Abstract
T cell infiltration in tumors has been investigated as a biomarker of response to checkpoint inhibitors. Neo-adjuvant studies in renal cell carcinoma (RCC) may provide a unique opportunity to compare T cell infiltration in a pretreatment renal mass biopsy to a posttreatment nephrectomy specimen, and thus evaluate the effects of immune checkpoint inhibitors. However, there are no data regarding the association of T cell infiltration in matched biopsy and nephrectomy samples without intervening treatment. Understanding this association will inform investigation of this potential biomarker in future studies.
Matched biopsy and nephrectomy samples (without intervening systemic therapy) were identified from patients with nonmetastatic RCC. Selected tissue sections from biopsy and nephrectomy samples were reviewed and marked for intratumoral lymphocytes by a pathologist. Immunohistochemistry (IHC) was utilized to stain for T cell markers (CD3, CD4, and CD8). Intratumoral staining was then quantified in the tissue sections as counts per total tumor area surveyed. Spearman correlation (r) was used to measure associations.
Thirty matched pairs were investigated. The median interval between biopsy and nephrectomy was 2.8 (0.2–87.7) months. Clear cell was the most common histology (29/30; 97%). There was a statistically significant positive correlation between the frequency of CD3 +and CD8+ T cells between matched biopsy and nephrectomy samples (r = 0.39; P = .036 and r = 0.38; P = .041, respectively).
The frequencies of CD8+ T cells in matched biopsy and nephrectomy samples in RCC in the absence of intervening treatment have been characterized and show a positive correlation between matched biopsy and nephrectomy samples.
Keywords: biomarker, biopsy, CD8+ T cells, immunotherapy, renal cell carcinoma, T cell infiltration
1. Introduction
The improved understanding of T cell function has led to the development of novel targeted immunotherapies which target immunosuppressive molecules like cytotoxic T lymphocyte antigen-4, programmed death 1 (PD-1), and PD-L1, called immune checkpoints, which play an important role in the evasion of cancer from the immune system by inhibiting the activity of antitumor T cells.[1] Nivolumab, a fully human IgG4 PD-1 check point inhibitor antibody, is the first approved checkpoint inhibitor for the treatment of metastatic RCC refractory to antiangiogenic therapy based on the overall survival advantage shown in a phase III clinical trial.[2]
Although checkpoint inhibitors have prompted a paradigm shift in the treatment of many cancers due to their ability to produce durable responses, only a subset of patients benefit from this treatment.[3–7] Therefore, identifying markers of response is critical and has been an area of active research. These potential biomarkers can not only provide prognostic and predictive information, but can also provide rationale for combination treatments. Previous studies have shown that information regarding tumor microenvironment can be useful in assessing the tumor response in relation to immune checkpoint inhibitors.[8–10] Specially, the role of CD8+ T cell infiltration, as a potential marker of response to PD-1 blockade, appears promising.[11]
Neo-adjuvant studies provide a unique opportunity to evaluate the effect of treatment on the tumor microenvironment before and after treatment, and thus help identify potential biomarkers. There are several neo-adjuvant studies (NCT03055013, NCT02762006, NCT03024996) of checkpoint inhibitors planned or ongoing where renal biopsy and/or nephrectomy samples will be collected to investigate potential biomarkers such as CD8+ T cell infiltration.[12–14] However, there are no existing data in RCC to characterize and associate baseline CD8+ T cells in biopsy and nephrectomy samples in the absence of an intervening treatment. Therefore, this study was aimed to investigate the CD8+ T cell infiltration and their association in matched biopsy and nephrectomy samples, in the absence of any systemic treatment. This information will also be instructive in further defining the role of biopsy in evaluating the dynamic changes of tumor microenvironment in RCC, in relation to treatment with immunotherapy.
2. Methods
2.1. Patients and pathology materials
Patients with nonmetastatic RCC who underwent a diagnostic renal biopsy and subsequent nephrectomy were retrospectively identified from the electronic medical records. All patients had matched biopsy and nephrectomy samples without intervening systemic therapy.
2.2. Evaluation of lymphocytes
Selected tumor tissue sections from biopsy and nephrectomy samples were stained with hematoxylin and eosin, and reviewed by the genitourinary pathologist (CP). The tissue sections, which showed highest lymphocytic infiltrate, were selected for evaluation. These sections were then marked for intratumoral and peri-tumoral lymphocytes. The intensity of the immune cell infiltration was divided into 4 categories including minimal, mild, moderate, and severe, defined as follows: minimal—very rare identifiable lymphocytes; mild—occasional small aggregates or individual lymphocytes without any obscuring of the background tumor or stromal cells; severe—dense lymphocytic infiltrates obscuring the background tumor or stromal cells; moderate—a degree of lymphocytic infiltrate between mild and severe.
2.3. IHC for subtyping of lymphocytes
Immunohistochemistry (IHC) was utilized to stain these selected tissue sections for T cell markers including CD3, CD4, and CD8 (Fig. 1). Antibodies used for each marker included: CD3—Ventana (Cat No. 790–4341, Prediluted), CD4—Novocastra (Cat No. NCL-CD4–1F6, Prediluted), and CD8—Biogenex (Cat No. MU422-UC, 1:10). Slides were scanned at ×20 magnification (Aperio AT2; Leica Biosystems). Image analysis software (Aperio ImageScope) was used to measure the number of positively stained cells within designated areas. A modified version of the Nuclear v9 algorithm was calibrated for each stain and utilized to detect marker positivity. CD3, CD4, and CD8 expression was assessed. For each marker, the intratumoral areas containing the highest density of positive cells were delineated with a square of fixed area (Fig. 1, green boxes). Each square was 1.0 mm2 and 0.1 mm2 for nephrectomy and biopsy cases, respectively, and did not overlap. The total number of squares delineated did not exceed 12, but fewer squares were marked if sample tumor areas were unable to accommodate this number. For each nephrectomy and biopsy case, the numbers of positive cells were summed and divided by the sum of the total area (mm2) from which the cells were counted. The results were reported as number of positive cells per mm2.
Figure 1.
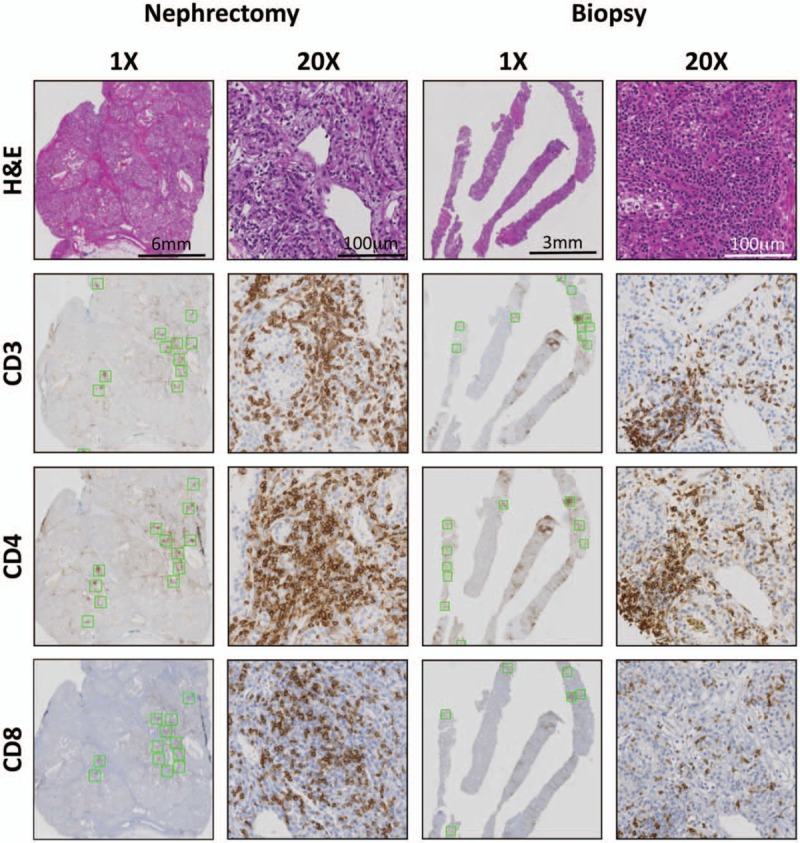
Intratumoral staining and quantification of immune infiltrates in matched nephrectomy and biopsy cases. H&E stain was performed to determine the degree of immune infiltration. Serial slides were stained for T cell markers CD3, CD4, and CD8 to assess the levels of T cell infiltrate in matched nephrectomy and biopsy material from untreated patients. Serial slides (green boxes, shown at ×1) represent areas of highest stain intensity and subsequently quantified for each stain. ×20 images illustrate T cell distribution within an intratumoral immune cell infiltrate in both nephrectomy and biopsy samples.
2.4. Statistical methods
Categorical clinicopathologic factors were summarized with frequency counts and percentages, and continuous factors with medians and ranges. The exact Wilcoxon signed-rank test was used to measure the difference in the degree of infiltration in matched samples. Kendall rank correlation coefficient was used to assess the consistency of the immune cell infiltration between biopsy and nephrectomy. Spearman rank correlations (r) were used to measure associations between the T cell subsets. Analyses were carried out using R version 3.4.3 and GraphPad Prism v 5.02.
3. Results
Thirty patients with matched biopsy and nephrectomy samples were investigated. The median interval between biopsy and nephrectomy was 2.8 (0.2–87.7) months. Forty-seven percent of patients underwent radical nephrectomy whereas 53% underwent partial nephrectomy. Clear cell was the most common histology (29/30; 97%) (Table 1). Only 5 of 30 cases had peri-tumoral tissue on the biopsy with any appreciable (greater than minimal) infiltrate, so further evaluation of peri-tumoral lymphocytes was not pursued. The degree of immune cell infiltration between matched biopsy and nephrectomy samples was 50% (15/30) concordant, and this correlation analysis was validated by using Kendall Tau test (P = .027) which showed consistency between 2 specimens (Table 2).
Table 1.
Patient characteristics and pathologic features.

Table 2.
Degree of immune cell in matched biopsy and nephrectomy samples.

The median number of CD3 +T cells in biopsy samples were 513.12 cells per mm2 and 1287.48 cells per mm2 in nephrectomy samples. A modest positive correlation was found between these frequencies (r = 0.39; P = .036). The median number of CD8 + T cells in biopsy samples were 288.96 cells per mm2 and 547.57 cells per mm2 in nephrectomy samples. These CD8 + T cell frequencies showed modest correlation with a spearman correlation r = 0.38, which was statistically significant (P = .041). On the other hand, CD4 + T cells did not show any significant correlation (Table 3).
Table 3.
Correlation of immune markers in matched biopsy and nephrectomy samples.

The correlation analyses were also performed based on the degree of immune cell infiltration in the biopsy samples. When the degree of immune cell infiltration was appreciable (defined as any immune cell infiltration greater than minimal, n = 16, 53%), a stronger and significant correlation was found between CD3+ and CD8+ T cell frequencies (r = 0.66; P = .006 and r = 0.78; P = .0004, respectively). CD4+ T cells did not show any correlation (r = 0.18, P = .49). On the other hand, in the presence of a minimal immune cell infiltration in the biopsy sample, no correlation was found between any of these cell frequencies (Table 4 and Fig. 2).
Table 4.
Correlation of immune markers in matched biopsy and nephrectomy samples based on immune cell infiltration of biopsy sample.

Figure 2.
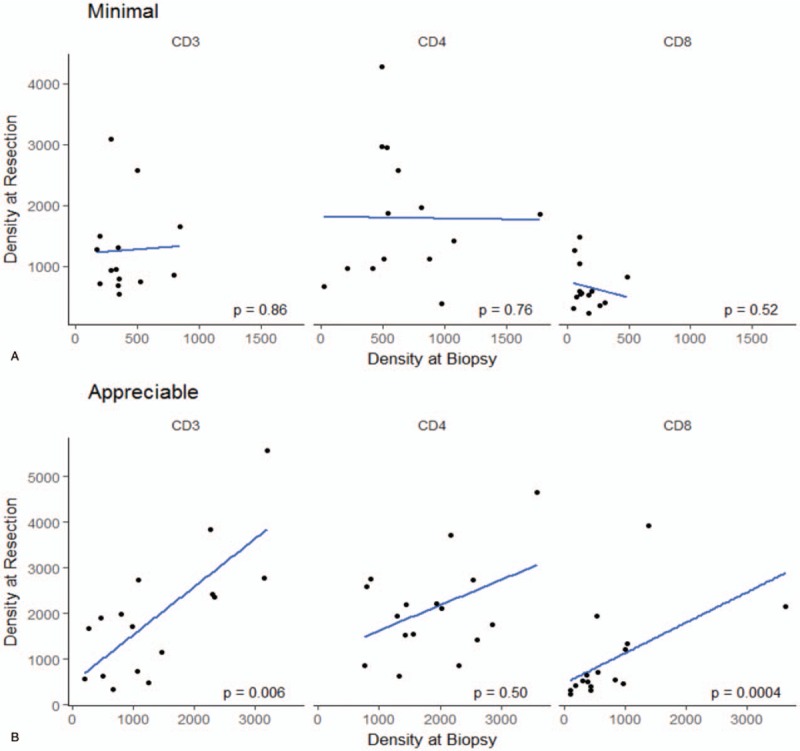
Scatter plot demonstrating correlation of CD3+, CD4+, and CD8+ T cells between biopsy and nephrectomy based on degree of immune cell infiltration. When the degree of immune cell infiltration was appreciable, a stronger and significant correlation was found between CD3+ and CD8+ T cell frequencies.
4. Discussion
This study characterized baseline T cell frequencies, and demonstrated a statistically significant positive correlation of CD8+ T cell infiltration in matched biopsy and nephrectomy samples in the absence of an intervening systemic treatment. Furthermore, it was shown that higher immune cell infiltration in the biopsy sample is associated with a stronger correlation between T cell markers.
Immune checkpoint inhibitors have shown promising clinical activity in many tumor types including RCC.[2] Several neo/adjuvant studies investigating the role of immune checkpoint inhibitors in RCC are ongoing with integrated biomarker analyses including CD8+ T cell infiltration.[12,14] Findings from the present analysis provide a baseline characterization of T cell infiltration in the absence of an intervening treatment.
Furthermore, this study has also demonstrated that needle biopsy can be informative regarding the tumor microenvironment. Using IHC, a biopsy can be utilized to measure the frequencies of T cells, especially CD8+ T cells, in the intratumoral region. This finding has practical implications in terms of using needle biopsy as a potential method to assess on-treatment changes in tumor microenvironment. These dynamic changes can not only provide prognostic and predictive information, but can also help enhance our mechanistic understanding of host-tumor responses in relation to immunotherapy. For example, Tumeh et al report a study wherein patients with metastatic melanoma being treated with pembrolizumab, an anti-PD-1 antibody, underwent serial biopsies during treatment, to quantify the frequencies of CD8+ T cells. Patients who responded to the treatment, not only had a higher pre-existing CD8+ T cells, but also showed proliferation of CD8+ T cells which correlated with radiographic response to treatment (Spearman correlation r = 0.07, P < .001). These findings indicate that simple core needle biopsy and IHC can provide important information regarding which patients will respond to the treatment. Similarly, in RCC, core needle biopsy has been used to assess the dynamic changes in tumor microenvironment in relation to nivolumab, an anti-PD-1 antibody.[15] Patients with metastatic RCC underwent baseline and on-treatment biopsies, and immunohistochemical analysis, to measure changes in T cell frequencies. An increase in all T cell frequencies, especially CD3+ and CD8+, were observed on treatment, indicating immune pharmacodynamic effects of nivolumab.[15]
However, there is a significant inter- and intratumoral heterogeneity in all tumors including RCC.[16,17] Therefore, accurate assessment of tumor microenvironment from a biopsy material can be challenging. For example, the present study demonstrated in matched samples that a biopsy only provides an accurate assessment of tumor microenvironment if it has more than minimal degree of immune infiltrate. This requirement perhaps ensures that the biopsy material represents an area of tumor with similar immune milieu. On the other hand, a biopsy material with minimal immune cell infiltrate may not truly represent immune infiltration of the tumor, perhaps due to sampling error. It is, however, reassuring that despite this limitation, biopsy material showed a positive correlation of CD3+ and CD8+ T cells with matched nephrectomy samples in the overall analysis, indicating its significance and utility in evaluating changes in tumor microenvironment.
Our study has several limitations including a small sample size. The evaluation of tissue sections for lymphocyte infiltration was carried out by a single pathologist, which limits its generalizability and reproducibility. Similarly, these samples were obtained from patients with localized RCC and may not represent tumor microenvironment of metastatic RCC patients. In addition, peri-tumoral areas were not included in the evaluation due to lack of T cell infiltrate.
In conclusion, the frequencies of CD8+ T cells in matched biopsy and nephrectomy samples in RCC in the absence of intervening treatment have been characterized and show a positive correlation. Biopsy material can be used for IHC analyses to provide insights into the tumor microenvironment, specifically CD8+ T cell infiltration, which can be instrumental in identification of potential markers of response to immunotherapy.
Author contributions
HZ contributed to the conception and design of the study, data acquisition, generation of tables, drafting, and final revision of the manuscript. PGP participated in the conception and design of the study, performed all histological quantification, generation of figures and tables, and the drafting and final revision of the manuscript. CP and JK performed all pathological assessments and contributed to the design of the study. LS participated in data acquisition. TR and XJ performed all statistical analyses and participated in the final revision of the manuscript. CMM participated in data acquisition and final revision of the manuscript. JF participated in final revision of the manuscript. PAR participated in data acquisition and final revision of the manuscript. TDG, PG, MCO, JAG participated in the design of the study and final revision of the manuscript. BIR contributed to the conception and coordination of the study, data acquisition, drafting, and final revision of the manuscript. All authors read and approved the manuscript.
Conceptualization: HZ, PGP Jr, TDG, PG, MCO, JAG, BIR.
Data curation: HZ, LS, CMD-M, PAR, BIR.
Formal analysis: HZ, PGP Jr, CP, JK, TR, XJ.
Methodology: PGP Jr, CP, JK.
Writing – original draft: HZ, PGP Jr, BIR.
Writing – review & editing: HZ, PGP Jr, TR, XJ, CMD-M, JF, PAR, TDG, PG, MCO, JAG, BIR.
Footnotes
Abbreviations: CTLA-4 = cytotoxic T lymphocyte antigen-4, IHC = immunohistochemistry, MDSC = myeloid-derived suppressive cells, PD-1 = programmed death-1, PD-L1 = programmed death ligand-1, RCC = renal cell carcinoma.
Reviewers
Saby George, MD, FACP, Associate Professor of Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY
Saby.George@RoswellPark.org
Eric Jonasch, MD, Professor, Department of Genitourinary Medical Oncology, Division of Cancer Medicine, The University Of Texas, MD Anderson Cancer Center, Houston, TX
ejonasch@mdanderson.org
Funding: Internal departmental funding only.
Ethics approval and consent to participate: Evaluation of archived pathology specimens described in this manuscript is covered under a study approved by the Cleveland Clinic Institutional Review Board which does not require individual patient consent.
Availability of data and material: The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
References
- [1].Carlo MI, Voss MH, Motzer RJ. Checkpoint inhibitors and other novel immunotherapies for advanced renal cell carcinoma. Nat Rev Urol 2016;13:420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 2015;33:1430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 2015;33:2013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Camisaschi C, Vallacchi V, Castelli C, et al. Immune cells in the melanoma microenvironment hold information for prediction of the risk of recurrence and response to treatment. Expert Rev Mol Diagn 2014;14:643–6. [DOI] [PubMed] [Google Scholar]
- [10].Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 2012;61:1019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nivolumab in Treating Patients With Localized Kidney Cancer Undergoing Nephrectomy (PROSPER). NCT03055013.Available at: https://clinicaltrials.gov/ct2/home. Last Accessed May 24, 2017. [Google Scholar]
- [13].A Phase Ib Trial of Neoadjuvant Durvalumab (MEDI4736) +/- Tremelimumab in Locally Advanced Renal Cell Carcinoma. NCT02762006.Available at: https://clinicaltrials.gov/ct2/home. Last Accessed May 24, 2017. [Google Scholar]
- [14].A Study of Atezolizumab as Adjuvant Therapy in Participants With Renal Cell Carcinoma (RCC) at High Risk of Developing Metastasis Following Nephrectomy (IMmotion010). NCT03024996 Available at: https://clinicaltrials.gov/ct2/home. Last Accessed May 24, 2017. [Google Scholar]
- [15].Choueiri TK, Fishman MN, Escudier B, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res 2016;22:5461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vaziri SA, Tavares EJ, Golshayan AR, et al. Differing von hippel lindau genotype in paired primary and metastatic tumors in patients with clear cell renal cell carcinoma. Front Oncol 2012;2:51. [DOI] [PMC free article] [PubMed] [Google Scholar]


