Abstract
Background:
Bone metastases are common in advanced breast cancer patients and frequently leading to skeletal-related morbidity and deterioration in the quality of life. Although chemotherapy and hormone therapy are able to control the symptoms caused by bone destruction, the underlying molecular mechanisms for the affinity of breast cancer cells towards skeletal bones are still not completely understood.
Methods:
In this study, bioinformatic analysis was performed on patients’ microarray gene expression data to explore the molecular mechanism associated with breast cancer bone metastasis. Microarray gene expression profile regarding patients with breast cancer and disseminated tumor cells was downloaded from Gene Expression Omnibus (GEO) database (NCBI, NIH). Raw data were normalized and differently expressed genes were identified by using Significance Analysis of Microarrays (SAM) methods. Protein interaction networks were expanded using String. Moreover, molecular functions, biological processes and signaling pathway enrichment analysis were performed using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG).
Results:
We identified 66 differentially expressed genes. After submitting the set of genes to String, genetic interaction network was expanded, which consisted of 110 nodes and 869 edges. Pathway enrichment analysis suggested that adhesion kinase, ECM-receptor interaction, calcium signaling, Wnt pathways, and PI3K/AKT signaling pathway are highly associated with breast cancer bone metastasis.
Conclusion:
In this study, we established a microarray genetic interaction network associated with breast cancer bone metastasis. This information provides some potential molecular therapeutic targets for breast cancer initiation and progression.
Keywords: bone metastasis, breast cancer, enrichment analysis, interaction network, microarray
1. Introduction
Globally, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer-related death in women.[1] Metastatic diseases occur in most women with advanced breast cancer and bone is one of the most preferential distant organs for metastasis of breast cancer. Evidence from clinical and postmortem studies suggests that 47% to 85% of breast cancer patients will have bone metastasis.[2] It has also been reported that the breast cancer tumor subtypes affect the metastases sites and rates. The lowest rate of bone metastases are patients with estrogen (ER)-negative/human epidermal growth factor receptor 2 (HER2)-negative tumors, which is 55.2%; meanwhile, this rate was significantly increased to 69.8% (HER2-positive tumors), 87.8% (ER-positive/HER2-negative/Ki67high tumors), and 73.1% (ER-positive/HER2-negative/Ki67low tumors). The most common sites of bone metastases are the spine, ribs, pelvis, proximal femur, and skull. The destruction of these bones frequently leads to excessive skeletal-related complication such as bone pain, pathological fractures, life-threatening hypercalcemia, spinal cord compression, and other nerve compression syndromes. Some of them can be fatal and significantly reduce the quality of life.
Bone metastasis is a complex, multistage process that requires breast cancer cells to detach from the primary tumor, travel through the blood or lymphatic system, survive in bone microenvironment, and then proliferate in bone tissue.[3] To date, genomic studies have suggested that each step of metastasis was associated with a series of molecular events. However, the interaction network of molecular mechanism associated bone metastases from breast cancer is still not completely understood. Motivated by this, we established a comprehensive protein interaction network by building a microarray gene expression profile originating from breast cancer patients with bone metastases, hoping to reveal the molecular mechanisms in breast cancer bone metastasis. In our analysis, 66 genes with significant expression changes were identified to confer bone metastasis. Pathway enrichment analysis highlighted that adhesion kinase, extracellular matrix (ECM)–receptor interaction, calcium signaling pathway, and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway are potential key regulators, which may involve in breast cancer bone metastasis. These results advanced our understanding of molecular information of bone metastasis from breast cancer and provided potential targets for clinical interventions.
2. Material and methods
2.1. Microarray dataset resources
After searching in Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/), a public functional genomics data repository, a microarray dataset was downloaded with the accession number GSE14776. In this study, Cawthorn et al[4] explored the analyzable yield of genetic material from human biopsy samples in order to describe differences in gene expression between disseminated tumor cells and bone metastatic tumor cells. Total RNA was extracted from disseminated tumor cells and bone metastatic tumor cells and mRNA array was performed on Illumina HumanRef-8 v3.0 platform. Other involved online databases were listed in the String website.
2.2. Aberrant expressed genes identification
To standardize the microarray data set, comparison of the gene expression profiles of metastatic tumor cells versus disseminated tumor cells was normalized using log2 transformation, a method previously developed by Fan et al.[5] Subsequently, Significance Analysis of Microarrays (SAM, http://statweb.stanford.edu/∼tibs/SAM/) was applied to produce a cluster of up- or downregulated variant genes according to previous publications.[6,7]
2.3. Functional protein association network construction
Protein–protein/Gene–protein interaction networks were expanded on the basis of the result from 2.2 using String consortium (http://string-db.org/).[8] Gene Ontology consortium (GO, http://www.geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/) functional enrichment were also applied via Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov/).[9,10]
2.4. Statistical analysis
Gene expression was considered to be significant if the threshold of false discovery rate (FDR) ≤5% and fold change ≥5. For GO and KEGG enrichment analysis, biological process, molecular function, and signaling pathways were identified as different if the P value was ≤5%.
2.5. Ethical Experimentation
The study does not involve any patient consent, so ethical approval is not necessary.
3. Results
3.1. Sixty-six genes were found to be significantly expressed in bone-specific metastatic breast tumor cells
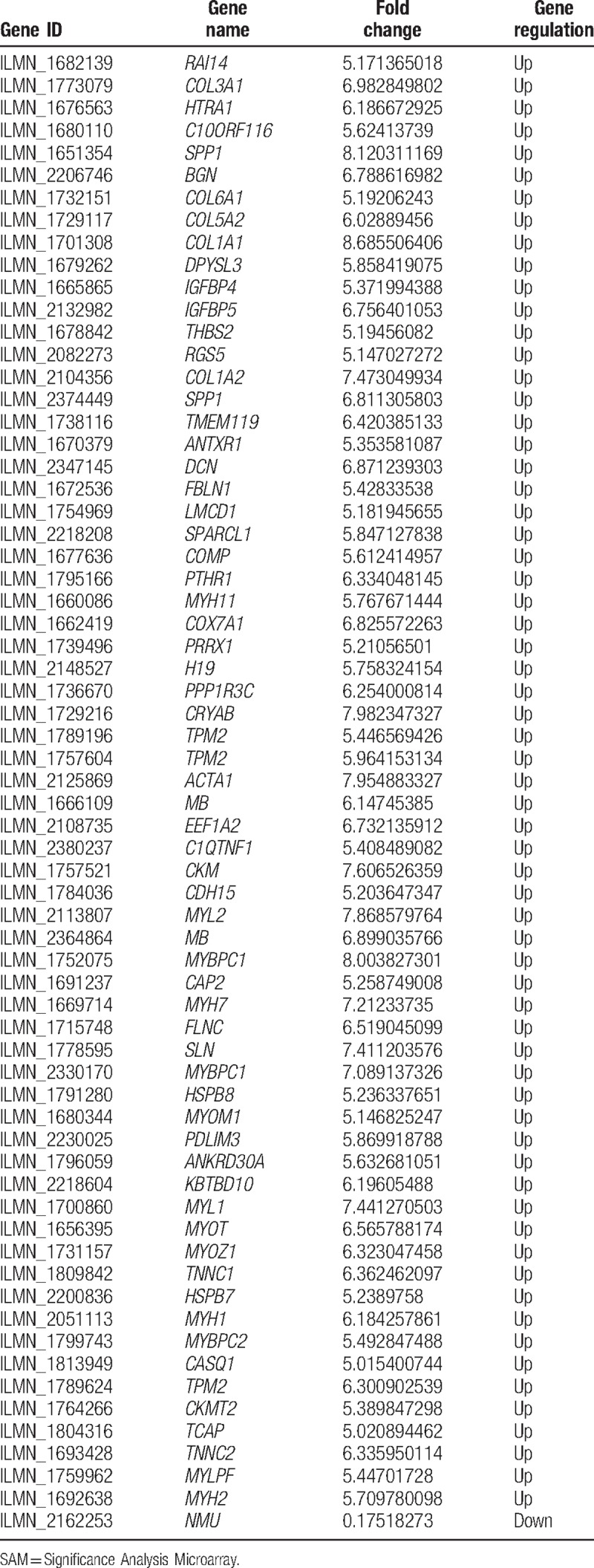
A total of 14 breast tumor samples were profiled in this study, consisting of 8 disseminated tumor cell samples and 6 metastatic tumor cell samples. After performing SAM, 66 genes were found to be differently expressed in metastatic tumor cells comparing to disseminated tumor cells as shown in Fig. 1 and Table 1. Totally, 65 genes increased and 1 gene decreased dramatically with the threshold of FDR ≤5% and fold change ≥5.
Figure 1.
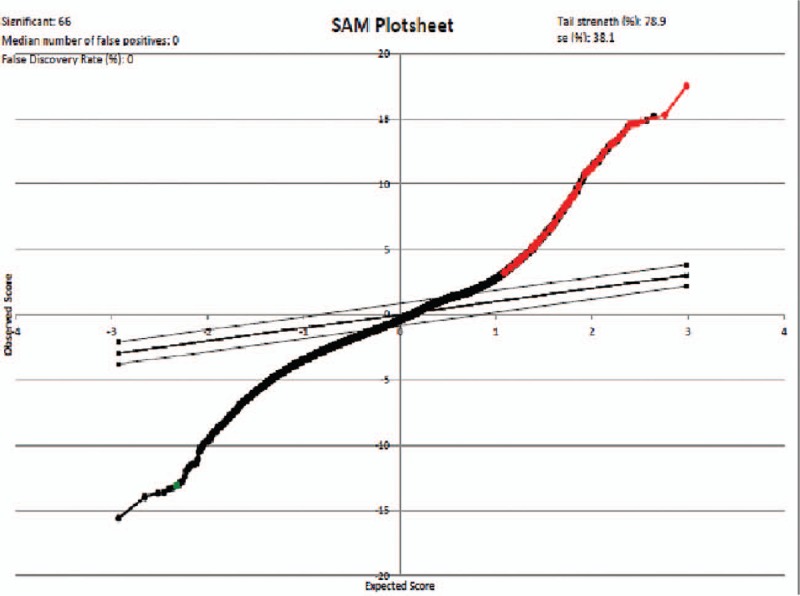
Significance analysis of microarrays (SAM) plot result generated by SAM plugin in Excel platform.
Table 1.
Significant genes identified by significant analysis of microarray (SAM) in metastatic tumor cells versus disseminated tumor cells.
3.2. Gene–gene interaction network construction associated with breast cancer bone metastasis
To better identify how these genes regulated breast cancer bone metastasis in a system biology perspective, all these significant genes were applied to String platform for further analysis. As shown in Fig. 2, the interaction network involved in bone metastasis consists of 110 nodes and 869 edges with the average node degree of 15.8. Network analysis also indicated that the clustering coefficient (cc) was 0.58, which means that the network has a reliable robustness Figure 3.
Figure 2.
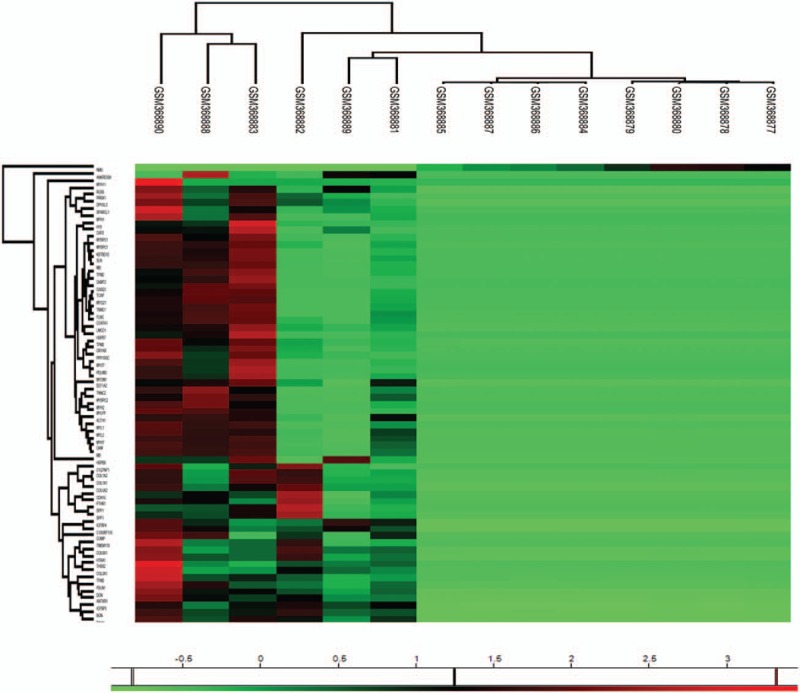
Heatmap visualization of the significant genes identified by SAM. All the detailed information can be seen in Table 1.
Figure 3.
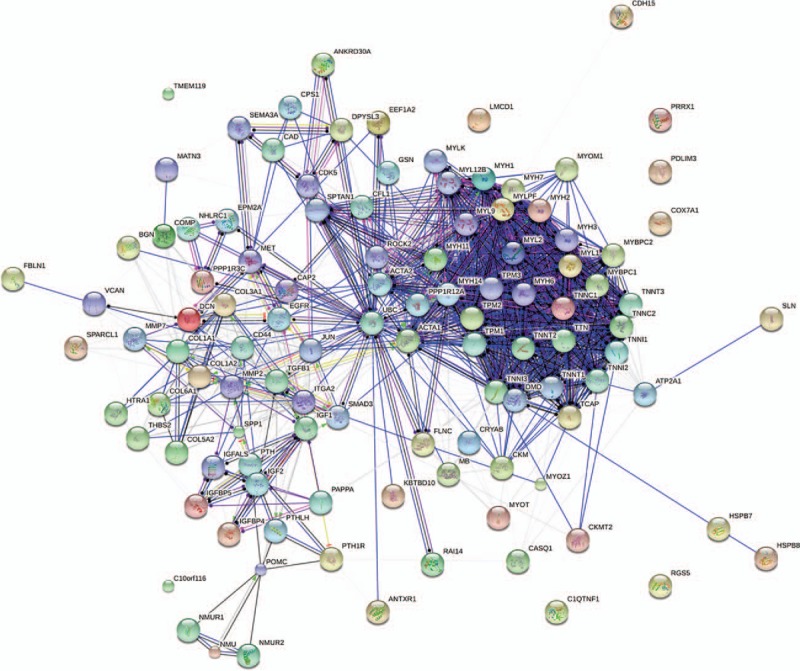
Gene to gene interaction network associated with breast cancer bone metastasis generating by String platform.
3.3. GO analysis in terms of molecular function and biological processes
To explore the genetic interaction network involved in bone metastasis in the context of GO, all the nodes were submitted to DAVID for functional annotation. As summarized in Table 2, molecular function analysis indicated that most of these genes regulated protein binding and activities. We also elevated the biological processes involved in this bone metastasis network (Table 3). Table 3 summarized all the potential biological processes for bone metastasis. In particular, all these genes seemed to be involved in skeletal muscle development and differentiation, and cell development Table 4.
Table 2.
Molecular function analysis of the genetic interaction network associated with metastatic tumor cells in terms of Gene Ontology (GO).
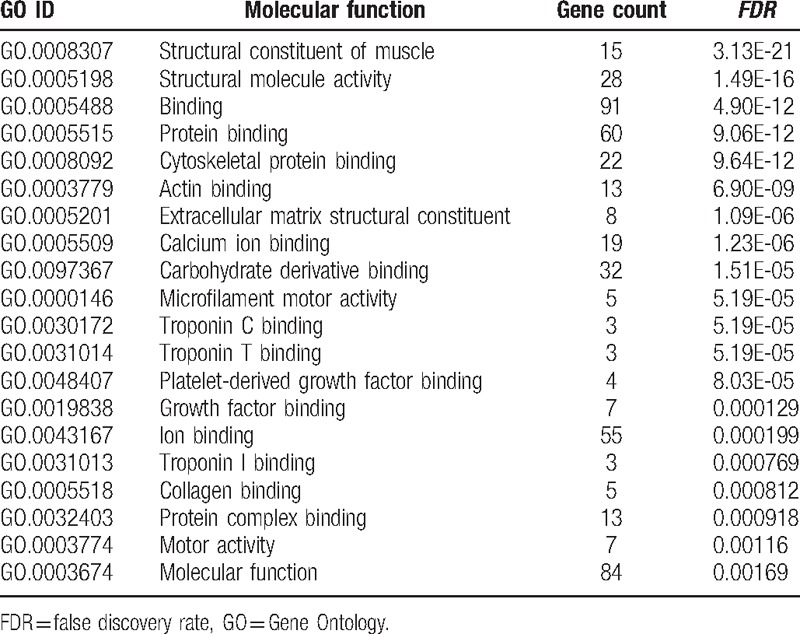
Table 3.
Biological process analysis of the genetic interaction network associated with metastatic tumor cells in terms of Gene Ontology (GO).
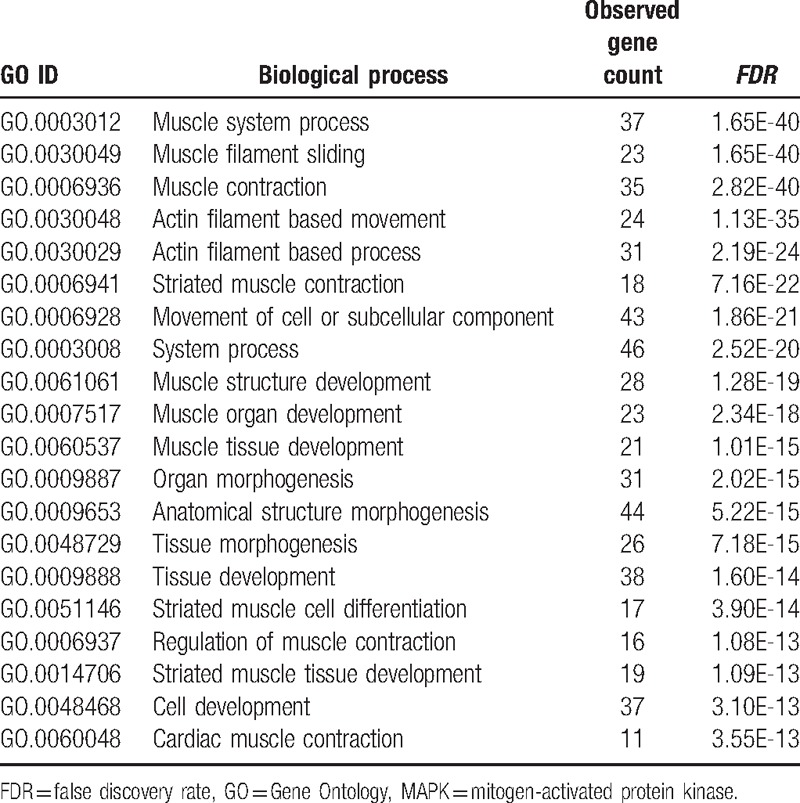
Table 4.
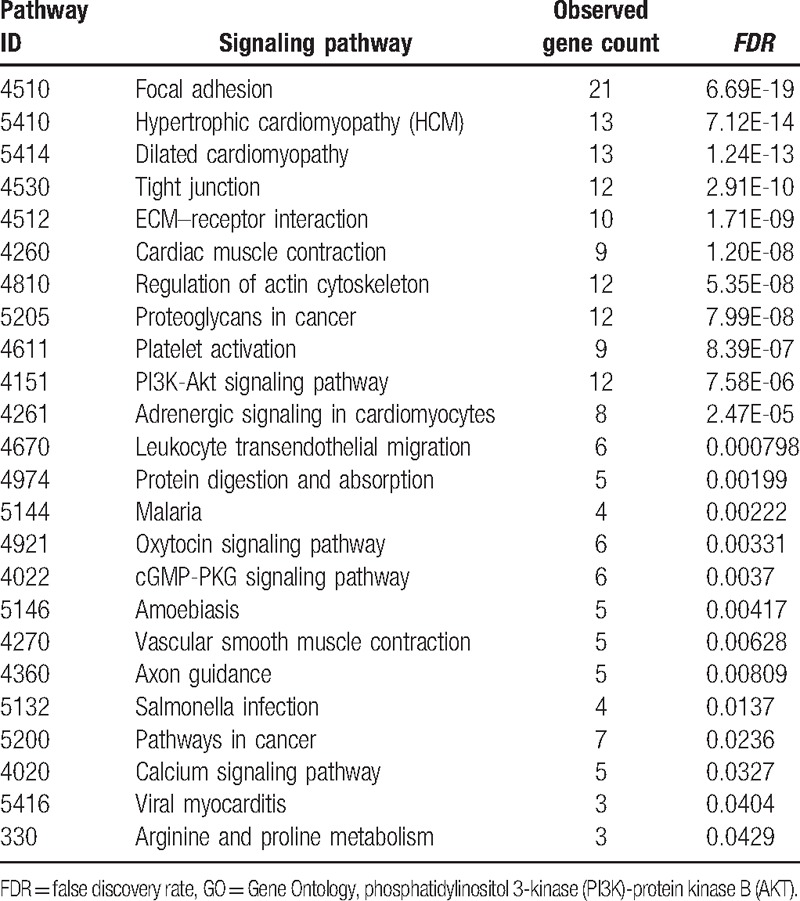
Signaling pathway analysis of the genetic interaction network associated with metastatic bone cells in terms of Gene Ontology (GO).
3.4. Signaling pathway enrichment analysis
To assess the relationship between the significantly expressed genes and bone metastasis, we also elevated the potential signaling pathways involved in this pathogenesis (Table 3). Notably, focal adhesion kinase (FAK), ECM–receptor interaction, calcium signaling pathway, and PI3K/AKT signaling pathways seem to confer bone metastasis in metastatic tumor cells.
4. Discussion
As we described previously, breast cancer bone metastasis is a complex process that includes tumor cells dissemination into circulation, homing to bone, and proliferation in bone tissue. Underlying these complicated, multistep scenarios, it has been known that a sophisticated network of molecular events is crucial in the development of metastasis to bone, which was not fully understood. In this literature, the authors identified a microarray gene expression profile and established a comprehensive genetic interaction network to reveal the molecular mechanisms in breast cancer bone metastasis. The results suggested that ECM–receptor interaction, FAK, calcium signaling pathway, and PI3K/AKT signaling pathway were highly associated with breast cancer bone metastasis.
Previous publications have already confirmed the role of ECM components in breast cancer dissemination and metastases.[11,12] As polysaccharides and fibrous proteins, ECM which induced by either cancer cells or stromal components is a crucial component of cancer microenvironment, initiating downstream signaling events that lead to the aggressive behavior of breast cancer.[13,14] The interaction of cancer cells and ECM components is profoundly altered at all steps of cancer metastasis, which include detachment from the primary tumor, migration through adjacent tissue, invasion into and extravasation from the vasculature.[15–17] Studies on interaction of tumor cells with ECM components showed increased extracellular protease activity mediated by the family of matrix metalloproteinases (MMPs).[18,19]
Several previously studies indicated that FAK mediated cancer metastasis in various cancers.[20–22] (FAK) is a nonreceptor protein tyrosine kinase that resides at the sites of at focal adhesions, which plays an essential role in cancer cells survival, proliferation, migration, and invasion.[23,24] FAK coordinates a signaling network that orchestrates these processes through both kinase-dependent and independent mechanisms.[25] FAK cooperates with SRC and leads to SRC phosphorylation and then FAK/SRC phosphorylation at multiple sites, relaying the external signal into cells associated with various genes and multiple signaling pathways, such as PI3K/AKT and MAPK.[26]
A previous study suggested the regulation of the metastasis formation either directly through mutations in the involved adhesion molecules or indirectly through impaired calcium signaling pathway.[27] The ubiquitous second messenger calcium is one of the crucial regulators that will be involved in several fundamental physiological functions, such as cell cycle control, survival, and cancer metastasis.[28,29] In multiple cancer metastasis stages, calcium signaling and cell adhesion interact in various ways with each other. E-cadherin, a calcium-dependent cell–cell adhesion molecule, is a major suppressor of metastasis, whose downregulation or inactivation in carcinomas has been reported to result in reduced cell adhesion, and essentially requires Ca2+-ions to form hemophilic interactions between 2 neighboring cells in adherens junctions.[30,31] Evidences suggest that Rap2B is an upstream target of the Ca2+-related ERK1/2 signaling pathway in cancer cells, contributing to important events during tumor progression, such as cell proliferation, migration, invasion, and metastasis,[32–35] which further attested our bioinformatic prediction.
The PI3K/AKT/mTOR pathway had been known to control many cellular functions such as proliferation, growth, survival, motility, and metabolism and proved to be related with cancer metastasis.[36,37] By stimulating the expressions of Receptor activator of nuclear factor kappa-B ligand (RANKL), parathyroid hormone-related protein (PTHrP), and bone morphogenetic protein 2 (BMP-2) partly through NF-kB, PI3K/AKT pathway had been proved to play an important role in prostate carcinoma bone metastasis.[38] Through the PI3K/AKT pathway, mPRα promoted the expression of MMP-9 during breast cancer cells invasion to local lymph nodes.[39] The angiogenesis and metastasis of breast cancer cells were inhibited by downregulating PI3K/AKT/ERK signaling pathway mediated by connective tissue growth factor.[40] Several drugs against PI3K, Mammalian target of rapamycin (mTOR), and AKT had already been invented and tested in clinical trials.
Besides the signaling pathways mentioned above, we also discovered many pathways, including tight junction, regulation of actin cytoskeleton, leukocyte transendothelial migration, etc, were involved in breast cancer bone metastasis. However, detailed information regarding the association between these pathways and bone metastasis has not been fully investigated.
In conclusion, using the integrated microarray gene expression profile and genetic interaction network, we characterized some molecular signaling pathways (ECM-receptor interaction, FAK, calcium signaling pathway, and PI3K/AKT signaling pathway), which may mediate the aggressive behavior of breast cancer in terms of bone polarization.
Acknowledgment
We thank GEO, SAM, and String databases for making their data readily available to the scientific community.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, and agree to be accountable for all aspects of the work.
Conceptualization: Zhi-hong Zheng.
Data curation: Xin-hua Chen.
Formal analysis: Zhe Pei, Hao Peng.
Methodology: Xin-hua Chen, Zhe Pei.
Project administration: Xin-hua Chen.
Resources: Xin-hua Chen, Zhi-hong Zheng, Zhe Pei.
Software: Xin-hua Chen, Hao Peng.
Supervision: Zhi-hong Zheng.
Writing – review & editing: Zhe Pei, Hao Peng.
Footnotes
Abbreviations: BC = breast cancer, cc = clustering coefficient, GEO = gene expression omnibus, GO =gene ontology, KEGG = Kyoto encyclopedia of genes and genomes, SAM = significance analysis of microarrays.
Funding/support: The fund was “Sponsored by National and Fujian Provincial Key Clinical Specialty Discipline Construction Program, P.R.C.”
The authors declare that they have no competing interests.
References
- [1].Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet 2011;378:1461–84. [DOI] [PubMed] [Google Scholar]
- [2].Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol 1991;9:509–24. [DOI] [PubMed] [Google Scholar]
- [3].Cheung KJ, Ewald AJ. A collective route to metastasis: seeding by tumor cell clusters. Science 2016;352:167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cawthorn TR, Amir E, Broom R, et al. Mechanisms and pathways of bone metastasis: challenges and pitfalls of performing molecular research on patient samples. Clin Exp Metastasis 2009;26:935–43. [DOI] [PubMed] [Google Scholar]
- [5].Fan S, Pan Z, Geng Q, et al. Layered signaling regulatory networks analysis of gene expression involved in malignant tumorigenesis of non-resolving ulcerative colitis via integration of cross-study microarray profiles. PLoS One 2013;8:e67142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fan S, Li X, Tie L, et al. KIAA0101 is associated with human renal cell carcinoma proliferation and migration induced by erythropoietin. Oncotarget 2016;7:13520–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fan S, Geng Q, Pan Z, et al. Clarifying off-target effects for torcetrapib using network pharmacology and reverse docking approach. BMC Syst Biol 2012;6:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zheng Z, Liu T, Zheng J, et al. Clarifying the molecular mechanism associated with carfilzomib resistance in human multiple myeloma using microarray gene expression profile and genetic interaction network. Onco Targets Ther 2017;10:1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li J, Fan S, Han D, et al. Microarray gene expression profiling and bioinformatics analysis of premature ovarian failure in a rat model. Exp Mol Pathol 2014;97:535–41. [DOI] [PubMed] [Google Scholar]
- [10].Fan S, Xu Y, Li X, et al. Opposite angiogenic outcome of curcumin against ischemia and Lewis lung cancer models: in silico, in vitro and in vivo studies. Biochim Biophys Acta 2014;1842:1742–54. [DOI] [PubMed] [Google Scholar]
- [11].Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012;196:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast 2013;22(suppl 2):S66–72. [DOI] [PubMed] [Google Scholar]
- [13].Harisi R, Jeney A. Extracellular matrix as target for antitumor therapy. Onco Targets Ther 2015;8:1387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Saha S, Lo PK, Duan X, et al. Breast tumour initiating cell fate is regulated by microenvironmental cues from an extracellular matrix. Integr Biol (Camb) 2012;4:897–904. [DOI] [PubMed] [Google Scholar]
- [15].Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature 2001;411:375–9. [DOI] [PubMed] [Google Scholar]
- [16].Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer 2001;1:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sasisekharan R, Shriver Z, Venkataraman G, et al. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat Rev Cancer 2002;2:521–8. [DOI] [PubMed] [Google Scholar]
- [18].Thorns V, Walter GF, Thorns C. Expression of MMP-2, MMP-7, MMP-9, MMP-10 and MMP-11 in human astrocytic and oligodendroglial gliomas. Anticancer Res 2003;23:3937–44. [PubMed] [Google Scholar]
- [19].Pal S, Moulik S, Dutta A, et al. Extracellular matrix protein laminin induces matrix metalloproteinase-9 in human breast cancer cell line mcf-7. Cancer Microenviron 2014;7:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Almeida EA, Ilic D, Han Q, et al. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol 2000;149:741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moon HS, Park WI, Choi EA, et al. The expression and tyrosine phosphorylation of E-cadherin/catenin adhesion complex, and focal adhesion kinase in invasive cervical carcinomas. Int J Gynecol Cancer 2003;13:640–6. [DOI] [PubMed] [Google Scholar]
- [22].Shibue T, Brooks MW, Inan MF, et al. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov 2012;2:706–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sood AK, Coffin JE, Schneider GB, et al. Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol 2004;165:1087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mon NN, Senga T, Ito S. Interleukin-1beta activates focal adhesion kinase and Src to induce matrix metalloproteinase-9 production and invasion of MCF-7 breast cancer cells. Oncol Lett 2017;13:955–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee TS, Lin JJ, Huo YN, et al. Progesterone inhibits endothelial cell migration through suppression of the Rho activity mediated by cSrc activation. J Cell Biochem 2015;116:1411–8. [DOI] [PubMed] [Google Scholar]
- [26].Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 2014;14:598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Woltmann A, Chen B, Lascorz J, et al. Systematic pathway enrichment analysis of a genome-wide association study on breast cancer survival reveals an influence of genes involved in cell adhesion and calcium signaling on the patients’ clinical outcome. PLoS One 2014;9:e98229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 2003;4:517–29. [DOI] [PubMed] [Google Scholar]
- [29].Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer 2011;11:609–18. [DOI] [PubMed] [Google Scholar]
- [30].Berx G, van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol 2009;1:a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 2004;4:118–32. [DOI] [PubMed] [Google Scholar]
- [32].Monteith GR, Davis FM, Roberts-Thomson SJ. Calcium channels and pumps in cancer: changes and consequences. J Biol Chem 2012;287:31666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Monteith GR, McAndrew D, Faddy HM, et al. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer 2007;7:519–30. [DOI] [PubMed] [Google Scholar]
- [34].Wang H, Wu Q, Liu Z, et al. Downregulation of FAP suppresses cell proliferation and metastasis through PTEN/PI3K/AKT and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death Dis 2014;5:e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Di J, Huang H, Qu D, et al. Rap2B promotes proliferation, migration, and invasion of human breast cancer through calcium-related ERK1/2 signaling pathway. Sci Rep 2015;5:12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Su W, Li S, Chen X, et al. GABARAPL1 suppresses metastasis by counteracting PI3K/Akt pathway in prostate cancer. Oncotarget 2017;8:4449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chao W, Deng JS, Li PY, et al. 3,4-dihydroxybenzalactone suppresses human non-small cell lung carcinoma cells metastasis via suppression of epithelial to mesenchymal transition, ROS-mediated PI3K/AKT/MAPK/MMP and NFkappaB signaling pathways. Molecules 2017;22:pii: E537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhu W, Hu X, Xu J, et al. Effect of PI3K/Akt signaling pathway on the process of prostate cancer metastasis to bone. Cell Biochem Biophys 2015;72:171–7. [DOI] [PubMed] [Google Scholar]
- [39].Wu X, Sun L, Wang X, et al. Breast cancer invasion and metastasis by mPRalpha through the PI3K/Akt signaling pathway. Pathol Oncol Res 2016;22:471–6. [DOI] [PubMed] [Google Scholar]
- [40].Chang CH, Ou TT, Yang MY, et al. Nelumbo nucifera Gaertn leaves extract inhibits the angiogenesis and metastasis of breast cancer cells by downregulation connective tissue growth factor (CTGF) mediated PI3K/AKT/ERK signaling. J Ethnopharmacol 2016;188:111–22. [DOI] [PubMed] [Google Scholar]


