Abstract
Rationale:
Membranoproliferative glomerulonephritis (MPGN) can be induced by autoimmune diseases, chronic infection, chronic hepatitis, and paraproteins (including cryoglobulinemia). In addition, the mixed cryoglobulinemic MPGN is reported to be highly correlated with hepatitis C virus (HCV) infection.
Patient concerns:
We reported a rare case of a 61-year-old woman without a history of viral hepatitis infection; she presented with bilateral leg edema and proteinuria. Renal pathology revealed MPGN with multiple positive immunofluorescent staining. The consequent serum survey revealed positive cryoglobulin and monoclonal gammopathy of kappa type of immunoglobulin M. However, bone marrow study showed no obvious plasma cell proliferation, indicating that multiple myeloma was less likely.
Diagnoses:
This patient's cryoglobulinemic MPGN could be related to monoclonal gammopathy of undetermined significance.
Interventions:
Oral immunosuppressant.
Outcomes:
After steroid treatment, her renal function normalized and proteinuria kept in low level.
Lessons:
We demonstrated a rare cause of cryoglobulinemic MPGN without HCV infection, which led to a favorable prognosis after receiving steroid therapy. Moreover, the diagnosis of monoclonal gammopathy should be considered when facing such case and aggressive steroid therapy might be beneficial.
Keywords: cryoglobulins, hepatitis C virus infection, membranoproliferative glomerulonephritis, monoclonal gammopathy, renal failure, renal pathology
1. Introduction
Membranoproliferative glomerulonephritis (MPGN) is characterized by the presence of mesangial hypercellularity, subendothelial deposition of immune complexes, and duplication of glomerular basement membrane.[1] Currently, MPGN is classified as immune-complex-mediated and complement-mediated MPGN; immune-complex-mediated MPGN is secondary to chronic infection, autoimmune disorders, and paraproteinemia (monoclonal gammopathy or cryoglobulinemia).[2–4] Mixed cryoglobulinemic MPGN, meaning that the cryoglobulins comprise more than 2 classes of immunoglobulin (Ig), has been mainly correlated with hepatitis C virus (HCV) infection.[5,6]
However, in this report, we presented a rare case of mixed cryoglobulinemic MPGN with contributions from monoclonal gammopathy of undetermined significance (MGUS) but not HCV infection.
2. Case presentation
A 61-year-old Taiwanese woman was admitted to the hospital because of bilateral leg edema, dizziness, and refractory hypertension. Her medical history was unremarkable, and there was no particular personal or family history of illness.
On admission in our hospital, a physical examination revealed cognition to be within normal limits, temperature of 36.2°C, heart rate of 92 beats/min, and a blood pressure of 190/100 mm Hg. Significant laboratory test findings included renal impairment (creatinine: 1.7 mg/dL), severe anemia (hemoglobin: 7.0 g/dL), hypoalbuminemia (albumin: 3.1 g/dL), and heavy proteinuria (2625 mg/24 h) (Table 1). Renal sonography showed bilateral kidneys of normal size (the right kidney was 12 cm and left kidney was 11 cm) but with increased echogenicity. Under the impression of acute renal failure due to glomerulonephritis, serum immunological markers were checked; they revealed elevated IgM and decreased complements levels. The serological test results for hepatitis B surface antigen, hepatitis C antibody, human immunodeficiency virus screens, antinuclear antibody, and antineutrophil cytoplasmic antibodies were all negative. However, serum immunoelectrophoresis was positive for the kappa type of monoclonal IgM and for the cryoglobulin (Table 1).
Table 1.
Laboratory data at admission.

Therefore, diagnostic renal biopsy was performed, and it showed lobulated, diffuse mesangial, and endocapillary proliferation with double-contour of the glomerular basement membrane (Fig. 1) and positive immunofluorescent staining of IgG, IgM, kappa, lambda, and C3 deposition (Fig. 2), which was compatible with the diagnosis of MPGN. Electron microscopy revealed a duplication of glomerular capillary walls with dense deposits in the subendothelial zone (Fig. 3). Further bone marrow examination was performed for monoclonal gammopathy, and it showed hypocellular marrow (cellularity: 30%) without an increased number of plasma cells, indicating the diagnosis of multiple myeloma was not favored.
Figure 1.
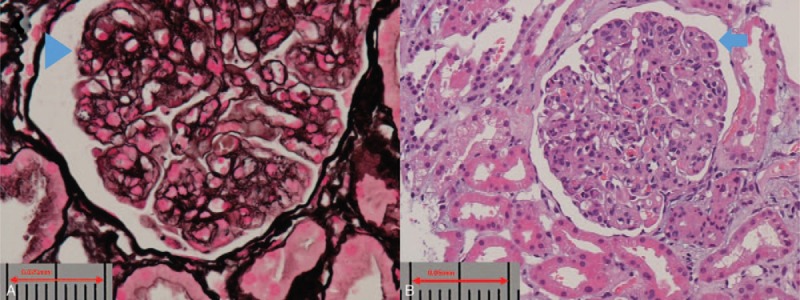
Light microscopy. (A) Silver stain, revealing double-contour of the glomerular basement membrane (arrow head). (B) Hematoxylin and eosin stain, showing lobulated, diffused mesangial and endocapillary proliferation (arrow).
Figure 2.
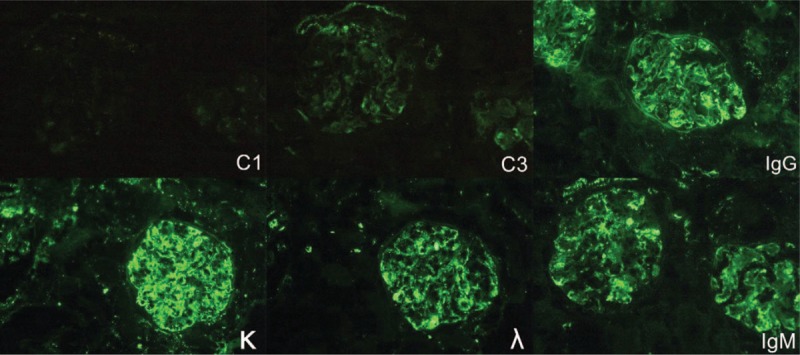
Immunofluorescence microscopy. The result shows a positive deposition of IgG, IgM, C3, κ, and λ.
Figure 3.
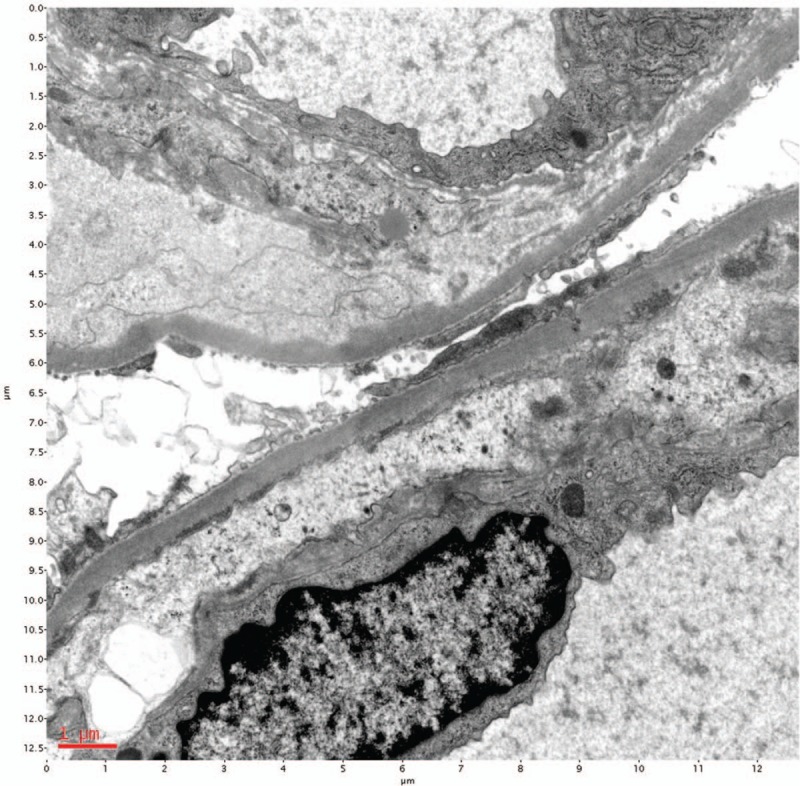
Electron microscopy. Duplication of glomerular capillary walls with dense deposits in the subendothelial zone.
Based on clinicopathologic correlation, cryoglobulinemic MPGN secondary to MGUS was impressed. Oral immunosuppressant therapy was adopted, including cyclosporine (25 mg daily for 3 months and then reduced to 25 mg every day for another 3 months) and prednisolone (20 mg daily for 3 months and then gradually tapered to 5 mg daily). She has continued the low-dose prednisolone therapy (5 mg daily) for approximately 4 years, and her renal function remained in the normal range with mild proteinuria (urine protein–creatinine ratio: 0.79 mg/mg).
3. Discussion
A majority of patients with MPGN are children, but the condition can occur at any age and the clinical presentation and course are variable. The proportions of males and females are nearly equal.[7] In general, one-third of patients with MPGN will have spontaneous remission, one-third will have progressive disease, and one-third will have disease processes that will undergo alternate increases and decreases but never completely disappear.[8]
Cryoglobulins precipitate at temperatures lower than normal body temperature (37°C) and are categorized as types I to III.[9] Type I cryoglobulinemia, in which the monoclonal Ig composes the cryoglobulin and most often due to underlying multiple myeloma or Waldenström macroglobulinemia.[10] Type II cryoglobulinemia, in which the cryoglobulin contains both a polyclonal IgG and a monoclonal IgM rheumatoid factor directed against the IgG, is most often due to chronic infection with HCV. Type III cryoglobulinemia, in which both IgG and IgM are polyclonal, is often seen in patients with chronic inflammatory and autoimmune diseases (such as systemic lupus erythematosus and Sjögren syndrome), lymphoproliferative malignancies, and HCV infection.[11–13] Types II and III were termed as the mixed cryoglobulinemia. In our case, the immunoelectrophoresis was not performed for his cryoglobulinemia, of which the type classification would be limited. However, according to the positive immunofluorescent staining of IgG, IgM, kappa, lambda, and C3 deposition in renal pathology and the evidence of serum monoclonal IgM, the type II cryoglobulinemic MPGN was impressed.
In patients with HCV infection, the immune complex consists of anti-HCV IgG and IgM anti-IgG (rheumatoid factor). The HCV may serve as the inciting agent because it has been found in high concentrations in the cryoprecipitate.[12,14] In addition to the increased production, a decreased clearance of the circulating immune complexes due to hepatic dysfunction may promote circulatory cryoglobulin accumulation and subsequent tissue deposition. This is supported by the known association between circulating cryoglobulins and prolonged liver disease and cirrhosis.[14]
In a report from the Mayo Clinic,[4] which included 68 patients with MPGN who were not infected with HCV, 28 patients (41%) presented with monoclonal and/or biclonal gammopathy, 24 were positive for monoclonal gammopathies, and 4 were positive for biclonal gammopathies. Out of 24 monoclonal gammopathies, 10 were positive for IgM κ, 9 were positive for IgG κ, 4 were positive for IgG λ, and 1 had only “light chains.” Out of 28 patients, 16 had MGUS, which was revealed on performing bone marrow biopsy. Cryoglobulin was positive in only 3 cases among the 28 patients. Two patients showed type I cryoglobulin, and 1 patient showed type II cryoglobulin. Moreover, Okura et al[15] had reported 81-year-old male with mixed cryoglobulinemic MPGN without HCV infection, who led a favorable outcome after a steroid therapy. Table 2 shows the published cases with cryoglobulinemic MPGN but without HCV infection in the literature, which just 4 cases have been reported. Our case was similar to the one reported by Okura et al,[15] who both had favorable response after steroid therapy.
Table 2.
Patients with mixed cryoglobulinemic membranoproliferative glomerulonephritis and negative hepatitis C virus infection.

Our patient was positive for cryoglobulinemia. Therefore, a careful follow-up is required because cryoglobulinemia is usually associated with lymphoproliferative disease (Waldenström macroglobulinemia or multiple myeloma). No standard treatment for monoclonal gammopathy-associated MPGN treatment was suggested and decisions will be made purely on the basis of clinical experience. Thereafter, there is a need for prospective, controlled studies in a larger cohort of patients with MPGN with monoclonal gammopathy to determine the optimal therapy.[16]
4. Conclusion
We reported a rare case of mixed cryoglobulinemic MPGN associated with underlying monoclonal gammopathy but not with HCV infection. Moreover, cryoglobulinemic MPGN seems to be associated with a favorable prognosis. Therefore, early diagnosis and treatment are essential. The aggressive steroid therapy may be beneficial for such case.
Author contributions
Conceptualization: Yu-Wei Fang, An-Hung Yang.
Data curation: Jung-Hui Hsu, An-Hung Yang.
Investigation: Ming-Hsein Tsai.
Supervision: Ming-Hsein Tsai.
Writing – original draft: Jung-Hui Hsu.
Writing – review & editing: Yu-Wei Fang, Ming-Hsein Tsai.
Footnotes
Abbreviations: HCV = hepatitis C virus, Ig = immunoglobulin, MGUS = monoclonal gammopathy of undetermined significance, MPGN = membranoproliferative glomerulonephritis.
Ethic approval was waived by the ethics committee at the Shin Kong Wu Ho-Su Memorial Hospital because our case study was based on chart review. However, the written informed consent was obtained from patient for publishing the related images and laboratory data.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Smith KD, Alpers CE. Pathogenic mechanisms in membranoproliferative glomerulonephritis. Curr Opin Nephrol Hypertens 2005;14:396–403. [DOI] [PubMed] [Google Scholar]
- [2].Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol 2011;31:341–8. [DOI] [PubMed] [Google Scholar]
- [3].Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med 2012;366:1119–31. [DOI] [PubMed] [Google Scholar]
- [4].Sethi S, Zand L, Leung N, et al. Membranoproliferative glomerulonephritis secondary to monoclonal gammopathy. Clin J Am Soc Nephrol 2010;5:770–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].D’Amico G, Fornasieri A. Cryoglobulinemic glomerulonephritis: a membranoproliferative glomerulonephritis induced by hepatitis C virus. Am J Kidney Dis 1995;25:361–9. [DOI] [PubMed] [Google Scholar]
- [6].Saadoun D, Sellam J, Ghillani-Dalbin P, et al. Increased risks of lymphoma and death among patients with non-hepatitis C virus-related mixed cryoglobulinemia. Arch Intern Med 2006;166:2101–8. [DOI] [PubMed] [Google Scholar]
- [7].Cameron JS, Turner DR, Heaton J, et al. Idiopathic mesangiocapillary glomerulonephritis. Comparison of types I and II in children and adults and long-term prognosis. Am J Med 1983;74:175–92. [DOI] [PubMed] [Google Scholar]
- [8].D’Amico G, Ferrario F. Mesangiocapillary glomerulonephritis. J Am Soc Nephrol 1992;2(suppl):S159–66. [DOI] [PubMed] [Google Scholar]
- [9].D’Amico G, Colasanti G, Ferrario F, et al. Renal involvement in essential mixed cryoglobulinemia. Kidney Int 1989;35:1004–14. [DOI] [PubMed] [Google Scholar]
- [10].Paueksakon P, Revelo MP, Horn RG, et al. Monoclonal gammopathy: significance and possible causality in renal disease. Am J Kidney Dis 2003;42:87–95. [DOI] [PubMed] [Google Scholar]
- [11].Monti G, Galli M, Invernizzi F, et al. Cryoglobulinaemias: a multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISC. Italian Group for the Study of Cryoglobulinaemias. QJM 1995;88:115–26. [PubMed] [Google Scholar]
- [12].Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med 1992;327:1490–5. [DOI] [PubMed] [Google Scholar]
- [13].Pozzato G, Mazzaro C, Crovatto M, et al. Low-grade malignant lymphoma, hepatitis C virus infection, and mixed cryoglobulinemia. Blood 1994;84:3047–53. [PubMed] [Google Scholar]
- [14].Lunel F, Musset L, Cacoub P, et al. Cryoglobulinemia in chronic liver diseases: role of hepatitis C virus and liver damage. Gastroenterology 1994;106:1291–300. [DOI] [PubMed] [Google Scholar]
- [15].Okura T, Jotoku M, Miyoshi K, et al. Case of membranoproliferative glomerulonephritis due to essential cryoglobulinemia without hepatitis C virus infection. Geriatr Gerontol Int 2009;9:92–6. [DOI] [PubMed] [Google Scholar]
- [16].Nasr SH, Satoskar A, Markowitz GS, et al. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol 2009;20:2055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


