Abstract
Background:
We aimed to estimate the association between dietary carrot intake and risk of breast cancer by conducting a meta-analysis of epidemiologic studies.
Methods:
Relevant studies were identified by searching databases through September 2017. We included studies that reported risk estimates with 95% confidence intervals for the association between dietary carrot intake and breast cancer risk. Random-effects models were used to calculate the summary risk estimates. Publication bias was estimated using Begg's funnel plot and Egger's regression asymmetry test.
Results:
A total of 10 articles met the eligibility criteria and were included in the meta-analysis involving 13,747 cases. The combined odds ratios (ORs) of breast cancer for the highest compared with the lowest dietary carrot intake was 0.79 (95% CI: 0.68, 0.90), and a significant heterogeneity was observed. In the subgroup analyses separated by study design, the inverse associations were more pronounced in the case–control studies than in the cohort studies, while the associations did not significantly differ by geographical region, study quality, exposure assessment. Omission of any single study had little effect on the combined risk estimate.
Conclusion:
The overall current literatures suggested that dietary carrot intake was associated with decreased risk of breast cancer.
Keywords: breast cancer, carrot, epidemiology, meta-analysis, nutrition
1. Introduction
Breast cancer is the most common female cancer worldwide. In 2012, 1.7 million women were diagnosed with breast cancer, and the estimated breast cancer deaths was 521,900 worldwide.[1] In Northern America, Australia/New Zealand, and Northern and Western Europe, the incidences of breast cancer are higher than those in other parts of the world.[1] International variation in breast cancer incidence might be explained by differences in the availability of early detection as well as risk factors. The impact of diet on breast cancer risk has been extensively studied, while only alcohol consumption has been unequivocally related to increased breast cancer risk.[2]
Carrot is commonly consumed around the world and contains many vitamins, fibers, minerals, and other phytochemicals, which may be beneficial for cancer prevention.[3] Evidence from meta-analysis also showed reduced risk of prostate and gastric cancers in the high carrot intake population.[4,5] Epidemiological studies have investigated the possible relationship between carrot intake and breast cancer risk. However, the results were not completely consistent, with some studies having found significant inverse association,[6,7] while others have not.[8,9] Due to the inconsistency and the insufficient statistical power of primary studies, we perform this meta-analysis aimed at determining whether high carrot intake is associated with reduced risk of breast cancer.
2. Methods
2.1. Literature search
The study was approved by the ethics institutional review board of the Affiliated Hospital of Medical School, Ningbo University. We conducted a systematic literature review of PubMed, Scopus, Embase, Web of Science, and the Cochrane register databases up till September 2017 for relevant citations in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[10] The free text words “vegetable or vegetables or carrot or carrots” and “breast cancer” were used for the search without any restriction. We also hand-searched the reference lists from retrieved articles to identify more studies.
2.2. Study selection
Two investigators independently reviewed all retrieved studies, and studies were included if they met the following criteria: the study design was prospective cohort, case-cohort, or nested case–control; the exposure of interest was carrot intake; the outcome of interest was breast cancer; the risk estimates with 95% confidence intervals (CI) was provided. When several studies were available for the same cohort, we retained the one with the largest number of cases for analysis.
2.3. Data extraction
Two investigators separately extracted basic characteristics and any discrepancy was resolved through a consensus discussion with a third reviewer. The extracted data including name of the first author, year of publication, study design, study location, year of follow-up (cohort studies) or year of data collection (case–control studies), number of cases and participants, dietary assessment, range of dietary carrot intake, and adjusted factors. For outcome statistics, we extracted risk estimates of the most fully adjusted model for the highest compared with the lowest carrot intake and the corresponding 95% CI. Considering that breast cancer is a relatively rare disease, the RR (relative risk) from cohort studies was assumed to be approximately equivalent to the OR (odds ratio), and the OR was used as the common measure of association between studies.[11]
2.4. Quality assessment
We assessed the methodological quality of each study using the Newcastle–Ottawa Quality Assessment Scale.[12] The checklist contains 8 items for the assessment of the patient selection, study comparability, and exposure (for case–control study) or outcome (for cohort study). The range of possible scores is 0 to 9. We assumed low quality studies as with a score < 7.
2.5. Statistics analysis
The summary ORs with 95% CIs were calculated using both a fixed-effects model or a random-effects model[13] depending on the heterogeneity between studies. Heterogeneity among studies was assessed with the Q-test and I2 score.[14] Statistical significance was considered while P < .05 or I2 > 50%. We conducted a sensitivity analysis to investigate the influence of any single study on the overall risk estimate by omitting one study sequentially. In addition to those methods, the Galbraith plot was also used to detect the possible sources of heterogeneity, and a re-analysis was conducted with exclusion of the studies possibly causing the heterogeneity.[15] We performed subgroup analyses stratified by study design, study region, study quality, method of exposure assessment, and the confounding factors. The meta-regression analysis was used to explore the source of heterogeneity. Publication bias was quantitatively assessed using the tests of Egger et al[16] and Begg and Mazumdar.[17] All statistical analyses were conducted using Stata Statistical Software, version 11.0.
3. Results
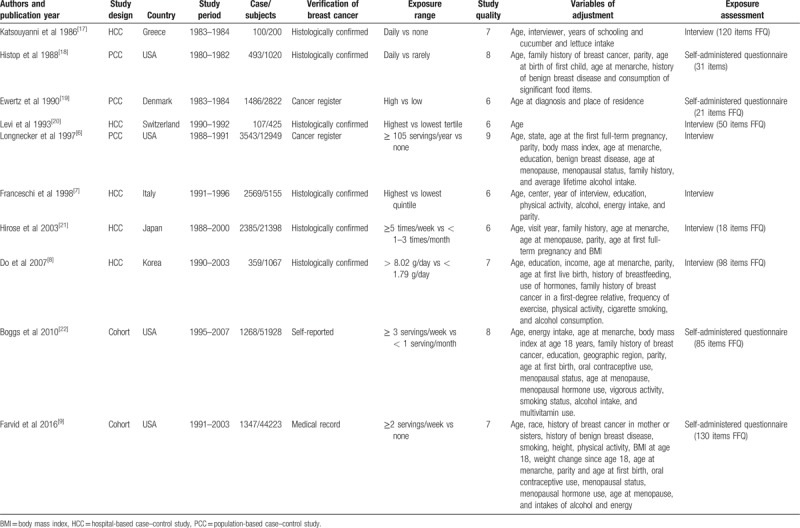
A flowchart showing the study selection process is presented in Figure 1. We finally identified 10 eligible studies,[6–9,18–23] containing 13,747 cases and 141,187 subjects. There were 2 cohort studies[9,23] and 8 case–control studies.[6–8,18–22] Two studies reported 2 separate outcomes (premenopausal and postmenopausal, estrogen receptor-positive, and estrogen receptor-negative),[19,22] thus there were 12 independent outcomes included in the meta-analysis. Four studies were conducted in the United States,[6,9,19,23] 4 studies in Europe,[7,18,20,21] and 2 in Asia.[8,22] The quality score ranged 6 to 9 stars, with a mean of 7 stars. Two studies reported ORs adjusted <3 factors,[20,21] whereas the other 8 studies adjusted for a wide range of potential confounding factors for breast cancer.[6–9,18,19,22,23] Information on carrot intake was obtained by interview[6–8,18,21,22] or self-administered questionnaire.[9,19,20,23] Detailed information of each of these studies is summarized in Table 1.
Figure 1.
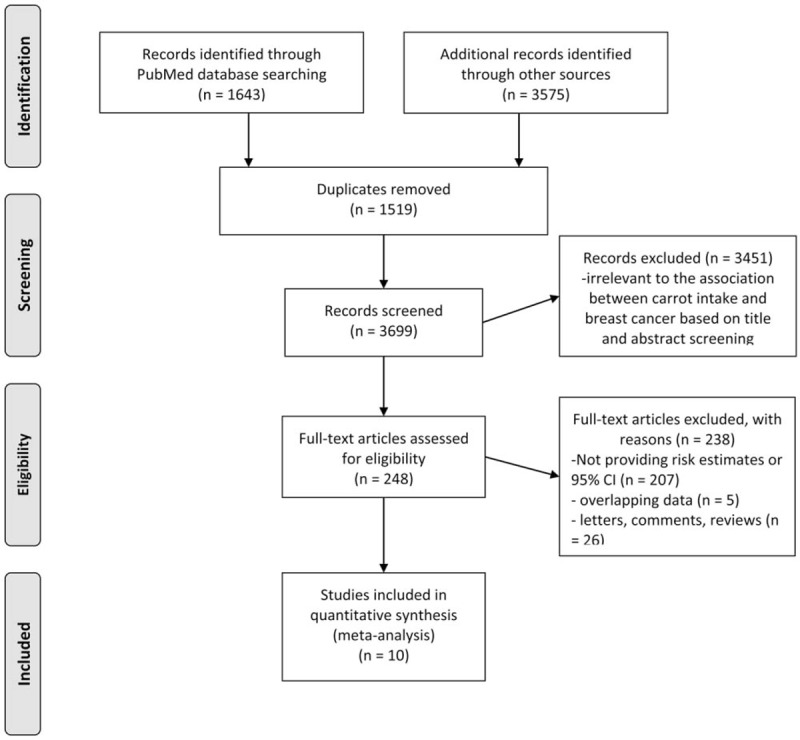
Flowchart of study selection.
Table 1.
Characteristics of included epidemiological studies on dietary carrot intake and risk of breast cancer.
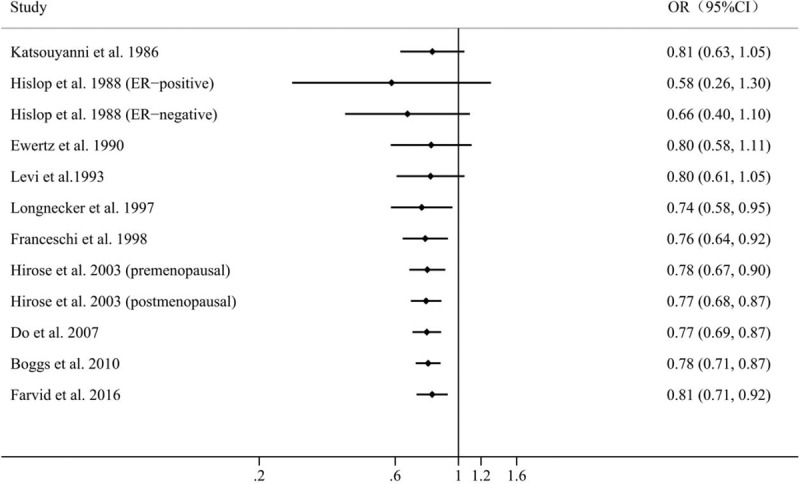
Results of pooled analysis are summarized in detail in Figure 2. Twelve independent outcomes reported the association between dietary carrot intake and risk of breast cancer. The pooled OR (95% CI) of breast cancer for the highest versus lowest categories of carrot intake was 0.79 (0.68, 0.90), indicating that high carrot consumption decreased the risk of breast cancer by 21%. A significant between-study heterogeneity was found between studies (P < .001, I2 = 75.1%).
Figure 2.
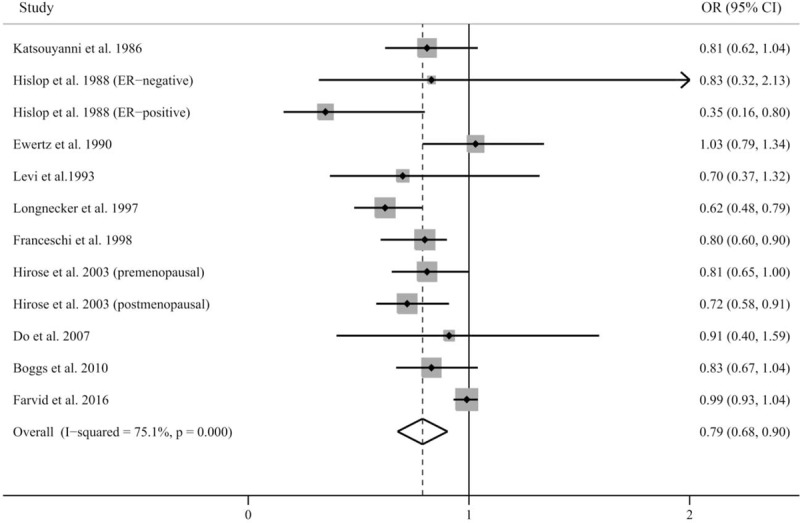
Pooled results for 10 epidemiological studies of dietary carrot intake and risk of breast cancer.
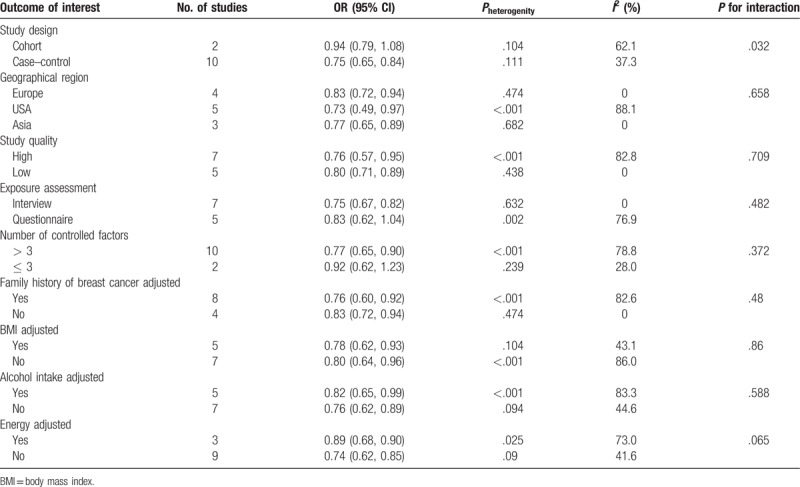
The results of subgroup analyses are presented in Table 2. Generally speaking, dietary carrot intake was consistently associated with reduced risk of breast cancer. In the subgroup analyses separated by study design, the inverse associations were more pronounced in the case–control studies (OR 0.75, 95% CI 0.65–0.84) than in the cohort studies (OR 0.94, 95% CI 0.79–1.08). The results were not significantly modified by geographical region, study quality or exposure assessment. We next investigated the impact of some confounding factors on the estimates of ORs. Also, whether adjusting for family history, BMI or alcohol intake did not affect the pooled OR, whereas more significant associations were observed in studies that adjusted for energy intake (OR 0.74, 95% CI 0.62–0.85) compared with studies that did not adjust for energy intake (OR 0.89, 95% CI 0.68–0.90). Furthermore, the risk estimate for studies that adjusted for more than 3 factors (OR 0.77, 95% CI 0.65–0.90) was much lower than studies that adjusted less than or equal to 3 factors (OR 0.92, 95% CI 0.62–1.23). The meta-regression analysis showed that the study design (case–control vs cohort) might be as a possible source of heterogeneity in the meta-analysis (P for interaction = .032).
Table 2.
Subgroup analysis of dietary carrot intake and risk of breast cancer by study design, geographical region, study quality, exposure assessment, and adjusted factors.
Sensitivity analysis was conducted to test the influence of individual studies on the overall results by repeating the meta-analysis while omitting each study at a time. The results showed that the pooled ORs of remaining studies kept consistency with before when omitting any single study (Fig. 3). The pooled ORs ranged from 0.76 (95% CI 0.67–0.84) when the study by Farvid et al[9] was omitted to 0.82 (95% CI 0.72–0.93) when the study by Hislop et al.[19] (ER-positive) was omitted, suggesting the high stability of the results. It was noted that when we omitted the study by Farvid et al[9], which was one of the 2 included cohort studies, the heterogeneity between studies were reduced and became insignificant (P = .131, I2 = 33.5%), indicating that this study was a major source of heterogeneity.
Figure 3.
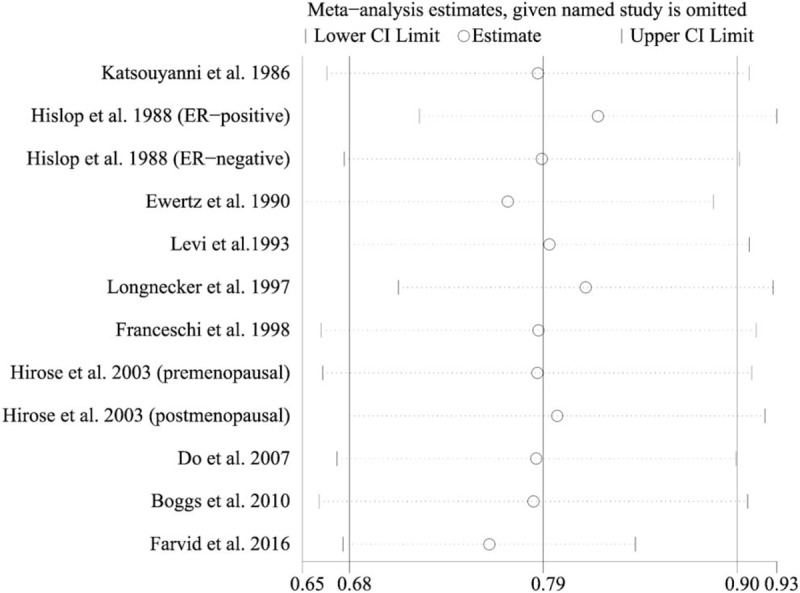
Sensitivity analysis was performed by removing each study in turn and recalculating the pooled risk estimates.
Through the Galbraith plot, we noted that 4 studies were the sources of heterogeneity (Fig. 4). There was no heterogeneity (P = .859, I2 = 0) after excluding these 4 studies, and the overall association was not materially changed (OR 0.81; 95% CI, 0.74–0.88).
Figure 4.
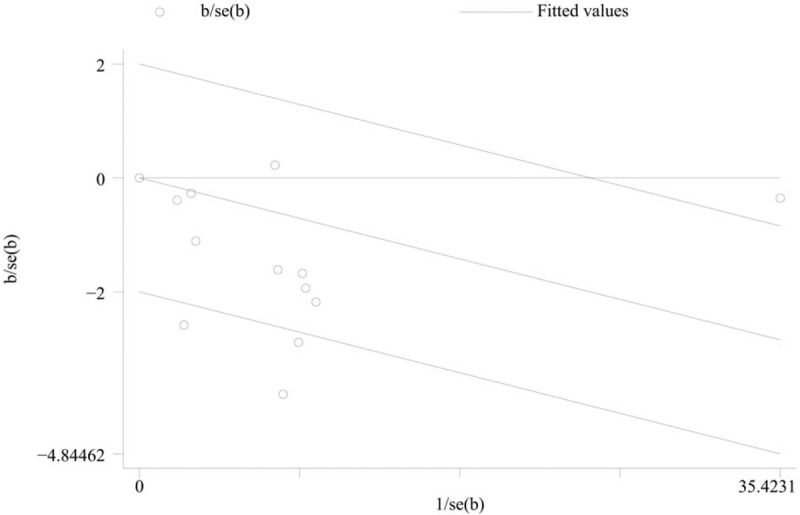
Galbraith plot showing that 4 studies might contribute to heterogeneity.
Cumulative meta-analyses were also performed via the assortment of studies by publication time (Fig. 5). The overall risk estimates were stable and the 95% CIs became increasingly narrower with accumulation of sample size over time before the latest study by Farvid et al.[9]
Figure 5.
A forest plot showing cumulative meta-analysis of dietary carrot intake and breast cancer risk.
We observed obvious asymmetry of Begg's funnel plot (Fig. 6A), and Egger’ test also showed significant publication bias for the analysis between dietary carrot intake and breast cancer incidence (P = .008). It was suggested that methods to test or adjust for publication bias in the presence of heterogeneity may not be powerful when the meta-analysis is not large.[24] Therefore, when we excluded the study by Farvid et al,[9] which was the main source of heterogeneity, there was no indication of publication bias from either visualization of the funnel plot (Fig. 6B) or Egger's test (P = .481).
Figure 6.
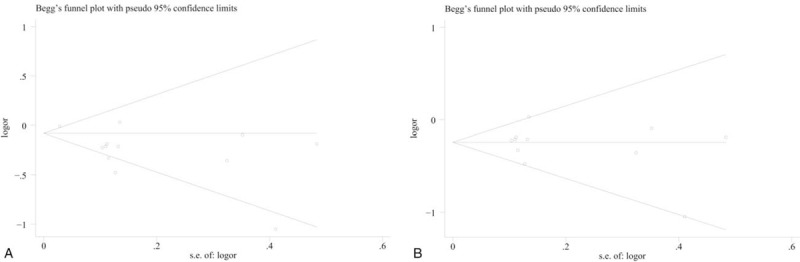
(A) Publication bias estimated by Begg's test. (B) Publication bias estimated by Begg's test when excluding the study by Farvid et al.
4. Discussion
The present meta-analysis, including 2 cohort studies and 8 case–control studies, explored the association between dietary carrot intake and breast cancer risk. To our knowledge, this is the first meta-analysis evaluating the relationship between carrot intake and incidence of breast cancer. The results showed that high carrot intake was associated with a 21% decreased risk of breast cancer.
The heterogeneity among studies should be mentioned. It seemed that the study by Farvid et al,[9] which was one of the 2 included prospective studies, might account for the major source of heterogeneity. In the sensitivity analysis, after excluding this study, the heterogeneity was reduced to a low level, and the inverse association between carrot intake and the risk of breast cancer became stronger. We further conducted a meta-regression analysis to explore the sources of heterogeneity, and the study design was identified as a possible source of heterogeneity. When we stratified by study design, the pooled analysis from the 8 case–control studies suggested an obvious reduction in risk, the results from the 2 cohort studies were nonsignificant, suggesting that our conclusion depend mainly on the case–control studies. However, cohort studies, which are less subjected to recall and selection biases, could provide more robust evidence than case–control studies. Therefore, further well-designed cohort studies are warranted to confirm the findings from our study.
The sensitivity analyses showed that the pooled OR was not materially changed when we omitted any single study, which suggested that our conclusion was statistically stable. In the subgroup analysis separated by geographical regions, study quality, exposure assessment, and adjustment of confounding factors, the results consistently showed significant inverse associations between dietary intake and breast cancer risk, and the pooled ORs were quite similar to the result from the main analysis, which further confirmed the reliability of the results of our study: that carrot intake is likely a protective factor against breast cancer.
A preventive role of carrot in the development of breast cancer is plausible. Carrot are rich in carotenoids as well as other vitamins and phenolic compounds that might have excellent cancer-fighting properties.[25,26] Epidemiological studies have shown that high overall dietary carotenoids intake is associated with a lower risk of breast cancer.[27] Carotenoids are hypothesized to reduce the risk of breast cancer due to their capacity for neutralizing different free radicals at different locations within membranes, inhibit cell proliferation, induce apoptosis, and suppress angiogenesis.[28,29] In vitro cell culture studies have showed that beta-carotene and lycopene could induce cell-cycle arrest and apoptosis in human breast cancer cell lines. In addition, carotenoids might also decrease the activity of cytochrome P450, an activator of procarcinogens,[30] and stimulate the expression of RB and p73, which are both tumor-suppressor genes.[31] In addition, phenolic compounds in carrot also have antioxidant, antimutagenic, and antitumor activities.[32]
There are several important limitations to be considered in interpreting the results of our meta-analysis. First, our meta-analysis only included published studies in English, and we did not search for unpublished studies or original data, which may result in the publication bias in the main analysis. However, the results of funnel plot and Egger's test should be interpreted cautiously due to the small number of included studies and the heterogeneity between studies, which may limit the ability to detect publication bias, and no publication bias was detected when the study by Farvid et al[9] was excluded. Second, breast cancer is a heterogeneous disease, and the estrogen receptor-positive (ER+) tumors are more strongly associated with hormone-related factors, such as late age at first birth and number of births, than ER-negative (ER-) tumors. However, carrot intake was not main focus of the included studies, relatively limited data prevented us from performing subgroup analysis according to ER status. Finally, residual confounding factors are always of concern in epidemiological studies. Compared with low carrot intake population, people who consume high amount of carrot are more likely to be with healthy behaviors, including higher levels of physical activity, lower prevalence of obesity, lower alcohol and fat consumption, and less smoking, but not all of the studies adjusted for these and other potential factors, which may confound the true relationship.
In summary, the results of the current meta-analysis indicated that high carrot intake was associated with decreased risk of breast cancer. These findings could have important public health implications given the high incidence and large burden of breast cancer. However, this evidence is mainly derived from case–control studies and further data from large cohorts are warranted to confirm the results.
Author contributions
Conceptualization: Qilong Miao, Fei Zhang.
Data curation: Fei Zhang.
Investigation: Faming Shao.
Methodology: Haichao Chen.
Software: Faming Shao.
Supervision: Qilong Miao, Fei Zhang.
Writing – original draft: Haichao Chen.
Writing – review & editing: Qilong Miao, Fei Zhang.
Footnotes
Abbreviations: CI = confidence interval, ER = estrogen receptor, OR = odds ratio.
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Vera-Ramirez L, Ramirez-Tortosa MC, Sanchez-Rovira P, et al. Impact of diet on breast cancer risk: a review of experimental and observational studies. Crit Rev Food Sci Nutr 2013;53:49–75. [DOI] [PubMed] [Google Scholar]
- [3].Li C, Ford ES, Zhao G, et al. Serum alpha-carotene concentrations and risk of death among US Adults: the Third National Health and Nutrition Examination Survey Follow-up Study. Arch Intern Med 2011;171:507–15. [DOI] [PubMed] [Google Scholar]
- [4].Xu X, Cheng Y, Li S, et al. Dietary carrot consumption and the risk of prostate cancer. Eur J Nutr 2014;53:1615–23. [DOI] [PubMed] [Google Scholar]
- [5].Fallahzadeh H, Jalali A, Momayyezi M, et al. Effect of carrot intake in the prevention of gastric cancer: a meta-analysis. J Gastric Cancer 2015;15:256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Longnecker MP, Newcomb PA, Mittendorf R, et al. Intake of carrots, spinach, and supplements containing vitamin A in relation to risk of breast cancer. Cancer Epidemiol Biomarkers Prev 1997;6:887–92. [PubMed] [Google Scholar]
- [7].Franceschi S, Parpinel M, La Vecchia C, et al. Role of different types of vegetables and fruit in the prevention of cancer of the colon, rectum, and breast. Epidemiology 1998;9:338–41. [PubMed] [Google Scholar]
- [8].Do MH, Lee SS, Jung PJ, et al. Intake of fruits, vegetables, and soy foods in relation to breast cancer risk in Korean women: a case-control study. Nutr Cancer 2007;57:20–7. [DOI] [PubMed] [Google Scholar]
- [9].Farvid MS, Chen WY, Michels KB, et al. Fruit and vegetable consumption in adolescence and early adulthood and risk of breast cancer: population based cohort study. BMJ 2016;353:i2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. W64. [DOI] [PubMed] [Google Scholar]
- [11].Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- [12].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [13].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [14].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [15].Bax L, Ikeda N, Fukui N, et al. More than numbers: the power of graphs in meta-analysis. Am J Epidemiol 2009;169:249–55. [DOI] [PubMed] [Google Scholar]
- [16].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [18].Katsouyanni K, Trichopoulos D, Boyle P, et al. Diet and breast cancer: a case-control study in Greece. Int J Cancer 1986;38:815–20. [DOI] [PubMed] [Google Scholar]
- [19].Hislop TG, Kan L, Coldman AJ, et al. Influence of estrogen receptor status on dietary risk factors for breast cancer. CMAJ 1988;138:424–30. [PMC free article] [PubMed] [Google Scholar]
- [20].Ewertz M, Gill C. Dietary factors and breast-cancer risk in Denmark. Int J Cancer 1990;46:779–84. [DOI] [PubMed] [Google Scholar]
- [21].Levi F, La Vecchia C, Gulie C, et al. Dietary factors and breast cancer risk in Vaud, Switzerland. Nutr Cancer 1993;19:327–35. [DOI] [PubMed] [Google Scholar]
- [22].Hirose K, Takezaki T, Hamajima N, et al. Dietary factors protective against breast cancer in Japanese premenopausal and postmenopausal women. Int J Cancer 2003;107:276–82. [DOI] [PubMed] [Google Scholar]
- [23].Boggs DA, Palmer JR, Wise LA, et al. Fruit and vegetable intake in relation to risk of breast cancer in the Black Women's Health Study. Am J Epidemiol 2010;172:1268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Terrin N, Schmid CH, Lau J, et al. Adjusting for publication bias in the presence of heterogeneity. Stat Med 2003;22:2113–26. [DOI] [PubMed] [Google Scholar]
- [25].Pelucchi C, Tramacere I, Bertuccio P, et al. Dietary intake of selected micronutrients and gastric cancer risk: an Italian case-control study. Ann Oncol 2009;20:160–5. [DOI] [PubMed] [Google Scholar]
- [26].Pelucchi C, Dal Maso L, Montella M, et al. Dietary intake of carotenoids and retinol and endometrial cancer risk in an Italian case-control study. Cancer Causes Control 2008;19:1209–15. [DOI] [PubMed] [Google Scholar]
- [27].Larsson SC, Bergkvist L, Wolk A. Dietary carotenoids and risk of hormone receptor-defined breast cancer in a prospective cohort of Swedish women. Eur J Cancer 2010;46:1079–85. [DOI] [PubMed] [Google Scholar]
- [28].Chew BP, Brown CM, Park JS, et al. Dietary lutein inhibits mouse mammary tumor growth by regulating angiogenesis and apoptosis. Anticancer Res 2003;23:3333–9. [PubMed] [Google Scholar]
- [29].Cui Y, Lu Z, Bai L, et al. beta-Carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor gamma expression and reactive oxygen species production in MCF-7 cancer cells. Eur J Cancer 2007;43:2590–601. [DOI] [PubMed] [Google Scholar]
- [30].Le Marchand L, Franke AA, Custer L, et al. Lifestyle and nutritional correlates of cytochrome CYP1A2 activity: inverse associations with plasma lutein and alpha-tocopherol. Pharmacogenetics 1997;7:11–9. [DOI] [PubMed] [Google Scholar]
- [31].Nishino H, Tokuda H, Murakoshi M, et al. Cancer prevention by natural carotenoids. Biofactors 2000;13:89–94. [DOI] [PubMed] [Google Scholar]
- [32].Sanchez-Carranza JN, Alvarez L, Marquina-Bahena S, et al. Phenolic compounds isolated from caesalpinia coriaria induce S and G2/M phase cell cycle Arrest differentially and trigger cell death by interfering with microtubule dynamics in cancer cell lines. Molecules 2017;22:E666. [DOI] [PMC free article] [PubMed] [Google Scholar]


