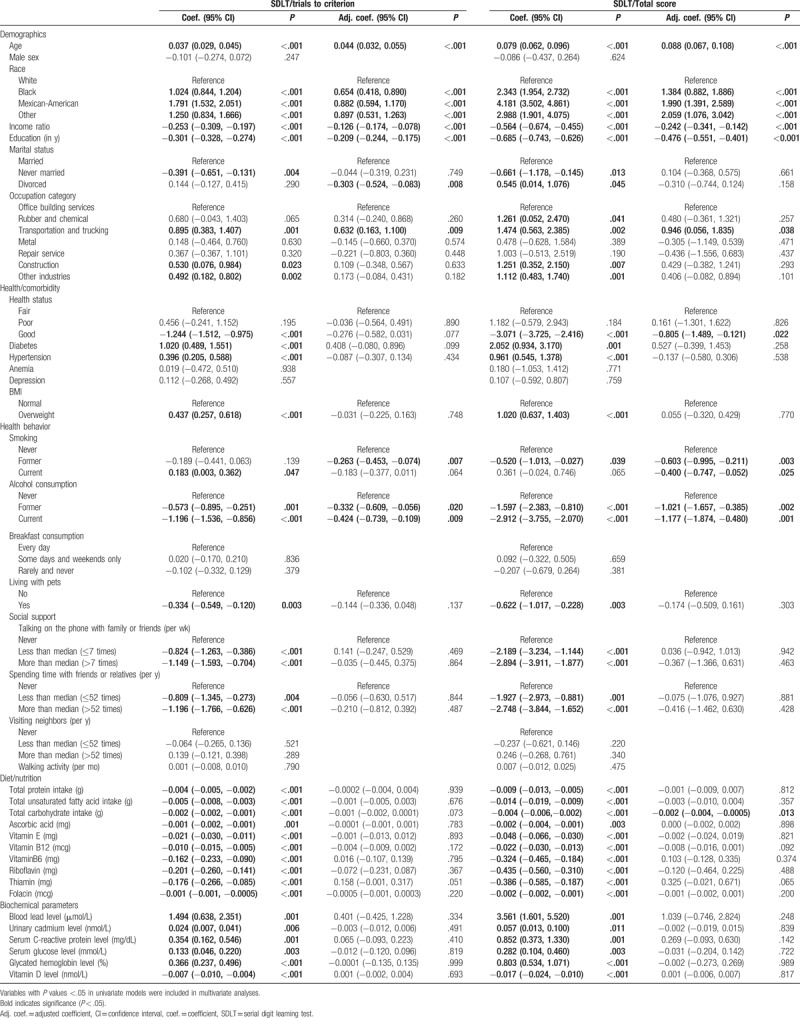
Multivariate linear regression analysis demonstrated that increased age was associated with increased number of trials needed to reach criterion in the SDLT (adjusted coefficient 0.044, 95% CI 0.032, 0.055, P < .001), whereas higher income ratio and higher education level were associated with reduced number of trials needed to reach criterion in the SDLT (adjusted coefficient −0.126, 95% CI −0.174, −0.078, P < .001; and adjusted coefficient −0.209, 95% CI −0.244, −0.175, P < .001, respectively). In addition, we found that race, marital status, and occupation category were associated with the number of trials needed to reach criterion in the SDLT. Specifically, black and Mexican-American participants needed more trials compared with white participants (adjusted coefficient 0.654, 95% CI 0.418, 0.890, P < .001; adjusted coefficient 0.882, 95% CI 0.594, 1.170, P < .001, respectively); divorced participants needed fewer trials than married participants (adjusted coefficient −0.303, 95% CI −0.524, −0.083, P = .008); participants working in the transportation and trucking needed more trials than office building services workers (adjusted coefficient 0.632, 95% CI 0.163, 1.100, P = .009). Health-related behaviors, such as smoking and alcohol consumption, were also associated with the variations in the number of trials needed to reach criterion in the SDLT. Participants who used to smoke and former and current drinkers required fewer trials compared with participants who never smoked and never consumed alcoholic beverages (adjusted coefficient −0.263, 95% CI −0.453, −0.074, P = .007; and adjusted coefficient −0.332, 95% CI −0.609, −0.056, P = .020 and coefficient −0.424, 95% CI −0.739, −0.109, P = .009, respectively).
3.2.5. SDLT/total score
The result of univariate linear regression analysis indicated that demographic and socioeconomic status, including age, race, income ratio, education level, marital status, and occupation category, were significantly associated with the total score on the SDLT. In addition, health status and lifestyle habits, such as diabetes diagnosis, hypertension, BMI, smoking, drinking, living with pets, talking on phone with family or friends, and spending time with friends or relatives, affected the total score on the SDLT. Dietary habits and biochemical parameters, including total protein intake, total unsaturated fatty acid intake, total carbohydrate intake, ascorbic acid, levels of vitamin E, vitamin B12, vitamin B6, riboflavin, thiamin, folacin, blood lead, urinary cadmium, serum C-reactive protein, serum glucose, glycated hemoglobin, and serum vitamin D, were also significantly associated with the total score on the SDLT (Table 4).
Multivariate linear regression analysis demonstrated that increased age was associated with higher total score on the SDLT (adjusted coefficient 0.088, 95% CI 0.067, 0.108, P < .001), whereas higher income ratio and higher education level were associated with lower score on the SDLT (adjusted coefficient −0.242, 95% CI −0.341, −0.142, P < .001; and adjusted coefficient −0.476, 95% CI −0.551, −0.401, P < .001, respectively). Race and occupation category were significantly associated with the total score on the SDLT, with black and Mexican-American participants having higher total scores than white participants (adjusted coefficient 1.384, 95% CI 0.882, 1.886, P < .001; adjusted coefficient 1.990, 95% CI 1.391, 2.589, P < .001, respectively); and transportation and trucking industry workers having higher total scores than office building services workers (adjusted coefficient 0.946, 95% CI 0.056, 1.835, P = .038). Good health status was associated with lower total scores than fair health status (adjusted coefficient −0.805, 95% CI −1.489, −0.121, P = .022). Smoking and drinking were also significantly associated with the total score on the SDLT. Current and former smokers was lower total score than never smoking participants (adjusted coefficient −0.400, 95% CI −0.747, −0.052, P = .025; adjusted coefficient −0.603, 95% CI −0.995, −0.211, P = .003, respectively); and former and current drinkers also had lower total score compared with never drinking individuals (adjusted coefficient −1.021, 95% CI −1.657, −0.385, P = .002; adjusted coefficient −1.177, 95% CI −1.874, −0.480, P = .001, respectively). Increased total carbohydrate intake was significantly associated with lower total score on the SDLT (adjusted coefficient −0.002, 95% CI −0.004, −0.0005, P = .013).
4. Discussion
Our results indicate that in 20 to 59-year-old NHANES III participants who underwent 3 computerized tests to evaluate cognitive functioning demographic and socioeconomic status, including older age, black ethnicity, lower income ratio, and lower education level, were associated with poorer neurocognitive function in all analyzed tests. In addition, participants with poor health, nonsmokers, and nondrinkers performed worse in all administered tests compared with individuals with good health, smokers, and participants consuming alcoholic beverages. Dietary and biochemical characteristics of the blood were not consistently associated with neurocognitive performance. Folacin intake was associated with faster reaction in SRTT, carbohydrate intake was associated with lower total score on the SDLT, while riboflavin consumption was associated with increased reaction time in SRTT. In addition, higher blood lead level was associated with increase in the mean total latency in the SDST. Overall, office building workers performed better in the administered neurocognitive tests. Specifically, office building services employees demonstrated faster reaction compared with metal industry workers in SRTT, and needed less trials to reach criterion and obtained lower total scores in SDLT than participants working in the transportation and trucking industry.
Advanced age was identified as a potential risk factor for neurocognitive decline in our study. Because population aging is taking place in nearly all countries of the world, considerable cognitive decline in a wide spectrum of cognitive abilities seen in older individuals is a major health and social issue.[21] Cognitive decline is closely linked to dementia and illness, and associated with increased mortality.[22] Therefore, further research is needed to understand the mechanisms of cognitive aging and the factors that contribute to its individual differences.
According to recent studies, differences seen in cognitive functioning of older individuals of different races could be attributed to social and cultural factors.[8–11] In general, the findings from previous research and our results indicate that older adults of African-American and Hispanic descent demonstrate lower performance on cognitive tests compared with whites.[23,24] The observed results can be due to substantial differences in the attainment of education, as it was shown before and further supported by our results that higher level of education is associated with better cognition and a decreased risk for dementia during old age.[25–27]
Similarly, evidence suggests that lower occupational status (eg, manual labor, trade, farmer) may be associated with poorer cognitive function,[28] and increased risk of dementia and Alzheimer's disease (AD), while occupations with higher mental or intellectual demands are associated with better cognitive performance and reduced risk of dementia.[22] Occupational exposures also contribute to cognitive performance in later life. Low-status employees are generally at higher risk for occupational exposures, and therefore more likely to suffer a nervous system damage.[22,29] Our results showed that office building service workers performed better in the administered neurocognitive tests compared with metal industry workers, and transportation and trucking industry workers.
Diet and lifestyle habits as modifiable factors that can play a role in cognitive aging have received a lot of interest in the scientific community and general public. It is reasonable to hypothesize that improving the diet of older people might help to delay the onset, or slow the progression of age-associated cognitive decline. However, despite widespread advertising of benefits associated with various vitamins and supplements, the solid scientific data supporting their use for cognitive health are limited. Goodwin et al[30] were among the first to show a role of folate, vitamin B12, vitamin B6, and omega-3s in cognitive function. The authors showed that healthy older subjects who had low blood levels of vitamins C, B12, riboflavin, and folic acid scored poorly on tests of memory and nonverbal abstract thinking.[3,30] Interestingly, it was demonstrated that supplementation of cobalamin-deficient patients with vitamin B12 lead to significant improvements in neuropsychiatric functions and cognitive recovery, suggesting that poor vitamin intake is at least partially responsible for the cognitive decline seen in some older persons.[3,31,32] B vitamins participate in regulating homocysteine levels, which is an independent risk factor for cognitive decline[3] and a stronger predictor of cognitive performance than either vitamin B12 or folate.[33] Homocysteine levels can be lowered by supplementation with B vitamins; however, it was shown that decrease of homocysteine levels does not improve cognitive function.[34] Moreover, a recent evidence-based review concluded that B12 supplementation did not improve cognitive function in patients with cognitive decline.[35] In addition, Malouf et al[36] showed that folic acid supplementation did not have any beneficial effect on measures of cognition or mood in older healthy women, and patients with mild to moderate cognitive decline and different forms of dementia. Altogether, experimental evidence supporting a beneficial effect of the micronutrient supplementation on cognitive health in older age is inconclusive. Possible reasons for inconsistent findings include different study designs, especially differences in the doses of the supplements and timing of interventions. It is also possible that some supplements may produce varying effect if received from food or obtained via supplementation. In addition, other active ingredients in the food or supplement itself may be responsible for the observed effects. It is possible that more complex interaction between dietary habits, that is, amounts of consumed proteins, fats, and carbohydrates, and also vitamins and minerals can influence the individual's response to aging. It was shown, for example, that dietary pattern with high caloric intake in the form of carbohydrates and low caloric intake in the form of fat and proteins may increase the risk of mild cognitive impairment or dementia in older persons.[37] Moreover, genetic background, especially metabolism-related polymorphisms, can also play a role.
In our study, we found that folacin intake was associated with faster reaction on the SRTT, while riboflavin consumption was associated with increased reaction time on the SRTT. In addition, we found that increased carbohydrate intake was associated with lower total score on the SDLT. Clearly, more controlled further studies focusing on individual response are needed to establish a more definitive role of vitamin supplementation and dietary habits in cognitive aging.
In conjunction with diet, other lifestyle factors such as smoking and drinking influence cognitive aging. While excessive alcohol consumption can cause long-term cognitive damage, evidence suggests that moderate levels of alcohol consumption in older people can be beneficial, possibly due to protective effects of ethanol on cardiovascular and cerebrovascular health.[38–41] Ganguli et al[42] showed that compared to no drinking, both minimal and moderate drinking were associated with better performance on cognitive tests. Interestingly, these associations were more pronounced when comparing current drinkers to former drinkers than to lifelong abstainers.[42] In agreement, our study showed that drinking was associated with better performance on all cognitive tests.
While smoking is a significant risk factor for heart disease, stroke, cancer, and other conditions, its effect on the cognitive function is controversial. Smoking increases the risk of stroke and therefore is expected to increase the risk of vascular dementia and cognitive decline. On the contrary, nicotine increases the release of acetylcholine, which can increase attention and information processing.[22] A recent systematic review showed that 16 out of the 29 selected cohort studies found the relationship between smoking and various cognitive outcomes, 4 found this relationship for some outcomes or certain subgroups, while the remaining 9 studies did not find an association or found an inverse association.[22] Only 2 out of 7 cross-sectional studies found an association between smoking and poor cognitive function. Our data demonstrate that smoking is associated with improved cognitive function.[22] Although further studies are needed to dissect the effect of smoking on cognition, its effect on other health aspects provides enough reasons to quit.
This study has several limitations. Because our study was a cross-sectional analysis by design, inferences regarding causality cannot be made. Moreover, because NHANES III is a US-based survey, the results need to be validated in other countries. Because only 3 cognitive function tests were administered as part of NHANES III, this study cannot provide a comprehensive cognitive assessment. In addition, there was an overall test nonresponse rate of 9%. Nonresponse rates increased with age, decreased with educational level, were higher for men, and were lower for non-Hispanic white individual than other ethnic groups, potentially introducing some bias into the study.[18]
Despite the limitations, our study has several strengths. NHANES III is a population-based survey that included validated examination measures, biological specimen collection, and limited measures of health status. Rigorous training in recruitment and data collection ensures high response rates, national representativeness, and high quality of collected data. The sample size is sufficient for precise prevalence measures at the national level. A large multiethnic population sample allowed us to explore the racial/ethnic heterogeneity in the association with neurocognitive function. This analysis was conducted in a nationally representative sample; therefore, our results may be generalized to the entire US adult population.
5. Conclusions
Our results indicate that age, ethnicity, income, education level, overall health, smoking status, drinking status, and dietary and biochemical characteristics of the blood, and job category were associated with neurocognitive performance in visual attention, learning, and concentration in a large, nationally representative sample of healthy, ethnically diverse 20 to 59-year-olds. Future studies are needed to understand the mechanisms of cognitive aging and the factors that contribute to its individual differences.
Acknowledgments
The authors acknowledge the efforts of the US National Center for Health Statistics (NCHS) in creation of the third National Health and Nutrition Examination Survey data. The interpretation and reporting of these data are the sole responsibility of the authors.
Author contributions
Conceptualization: Shibing Zhu, Jing Zhao, Zhiming Chen.
Data curation: Zhiming Chen, Yanpeng Wang.
Formal analysis: Shibing Zhu, Jing Zhao, Yanpeng Wang.
Supervision: Shibing Zhu.
Writing – original draft: Shibing Zhu, Jing Zhao.
Writing – review & editing: Shibing Zhu, Jing Zhao, Zhiming Chen, Yanpeng Wang.
Footnotes
Abbreviations: CNS = central nervous system, NCHS = the National Center for Health Statistics, NHANES III = the Third National Health and Nutrition Examination Survey, SDLT = serial digit learning test, SDST = symbol digit substitution test, SRTT = simple reaction time test.
Funding: This work was supported by the Hangzhou Red Cross Hospital for Medicine and Health Care in Zhejiang Province general studies program (Grant no: 2015116934 to JZ).
The authors report no conflicts of interest.
References
- [1].Frechette JD, Marraccini ME. Role of nutrition in the prevention of cognitive decline. Ann Long Term Care Clin Care Aging 2014;22:41–8. [Google Scholar]
- [2].Huang P, Fang R, Li BY, et al. Exercise-related changes of networks in aging and mild cognitive impairment brain. Front Aging Neurosci 2016;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Selhub J, Bagley LC, Miller J, et al. B vitamins, homocysteine, and neurocognitive function in the elderly. Am J Clin Nutr 2000;71:614s–20s. [DOI] [PubMed] [Google Scholar]
- [4].Lipnicki DM, Sachdev PS, Crawford J, et al. Risk factors for late-life cognitive decline and variation with age and sex in the Sydney Memory and Ageing Study. PLoS One 2013;8:e65841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].National Research Council Committee on Future Directions for Cognitive Research on A. The National Academies Collection: Reports funded by National Institutes of Health. In: Stern PC, Carstensen LL, eds. The Aging Mind: Opportunities in Cognitive Research.. Washington (DC): National Academies Press (US) National Academy of Sciences; 2000. [PubMed] [Google Scholar]
- [6].Hartman DE. Neuropsychological toxicology: identification and assessment of neurotoxic syndromes. Arch Clin Neuropsychol 1987;2:45–65. [PubMed] [Google Scholar]
- [7].Bondi MW, Salmon DP, Monsch AU, et al. Episodic memory changes are associated with the APOE-epsilon 4 allele in nondemented older adults. Neurology 1995;45:2203–6. [DOI] [PubMed] [Google Scholar]
- [8].SB G, Choi S, Krishnan J, et al. Cigarette smoke and related risk factors in neurological disorders: an update. Biomed Pharmacother 2017;85:79–86. [DOI] [PubMed] [Google Scholar]
- [9].Woods AJ, Porges EC, Bryant VE, et al. Current heavy alcohol consumption is associated with greater cognitive impairment in older adults. Alcohol Clin Exp Res 2016;40:2435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Smith MA, Scholey AB. Nutritional influences on human neurocognitive functioning. Front Hum Neurosci 2014;8:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ji X, Cui N, Liu J. Neurocognitive function is associated with serum iron status in early adolescents. Biol Res Nurs 2017;19:269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schlogl M, Holick MF. Vitamin D and neurocognitive function. Clin Interv Aging 2014;9:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hardman RJ, Kennedy G, Macpherson H, et al. Adherence to a Mediterranean-style diet and effects on cognition in adults: a qualitative evaluation and systematic review of longitudinal and prospective trials. Front Nutr 2016;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Smith PJ, Blumenthal JA. Diet and neurocognition: review of evidence and methodological considerations. Curr Aging Sci 2010;3:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cox CS, Rothwell ST, Madans JH, et al. Plan and operation of the NHANES I Epidemiologic Followup Study, 1987. Vital Health Stat 1992;1:1–90. [PubMed] [Google Scholar]
- [16].Ezzati TM, Massey JT, Waksberg J, et al. Sample design: Third National Health and Nutrition Examination Survey. Vital Health Stat 1992;2:1–35. [PubMed] [Google Scholar]
- [17].Yang L, Koyanagi A, Smith L, et al. Hand grip strength and cognitive function among elderly cancer survivors. PLoS One 2018;136:e0197909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Krieg EF, Jr, Chrislip DW, Letz RE, et al. Neurobehavioral test performance in the third National Health and Nutrition Examination Survey. Neurotoxicol Teratol 2001;23:569–89. [DOI] [PubMed] [Google Scholar]
- [19].Statistics NCfH. NHANES III Examination Data File Documentation: Age Two Months and Older. Hyattsville, MD: National Center for Health Statistics; 1996. [Google Scholar]
- [20].Hnizdo E, Sullivan PA, Bang KM, et al. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2002;156:738–46. [DOI] [PubMed] [Google Scholar]
- [21].Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med 2013;29:737–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hughes TF, Ganguli M. Modifiable midlife risk factors for late-life cognitive impairment and dementia. Curr Psychiatry Rev 2009;5:73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Castora-Binkley M, Peronto CL, Edwards JD, et al. A longitudinal analysis of the influence of race on cognitive performance. J Gerontol B Psychol Sci Soc Sci 2015;70:512–8. [DOI] [PubMed] [Google Scholar]
- [24].Early DR, Widaman KF, Harvey D, et al. Demographic predictors of cognitive change in ethnically diverse older persons. Psychol Aging 2013;28:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Alley D, Suthers K, Crimmins E. Education and cognitive decline in older americans: results from the AHEAD sample. Res Aging 2007;29:73–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Diaz-Venegas C, Downer B, Langa KM, et al. Racial and ethnic differences in cognitive function among older adults in the USA. Int J Geriatr Psychiatry 2016;31:1004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stern Y, Gurland B, Tatemichi TK, et al. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994;271:1004–10. [PubMed] [Google Scholar]
- [28].Jorm AF, Rodgers B, Henderson AS, et al. Occupation type as a predictor of cognitive decline and dementia in old age. Age Ageing 1998;27:477–83. [DOI] [PubMed] [Google Scholar]
- [29].Qiu C, Karp A, von Strauss E, et al. Lifetime principal occupation and risk of Alzheimer's disease in the Kungsholmen project. Am J Ind Med 2003;43:204–11. [DOI] [PubMed] [Google Scholar]
- [30].Goodwin JS, Goodwin JM, Garry PJ. Association between nutritional status and cognitive functioning in a healthy elderly population. JAMA 1983;249:2917–21. [PubMed] [Google Scholar]
- [31].Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med 1988;318:1720–8. [DOI] [PubMed] [Google Scholar]
- [32].Martin DC, Francis J, Protetch J, et al. Time dependency of cognitive recovery with cobalamin replacement: report of a pilot study. J Am Geriatr Soc 1992;40:168–72. [DOI] [PubMed] [Google Scholar]
- [33].Riggs KM, Spiro A, 3rd, Tucker K, et al. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr 1996;63:306–14. [DOI] [PubMed] [Google Scholar]
- [34].Clarke R, Bennett D, Parish S, et al. Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr 2014;100:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser 2013;13:1–45. [PMC free article] [PubMed] [Google Scholar]
- [36].Malouf M, Grimley EJ, Areosa SA. Folic acid with or without vitamin B12 for cognition and dementia. Cochrane Database Syst Rev 2003. Cd004514. [DOI] [PubMed] [Google Scholar]
- [37].Roberts RO, Roberts LA, Geda YE, et al. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J Alzheimers Dis 2012;32:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bartley PC, Rezvani AH. Alcohol and cognition - consideration of age of initiation, usage patterns and gender: a brief review. Curr Drug Abuse Rev 2012;5:87–97. [DOI] [PubMed] [Google Scholar]
- [39].Jayasekara H, English DR, Room R, et al. Alcohol consumption over time and risk of death: a systematic review and meta-analysis. Am J Epidemiol 2014;179:1049–59. [DOI] [PubMed] [Google Scholar]
- [40].Beydoun MA, Gamaldo AA, Beydoun HA, et al. Caffeine and alcohol intakes and overall nutrient adequacy are associated with longitudinal cognitive performance among U.S. adults. J Nutr 2014;144:890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Peters R, Peters J, Warner J, et al. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing 2008;37:505–12. [DOI] [PubMed] [Google Scholar]
- [42].Ganguli M, Vander Bilt J, Saxton JA, et al. Alcohol consumption and cognitive function in late life: a longitudinal community study. Neurology 2005;65:1210–7. [DOI] [PubMed] [Google Scholar]


