Supplemental Digital Content is available in the text
Keywords: albendazole, eosinophilia, toxocariasis
Abstract
Blood eosinophilia is a common clinical finding. Helminthic infections, including toxocariasis, are a common cause of eosinophilia; however, the clinical course of toxocariasis associated with eosinophilia is not fully understood. Thus, controversies exist regarding treatment indications.
To evaluate the clinical features and natural course of various types of eosinophilia, with a particular focus on toxocariasis, we retrospectively reviewed the medical records of 1000 patients with peripheral blood eosinophilia who were referred to the allergy clinic at Asan Medical Center between 2007 and 2012. Clinical parameters and imaging study findings were evaluated. The treatment response to albendazole and resulting changes in eosinophilia and imaging studies were analyzed in patients diagnosed with toxocariasis.
Among the 1000 subjects, toxocariasis was the most common cause of eosinophilia (n = 534; 53.4%), followed by allergic disease and adverse drug reactions. The majority of patients with toxocariasis were men, and they were mostly asymptomatic. More than one-third of patients (n = 215; 40.3%) with toxocariasis exhibited organ involvement, particularly hepatic involvement. In most cases of eosinophilia and organ involvement due to toxocariasis, the symptoms normalized regardless of treatment.
Most cases of eosinophilia related to toxocariasis displayed a self-remitting course regardless of treatment. With the exception of several clinical situations, including ocular involvement, the clinical need for anti-helminthic therapy in toxocariasis is not that significant.
1. Introduction
Eosinophilia is a common condition in clinical practice and is accompanied by various symptoms and signs, depending on the cause. Common causes of blood eosinophilia include parasitic infections, atopy, allergic diseases, and adverse drug reactions.[1] Toxocariasis, one of the most common worldwide helminthozoonoses, is caused by the migration of Toxocara canis or T cati. Human toxocariasis usually occurs through the ingestion of eggs from soil contaminated with the excrement of potential hosts, such as dogs, cats, and cows, or through the consumption of raw meat or uncooked liver.[2–4] The clinical manifestations of toxocariasis vary greatly, ranging from asymptomatic cases to organ damage that results in serious sequelae.[5,6]
Seroepidemiological data vary by country and environment. The rate of seropositivity is usually 2% to 5% in urban and 14.2% to 37% in rural areas of Europe compared with 50% to 80% in developing tropical countries.[7] One recent article described the prevalence of toxocariasis in patients with unknown eosinophilia as 70%.[8] Nevertheless, the current data related to toxocariasis—including the clinical features and natural course of illness—are limited; therefore, the establishment of appropriate clinical diagnoses, treatments, and long-term follow-up plans is challenging. Albendazole is known as the drug of choice for the treatment of toxocariasis,[9] but there are no standard guidelines for the exact dose or duration of treatment. Hence, the excessive use of drugs or early interruption of the treatment regimen is a concern.
The aims of the present study were to better understand the clinical course and features of toxocariasis and to determine the ideal diagnostic approach and treatment for toxocariasis in terms of eosinophilia and eosinophilic organ infiltration.
2. Methods
2.1. Subjects and study design
We conducted a retrospective study of adult patients who were referred to the allergy clinic at Asan Medical Center owing to eosinophilia between January 2007 and December 2012. Patient medical records were investigated for clinical features, including age, sex, history of allergic disease, drug usage, symptoms, history of ingesting raw beef or viscera in the previous 6 months, and type of prescribed medication if eosinophilia treatment had been administered. Peripheral blood eosinophil counts, serum total immunoglobulin (Ig)E levels, and enzyme-linked immunosorbent assay (ELISA) tests for toxocariasis, clonorchiasis, paragonimiasis, cysticercosis, and sparganosis were also evaluated. To determine the presence of organ involvement, we obtained the results of chest and abdominal computed tomography (CT) scans. Peripheral blood eosinophil counts assessed at 3, 6, 9, and 12 months and follow-up CT results obtained within 1 year after enrollment were used for outcome measurement if the patients had undergone follow-up examinations.
Patients diagnosed with eosinophilia were treated according to the judgment of the clinician. Of these patients, we investigated the medical records of those with toxocariasis who had been prescribed drugs—oral albendazole and steroids—to analyze the effects of these drugs.
All study subjects were fully informed of the study protocol, and they provided written, signed statements of informed consent. The protocol was approved by the Internal Review Board and Ethics Committee of Asan Medical Center in Seoul, Korea (approval number: 2010–0525).
2.2. Definition of eosinophilia
Peripheral eosinophilia is defined as blood eosinophil counts of >0.5 × 109/L. Patients who had a positive result on the Toxocara ELISA were diagnosed with toxocariasis. Other parasitic infections were defined based on the presence of one or more positive parasite serologic test results for Clonorchissinensis, Paragonimus westermani, Cysticercus, and/or Sparganum. In addition to these 2 tests, we allocated study subjects to groups according to the suspected cause of eosinophilia based on demographic and laboratory findings as follows: toxocariasis, other parasitic infections, drugs, allergic disease, pulmonary diseases, hypereosinophilic syndrome, malignancy, and unidentified.[10] Pulmonary diseases were characterized by the appearance of large numbers of eosinophils infiltrating into the airways and parenchyma of the lungs with respiratory symptoms, such as acute/chronic eosinophilic pneumonia, Churg–Strauss syndrome, and allergic bronchopulmonary aspergillosis.[11–14] Patients who fulfilled the criteria for a sustained eosinophilia (≥1.5 × 109/L) in the peripheral blood; signs and symptoms of organ involvement related to eosinophilia; and a lack of an attributable cause for the eosinophilia such as parasitic illness, drugs, or others were assessed as having hypereosinophilic syndrome (HES).[15,16] Study subjects who had no underlying cause of eosinophilia and also did not meet the diagnostic criteria for HES were classified as unidentified. To avoid misclassification, patients with symptoms meeting the criteria for more than one cause (eg, toxocariasis and HES) were excluded from this study.
2.3. Outcome measurement
The clinical course of toxocariasis was assessed based on the improvements in blood eosinophilia and organ involvement. A decline in blood eosinophils of >50% compared with the baseline value or a normalization of eosinophilia (<0.5 × 109/L) within 12 months was considered improvement. Improvement of organ involvement was evaluated with follow-up radiologic imaging findings and categorized into four groups: complete resolution (CR, disappearance of all target lesions), partial resolution (PR, a decrease of 20% to ≥50% in the target lesions), stationary (a decrease of <20% in the target lesions), and aggravation. We also evaluated the effects of albendazole and steroid treatment on the outcomes of patients with toxocariasis.
2.4. Statistical analysis
Data were analyzed using the SPSS 18.0 software package (SPSS Inc., Chicago, IL). Kaplan–Meier survival analysis and Pearson χ2 test were performed to evaluate the improvement in blood eosinophilia and organ involvement, respectively. Continuous variables, including age, blood eosinophil counts, and serum IgE levels, were compared using analysis of variance or the Kruskal–Wallis test. For categorical variables, a χ2 test was performed. We also conducted multivariate analysis using a Cox proportional hazards regression model and logistic regression model to assess the effect of steroid and anti-parasitic treatments in toxocariasis. Age and sex were considered as potential confounders. A P-value of <.05 was regarded as statistically significant.
3. Results
3.1. Causes of eosinophilia
Between January 2007 and December 2012, 1023 patients visited the allergy clinic with eosinophilia, and 1000 patients who underwent adequate evaluation for eosinophilia were included in this analysis.
Among these 1000 patients, toxocariasis was the most common cause of eosinophilia (53.4%). Similar proportions of allergic disease, HES, and drugs were noted (7.1%, 6.7%, and 6.4%, respectively). Pulmonary diseases and malignancy were the least common (Table 1). The median age of the 1000 subjects was 53 years (range, 29–79 years), and the population was predominantly male (86%). The median peak eosinophil count was 971 μL (range, 510–31,840), and the mean serum IgE level was 1476.6 IU/mL.
Table 1.
Causes of eosinophilia.

3.2. Characteristics according to the cause of eosinophilia
The characteristics of each group are presented in Table 2. The median age of each group ranged from 46 to 64 years; the subjects in the allergic and pulmonary diseases group were the youngest. Men constituted the greatest proportion of most groups (52.1%–86%), except for the group with drugs as the cause. The peak peripheral eosinophil count was the highest in the HES and pulmonary diseases groups (3465 and 2948 μL), whereas the toxocariasis and allergic disease groups had the lowest counts (971 and 832 μL). Interestingly, serum total IgE levels were higher in the HES, pulmonary diseases, and toxocariasis groups. The frequency of raw meat ingestion was highest in both the parasitic infection groups (96.8% and 88.9%), whereas the drugs group had the lowest frequency (13.3%). There were significant differences in age, sex, peak peripheral eosinophil count, serum IgE level, history of allergic diseases, frequency of raw meat intake, and symptoms among the groups. The toxocariasis and other parasitic infections groups had a relatively high incidence (40.3% and 48.6%, respectively) of organ involvement. The lung was the most commonly involved organ in the other groups, whereas hepatic involvement was most commonly observed in the toxocariasis and other parasites groups (67.4% and 76.5%, respectively; Table 2).
Table 2.
Characteristics of eosinophilia according to the underlying cause.
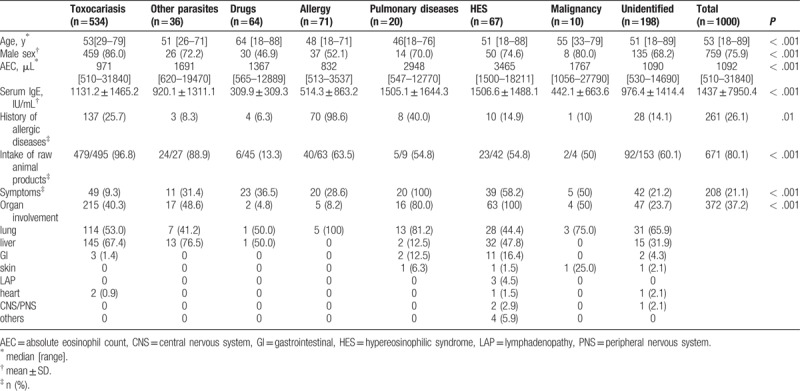
Clinical symptoms were most commonly present in the HES and pulmonary diseases groups (58.2% and 100%), and the most frequent symptoms were respiratory and cutaneous manifestations (eTable 1). Gastrointestinal symptoms occurred more often in the HES and other parasitic infections groups than in the groups with eosinophilia owing to other causes. Toxocariasis exhibited the lowest incidence of clinical symptoms (9.3%), but the distribution of symptoms was similar to that in the other groups. The incidence rate of organ involvement ranged from 4.8% to 81.2%. The malignancy group had the highest rate; however, the sample size was too small for evaluation.
3.3. Validation of the diagnosis of current toxocariasis
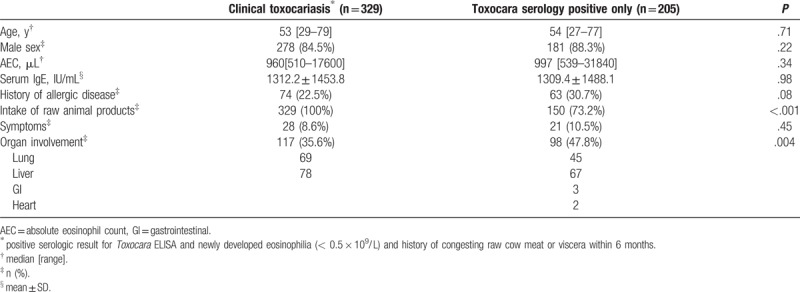
To determine the validity of the current infection assessment performed using the toxocariasis ELISA test, the diagnostic criterion used in this study, we defined another set of diagnostic criteria for further analysis. One diagnostic scheme included patients with eosinophilia who had positive Toxocara ELISA results only, and the other diagnostic scheme included all 3 measures: positive results for the Toxocara ELISA test, newly developed eosinophilia, and a history of ingesting raw beef or viscera within the past 6 months. We compared the clinical characteristics of these 2 groups.
Among 534 patients with toxocariasis, 329 fulfilled all three criteria, and 205 patients had positive results for the serologic test only. Most of the parameters, including age, sex, blood eosinophil counts, serum IgE levels, and symptoms, were not significantly different between the groups, except for the history of consumption of raw animal products and presence of organ involvement. Patients who had only a positive result for the Toxocara ELISA displayed a higher prevalence of organ involvement (35.6% and 47.8%). The liver was the most frequently affected organ in both groups (Table 3).
Table 3.
Clinical characteristics of toxocariasis according to two different diagnostic criteria.
3.4. Clinical course of untreated toxocariasis
To investigate the natural history of toxocariasis-induced eosinophilia, we compared the natural course of untreated patients with toxocariasis-induced eosinophilia with those of untreated patients with other causes of eosinophilia.
Of 534 patients with toxocariasis, 49 (9.2%) received follow-up without any specific treatment. Among these, 39 (39/49, 79.6%) achieved normalization of blood eosinophilia, and the median time to normalization was 3.0 (range, 0.5–12) months.
Of 466 patients with eosinophilia due to other causes, 261 received no medication, and eosinophil counts were followed in 196 of the 261 untreated subjects (75.1%). Blood eosinophilia resolved in 135 patients (135/196, 68.8%), and the median time to resolution was 6.3 (range, 0.5–11.5) months (Table 4).
Table 4.
Clinical course of untreated toxocariasis and other causes of eosinophilia.
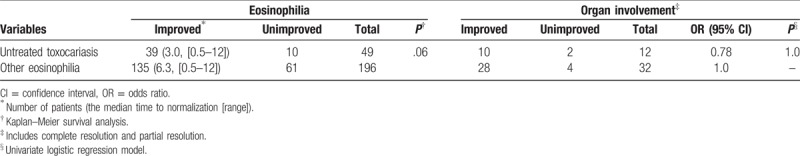
Survival analysis revealed that toxocariasis-induced eosinophilia tended to normalize in a greater number of patients within a shorter period, but this result was without statistical significance (P = .06, Fig. 1). In terms of organ involvement, 12 (24.5%) of 49 patients with toxocariasis without treatment exhibited organ involvement, and 10 patients (10/12, 83.3%) achieved CR. Organ involvement occurred in 32 of the 261 subjects with eosinophilia due to other etiologies (32/261, 12.3%), and 28 experienced improvement. Among these, 26 subjects achieved CR, whereas 2 experienced PR (Table 4). Statistical analysis could not be performed on these data because the sample size was too small; furthermore, toxocariasis improved in all the patients of the untreated group.
Figure 1.
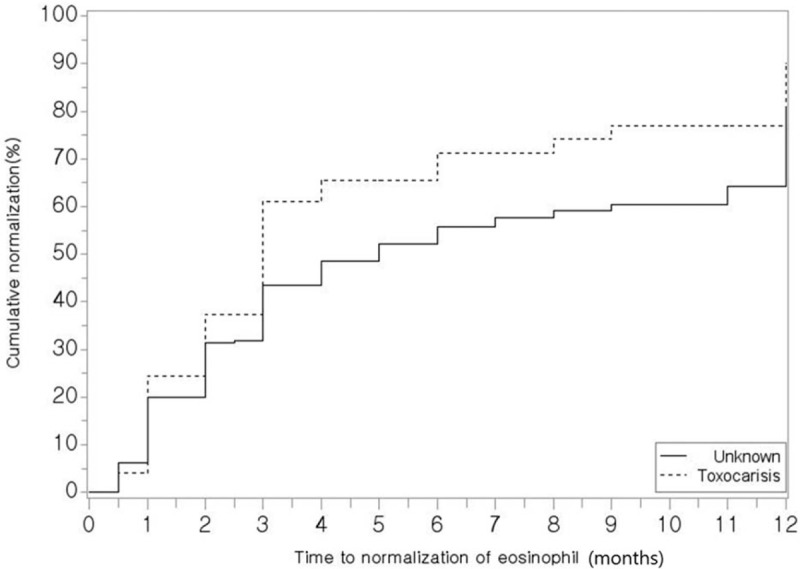
Normalization rates of eosinophilia in patients with untreated toxocariasis and other etiologies of eosinophilia. ∗Data were analyzed using Kaplan–Meier survival analysis.
3.5. Effect of albendazole treatment on toxocariasis
The majority (82.9%) of the 443 patients with toxocariasis who did not receive steroid treatment were followed using blood eosinophil counts. Of these, 391 were treated with 800 mg/day of albendazole, and the remaining 49 did not receive any treatment. The improvement rates of eosinophilia were 75.2% (294/391) and 79.6% (39/49), and the median time to normalization was 3 months (range, 0.5–12). In the multivariate analysis, the albendazole-treated group displayed a lower rate of improvement that was not statistically significant (75.2% vs 79.6%, hazard ratio [HR] 0.78, P > .05; Table 5). When we divided the patients into 3 groups according to the duration of treatment (namely, 800 mg/day of albendazole administered for <1 week or >1 week and no treatment), we detected no significant association between albendazole treatment and the rate of eosinophilia improvement (eTable 2).
Table 5.
Effect of albendazole treatment on toxocariasis-related eosinophilic disorder.
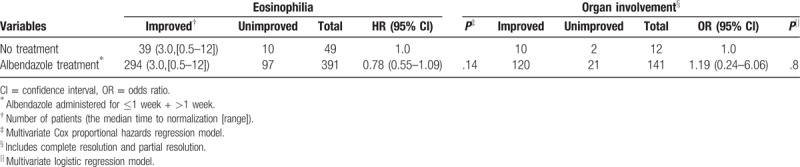
Organ involvement was observed in the 153 patients with toxocariasis (28.7%) who were not treated with steroids, and this aspect of their disease was followed during the study period. Of the patients with organ involvement, 141 were treated with albendazole, and 12 did not receive any treatment. The improvement rates of the treated and untreated patients were 85.1% (120/141) and 83.3% (10/12), respectively; in addition, 108 and 9 patients in the treated and untreated groups achieved CR, respectively. No difference in the radiologic resolution rate was observed between the 2 groups (P = .8, Table 5). In a subgroup analysis of the 3 groups according to treatment duration, no significant association was identified between albendazole treatment and the resolution of organ involvement (eTable 2).
3.6. Effect of steroid treatment on toxocariasis
Additionally, we evaluated the effect of steroid treatment on eosinophilia and organ involvement in toxocariasis. Fifty-four patients (10.1%) without albendazole treatment were followed using blood eosinophil counts; five patients were administered steroids, and the remaining 49 did not receive any medication. The improvement rates of eosinophilia were 100% (5/5) and 79.6% (39/49), and the median times to normalization were 1 (range, 1) and 2 (range, 0.5–12) months, respectively. A positive correlation was identified between steroid treatment and the rate of eosinophilia normalization (P = .02). Of the 413 patients who were treated with albendazole, 22 were co-treated with steroids, and the remaining 391 received albendazole only. Eosinophilia normalized in 86.4% (19/22) of the patients who received steroids and albendazole and in 75.2% (294/391) of the patients in the albendazole only group; the median times to normalization were 2 (range, 0.5–12) and 3.5 (range, 0.5–12) months, respectively. Steroid therapy did not significantly affect the normalization rate in the albendazole-treated group (P = .22, Table 6).
Table 6.
Effect of steroid treatment on toxocariasis-related eosinophilic disorder.
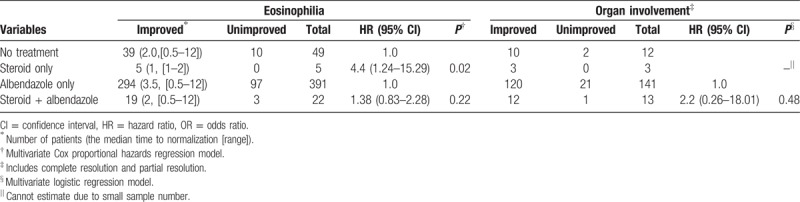
Regarding organ involvement, 15 patients followed the organ involvement without taking albendazole, 3 of which received steroids, and 12 observed any medication. The improvement rates of organ involvement were 100% (3/3) and 83.3% (10/12), respectively. The impact of albendazole on organ involvement was not analyzed due to the small sample size of steroid only group. Of the 154 patients who received albendazole, 141 received the albendazole alone, while the other 13 received both steroids and albendazole. Organ involvements were improved in 85.1% (120/141) of albendazole only group and in 92.3% (12/13) of patients who received both albendazole and steroids. There was no statistically significant difference between the 2 groups (HR 2.2, P = .48, Table 6).
4. Discussion
In the present study, toxocariasis was found to be the most common cause of eosinophilia, and 53.4% patients with eosinophilia were diagnosed with this condition. The majority of patients with toxocariasis in the current study followed a benign course, with 79.6% displaying normalization of their peripheral eosinophil counts. Toxocariasis-induced eosinophilia exhibited a significantly higher normalization rate than did eosinophilia due to other causes and resolved more quickly without medical treatment. Eosinophilia with organ involvement was observed more frequently in cases of toxocariasis than in cases resulting from other causes such as drugs or allergic diseases. Most cases of organ involvement in toxocariasis were limited to the liver and lung.
Human toxocariasis was observed to be most common in middle-aged male subjects, and most patients were asymptomatic. Several publications have reported a relationship between raw meat consumption and toxocariasis.[7,17–19] Consistent with previous results, we observed a strong correlation between raw meat consumption and Toxocara infection.
The prevalence of Toxocara infection in this study was very high in cases of eosinophilia, which is consistent with recent studies indicating a prevalence of >5%.[20–22] In particular, considering that most of the patients followed at our center are urban residents, the current prevalence of toxocariasis is likely to be an underestimate in both urban and rural areas. Therefore, Toxocara infection should be considered a potential cause of clinical eosinophilia, particularly in male patients with a recent history of raw meat ingestion. If toxocariasis is suspected, physicians should consider evaluating the patient for possible organ involvement using CT or ultrasonography.
Currently, the most accurate diagnosis of Toxocara infection is made through tissue biopsy and confirmation of larva; however, such a diagnostic approach is difficult to perform and invasive. Hence, the detection of IgG antibodies against the excretory-secretory antigens (ES Ag) of T canis using ELISA is the mainstay of diagnosis.[18] The Toxocara ELISA is known to have up to 90% sensitivity and specificity; however, this test has some limitations such as its inability to distinguish current disease from previous infections. It also has the potential to produce false-positive results for strongyloidiasis, trichinelliasis, and fascioliasis.[5,23] To compensate for these shortcomings, clinicians often conduct simultaneous Western blotting and ELISA or use recombinant TES Ag, but such an approach involves practical difficulties related to cost and time.[3] To establish more efficient clinical diagnostic criteria and to validate the serological methods for current Toxocara infection, we divided Toxocara seropositive patients into 2 groups: patients with a positive serological result only and patients who fulfilled the clinical toxocariasis criteria adopted in this study. Our results revealed no differences in the clinical characteristics of these 2 groups, including their age and sex or laboratory data such as peripheral eosinophil counts, IgE levels, and frequency of organ involvement. Compared with other patients with different causes of eosinophilia, the patients who only had a seropositive result displayed a higher rate of raw meat intake within the prior 6 months. Further assessments are clearly required, but the current serological method is believed to be a relatively reasonable diagnostic tool, particularly when considering clinical factors such as newly developed eosinophilia or a history of raw meat ingestion in combination.
The average eosinophil count of patients with toxocariasis in the present study was 971 μL, which is consistent with a previous study that reported a range of 400 to 1000 μL in most asymptomatic cases.[5] In addition to peripheral eosinophil counts and serological methods, serum IgE and eosinophil cationic protein levels are alternative measures that can assist in the diagnosis of a current Toxocara infection.[24] According to the findings of Obwaller et al[25] and Magnaval et al[26] in the 1990 s, the level of serum IgE is a less effective marker than is the intensity of eosinophilia for the evaluation of toxocariasis, but higher IgE levels are more frequently observed in patients with toxocariasis with cutaneous signs. In the current study, however, patients with toxocariasis displayed high IgE levels despite most cases being asymptomatic. More interestingly, the average IgE level of the current toxocariasis patients was >1300 IU, which is similar to the levels observed in the HES group and indicates a marked increase in eosinophil counts. Toxocara larvae are tissue-penetrating parasites, so the peripheral eosinophil count may not fully reflect the true infectious status, owing to potential sequestration in infected tissues.[13] In such cases, we suggest that an evaluation of serum IgE levels could be helpful in making a diagnosis.
Even after the confirmation of Toxocara infection, deciding to treat and selecting the appropriate treatment for the infection can be challenging. Most clinicians agree that treatment should be initiated when involvement of vital organs such as the brain or eye is confirmed or the infection is symptomatic. Currently, the administration of albendazole, known as treatment of choice in toxocariasis, has reported effective symptom improvement and relapse prevention effect.[5,27,28]
However, in cases of asymptomatic infection that may only show a high TES antibody titer, conflicting opinions exist. Some authors argue against treatment on the basis that the majority of toxocariasis is self-limiting or subclinical. There is also concern regarding allergic reactions following anti-parasitic therapy.[29,30] Conversely, other authors insist that anti-parasitic therapy is necessary for the treatment of toxocariasis as a persistent, chronic infection to prevent the migration of reactivated larvae into vital organs.[5,31,32] Most patients with toxocariasis in the present study were asymptomatic, but >80% of these patients received treatment. Several studies have recommended albendazole (800 mg/day for 5 days or 10 mg/kg for 14 days) as the standard treatment regimen for systemic toxocariasis.[18,33,34] Based on this recommendation, we assessed the effect of albendazole treatment on the normalization rate of eosinophilia and organ involvement, according to the treatment duration (untreated, ≤1 week, >1 week). Nevertheless, we observed that anti-parasitic therapy did not affect the course of eosinophilia or the degree of organ involvement regardless of the period of treatment, and most patients demonstrated improvement without treatment. By contrast, patients in the current study who were prescribed an oral steroid demonstrated a shorter period to normalization in blood eosinophilia. However, because the number of patients analyzed in the present study was too small to allow statistical analysis, further studies are needed to corroborate this finding. Clinical determination of the appropriate follow-up period for patients with eosinophilia is also challenging. We suggest that a 3 to 4-month interval is a suitable period for patient follow-up, because the median time for normalization of eosinophilia was 4.3 months in the untreated group in the present study.
There are some limitations of the current study. First, owing to the retrospective nature of our analysis, follow-up eosinophil counts were assessed in an irregular manner and some loss of data occurred, which may have affected the results. For toxocariasis-induced organ involvement, the number of patients was too small, and the follow-up period was irregular, so it was not possible to determine the average time until improvement. Nevertheless, this is the first large-scale study to evaluate the clinical course and treatment outcomes of Toxocara infection, and we evaluated the validity of the current diagnostic tools and treatment.
In summary, the prevalence of human toxocariasis is high; hence, clinicians need to consider toxocariasis as a common cause of eosinophilia, even in urban areas. Existing diagnostic tools, including a serological test and clinical factors, are reasonably effective for detecting current toxocariasis. In addition, in most cases of asymptomatic human toxocariasis, empirical anti-parasitic therapy considered unnecessary even if they have eosinophilia or organ involvement. Clinicians should be more prudent in making treatment decisions and appropriately selecting patients.
Author contributions
Data curation: Sun-Young Yoon, Seunghee Baek.
Formal analysis: Sun-Young Yoon, Seunghee Baek.
Funding acquisition: Tae-Bum Kim.
Investigation: Sun-Young Yoon, So Y. Park, Bomi Shin, Hyouk-Soo Kwon, Tae-Bum Kim.
Methodology: Sun-Young Yoon, So Y. Park, Hyouk-Soo Kwon, You S. Cho, Tae-Bum Kim.
Project administration: Tae-Bum Kim.
Resources: Tae-Bum Kim.
Supervision: Hyouk-Soo Kwon, You S. Cho, Hee-Bom Moon.
Writing - original draft: Sun-Young Yoon.
Writing - review & editing: Tae-Bum Kim.
Supplementary Material
Footnotes
Abbreviations: AEC = absolute eosinophil count, CNS = central nervous system, CR = complete resolution, GI = gastrointestinal, HES = hypereosinophilic syndrome, LAP = lymphadenopathy, PNS = peripheral nervous system, PR = partial resolution.
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HC15C1335). Tae-Bum Kim orcid: 0000-0001-5663-0640.
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Roufosse F, Weller PF. Practical approach to the patient with hypereosinophilia. J Allergy Clin Immunol 2010;126:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Choi D, Lim JH, Choi DC, et al. Toxocariasis and ingestion of raw cow liver in patients with eosinophilia. Korean J Parasitol 2008;46:139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fillaux J, Magnaval JF. Laboratory diagnosis of human toxocariasis. Vet Parasitol 2013;193:327–36. [DOI] [PubMed] [Google Scholar]
- [4].Hoffmeister B, Glaeser S, Flick H, et al. Cerebral toxocariasis after consumption of raw duck liver. Am J Trop Med Hyg 2007;76:600–2. [PubMed] [Google Scholar]
- [5].Pawlowski Z. Toxocariasis in humans: clinical expression and treatment dilemma. J Helminthol 2001;75:299–305. [DOI] [PubMed] [Google Scholar]
- [6].Rubinsky-Elefant G, Hirata CE, Yamamoto JH, et al. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol 2010;104:3–23. [DOI] [PubMed] [Google Scholar]
- [7].Magnaval JF, Glickman LT, Dorchies P, et al. Highlights of human toxocariasis. Korean J Parasitol 2001;39:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kwon NH, Oh MJ, Lee SP, et al. The prevalence and diagnostic value of toxocariasis in unknown eosinophilia. Ann Hematol 2006;85:233–8. [DOI] [PubMed] [Google Scholar]
- [9].Othman AA. Therapeutic battle against larval toxocariasis: are we still far behind? Acta Trop 2012;124:171–8. [DOI] [PubMed] [Google Scholar]
- [10].Tefferi A. Blood eosinophilia: a new paradigm in disease classification, diagnosis, and treatment. Mayo Clin Proc 2005;80:75–83. [DOI] [PubMed] [Google Scholar]
- [11].Watts R, Lane S, Hanslik T, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007;66:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Agarwal R. Allergic bronchopulmonary aspergillosis. Chest 2009;135:805–26. [DOI] [PubMed] [Google Scholar]
- [13].Tazelaar HD, Linz LJ, Colby TV, et al. Acute eosinophilic pneumonia: histopathologic findings in nine patients. Am J Respir Crit Care Med 1997;155:296–302. [DOI] [PubMed] [Google Scholar]
- [14].Carrington CB, Addington WW, Goff AM, et al. Chronic eosinophilic pneumonia. N Engl J Med 1969;280:787–98. [DOI] [PubMed] [Google Scholar]
- [15].Chusid MJ, Dale DC, West BC, et al. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore) 1975;54:1–27. [PubMed] [Google Scholar]
- [16].Amy DK, Peter FW. Eosinophilia and eosinophil-related disorders. Middleton's Allergy: Principles, Practice. 8th ed.2013;Philadelphia, PA: Saunders, an imprint of Elsevier Inc, 1205–23. [Google Scholar]
- [17].Sturchler D, Schubarth P, Gualzata M, et al. Thiabendazole vs. albendazole in treatment of toxocariasis: a clinical trial. Ann Trop Med Parasitol 1989;83:473–8. [DOI] [PubMed] [Google Scholar]
- [18].Deutz A, Fuchs K, Auer H, et al. Toxocara-infestations in Austria: a study on the risk of infection of farmers, slaughterhouse staff, hunters and veterinarians. Parasitol Res 2005;97:390–4. [DOI] [PubMed] [Google Scholar]
- [19].Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev 2003;16:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Obwaller A, Jensen-Jarolim E, Auer H, et al. Toxocara infestations in humans: symptomatic course of toxocarosis correlates significantly with levels of IgE/anti-IgE immune complexes. Parasite Immunol 1998;20:311–7. [DOI] [PubMed] [Google Scholar]
- [21].Park HY, Lee SU, Huh S, et al. A seroepidemiological survey for toxocariasis in apparently healthy residents in Gangwon-do, Korea. Korean J Parasitol 2002;40:113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim HB, Seo JW, Lee JH, et al. Evaluation of the prevalence and clinical impact of toxocariasis in patients with eosinophilia of unknown origin. Korean J Intern Med 2017;32:523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jacquier P, Gottstein B, Stingelin Y, et al. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol 1991;29:1831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Magnaval JF, Berry A, Fabre R, et al. Eosinophil cationic protein as a possible marker of active human Toxocara infection. Allergy 2001;56:1096–9. [DOI] [PubMed] [Google Scholar]
- [25].Magnaval JF, Fabre R, Maurieres P, et al. Evaluation of an immunoenzymatic assay detecting specific anti-Toxocara immunoglobulin E for diagnosis and posttreatment follow-up of human toxocariasis. J Clin Microbiol 1992;30:2269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ahn SJ, Woo SJ, Jin Y, et al. Clinical features and course of ocular toxocariasis in adults. PLoS Negl Trop Dis 2014;8:e2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rytomaa T. Organ distribution and histochemical properties of eosinophil granulocytes in rat. Acta Pathol Microbiol Scand Suppl 1960;50:1–18. [PubMed] [Google Scholar]
- [28].Barisani-Asenbauer T, Maca SM, Hauff W, et al. Treatment of ocular toxocariasis with albendazole. J Ocul Pharmacol Ther 2001;17:287–94. [DOI] [PubMed] [Google Scholar]
- [29].Mikulecky M, Mikulecky M. A biometrical view on chemotherapy of toxocariasis. Acta Pediatr 1994;83:1232. [DOI] [PubMed] [Google Scholar]
- [30].Kazura JW. Mahmoud AA. Visceral larva migrans and other tissue nematodes ???. Marcel Dekker Inc., New York:1997. [Google Scholar]
- [31].Winiewska-Ligier M, Wo?niakowska-G?sicka T, Sobolewska-Dryja?ska J, et al. Analysis of the course and treatment of toxocariasis in children-a long-term observation. Parasitol Res 2012;110:2363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Magnaval JF, Dorchies P, Glickman LT. Antimicrobial Therapy and Vaccines. 2nd ed.Pittsburgh, PA: ESun Technologies LLC; 2005. [Google Scholar]
- [33].Hotez PJ. Gellis and Kaganís Current Pediatric Therapy. 15th ed.Philadelphia, PA: W.B. Saunders Pubs; 1995. [Google Scholar]
- [34].Kim YH, Huh S. Prevalence of Toxocara canis, Toxascaris leonina and Dirofilaria immitis in dogs in Chuncheon, Korea (2004). Korean J Parasitol 2005;43:65–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


