Abstract
Olanzapine is an atypical antipsychotic that has shown efficacy for the treatment of nausea, anxiety, and insomnia. This study was conducted to evaluate the efficacy of olanzapine (5 mg) combined with 5-HT3 receptor antagonists and dexamethasone for the prevention of chemotherapy-induced nausea and vomiting (CINV) in lung patients receiving cisplatin-based (25 mg/m2 d1-3) highly emetogenic chemotherapy (HEC).
Olanzapine (5 mg) was administered a day prior to cisplatin administration and continued on days 1 to 5. We evaluated complete response (CR) rate and rates of no nausea and no vomiting in 3 periods. In addition, Self-Rating Anxiety Scale (SAS), Self-rating Depression Scale (SDS), and The Functional Living Index-Emesis (FLIE) questionnaire were also assessed.
A total of 40 lung cancer patients were included. CR for acute, delayed, and over all phases were 82.5%, 75.0%, and 70.0%, respectively. The rate of no nausea in the acute phase was 70.0% and 62.5% in delayed phase. The rate of no vomiting in the acute phase was 85.0%, and 77.5% in delayed phase. The rate of no nausea and no vomiting in the overall phase were 57.5% and 75.0%, respectively. The median SAS and SDS score were 37.9 and 41.6 in pre-chemotherapy, respectively. Up to day 6 after chemotherapy treatment, the median SAS and SDS score were 36.9 and 42.0, respectively. The median FLIE score was 111.7. The main side effects were grade 1 somnolence (35.0%) and mild constipation (52.5%).
Around 5 mg olanzapine may be used as a potential, safe, and cost-beneficial alternative to prevent nausea and vomiting for HEC, particular for multiday chemotherapy regimen.
Keywords: chemotherapy induced, nausea, olanzapine, prevention, vomiting
1. Introduction
Lung cancer is one of the most common types of malignant tumor.[1] In the past decades, advances in molecular analysis and the development of targeted therapies have changed the treatment and the survival for lung cancer. Even so, part of lung cancer patients is without driver genes, chemotherapy remains the most important approach. The side effects of chemotherapy are more severe than targeted drugs. Chemotherapy-induced nausea and vomiting (CINV) is frequent; and without proper antiemetic treatment, the incidence of CINV could be 70% to 80%.[2] Three international guidelines[3–5] have recommend combinations of 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists, dexamethasone, and neurokinin-1 (NK1) receptor antagonists to prevent CINV in patients receiving highly emetogenic chemotherapy. However, nausea remains a major problem for many patients.
Olanzapine is an atypical antipsychotic drug and antagonizes multiple neuronal receptors including dopamine (D1, D2, D4), serotonin (5HT2A, 5HT2C, 5HT3), histamine (H1) alpha1-adrenergic receptors; acetylcholine at muscarinic receptors that has been approved by the Food and Drug Administration (FDA) for the treatment of psychotic disorders.[6,7] Side effects include mild short-term sedation, weight gain, and an increased risk of hyperglycemia.[8–11] Olanzapine may have correlation with nausea and vomiting and it might have clinically significant in antiemetic effect. Nowadays, there have some researches to explore the activity of olanzapine on CINV, particularly in the delayed phase.[12–17] There are some reports to compare the efficacy of 10 mg olanzapine versus aprepitant, each combining with palonosetron (PAL) and dexamethasone (DEX), in the prevention of CINV in patients receiving HEC and results showed olanzapine combining with DEX and PAL was very effective at controlling CINV.
Due to a study that suggested use 5 mg olanzapine for the prevention of CINV because there can be a possible potential for decreasing side effects.[13] Another research, a double-blind randomized phase II study of olanzapine 10 mg versus 5 mg for emesis induced by highly emetogenic chemotherapy (HEC) completed.[18] They conducted the study to base on olanzapine in combination with standard antiemetic treatment (aprepitant, palonosetron, and dexamethasone). Therefore, we explore the efficacy and safety of the dose of 5 mg olanzapine with palonosetron and dexamethasone for the prevention of HEC in lung cancer patients. In addition, anxiety and dressed symptoms were assessed by the Zung Self-Rating Anxiety Scale (SAS)[19] and the Zung Self-rating Depression Scale (SDS).[20] SAS and SDS are commonly used assessment tools in China with good validity and reliability.[21,22] The current researches have no analysis with the relationship between CINV and depression and anxiety. This study aims to explore the associations of CINV with depressive and anxiety symptoms, whether olanzapine could mediate the effect of the psychological stress on depressive and anxiety.
2. Material and methods
The research was conducted between April 2016 and April 2017 at Zhejiang Cancer Hospital. The subject sample consisted of chemotherapy-naïve patients with lung cancer including nonsmall cell lung cancer and small cell lung cancer receiving chemotherapy with highly emetogenic chemotherapy (HEC) containing cisplatin at a dose of 25 mg/m2 d1-3. The enrollment eligibility criteria of patients were as follows: aged 20 to 75 years; an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 to 1; bone marrow activity met the criteria for starting administration of chemotherapy; liver and renal function met the criteria for starting administration of chemotherapy; and provided written and informed consent. The exclusion criteria of patients were as follows: had previously received chemotherapy; were receiving chemotherapy in combination with radiation therapy; had diabetes; had creatine phosphokinase ≤1.5 mg/dL; had emetic episodes requiring administration of antiemetics the day prior to chemotherapy; had psychiatric disorders; had a family history of neuroleptic malignant syndrome; had active infection; and had gastrointestinal obstruction or narrow-angle glaucoma; has symptomatic brain metastases or requiring anticonvulsant medication; had history of hypersensitivity or allergy to the study drugs or similar compounds. Also excluded were women who were pregnant, were hoping to become pregnant, and were breastfeeding.
2.1. Ethics approval and consent to participate
This study received ethical approval from Zhejiang Cancer Hospital. The ethical approval was obtained from an institutional ethics committee within the Zhejiang Cancer Hospital. Written informed consent was obtained from all individual participants included in the study.
2.2. Antiemetic treatment
All patients who enrolled in this trial were administered double therapy and olanzapine. 5-HT3 RA was administered in accordance with recommendations in National Comprehensive Cancer Network (NCCN).[23] Ondansetron was administered intravenously on day 1 to 3 at a dose of 8 mg twice daily, 30 to 60 minutes prior to cisplatin administration. Dexamethasone was intravenously administered 30 to 60 minutes prior to cisplatin administration at a dose of 5 mg on day 1 to 3 and then intravenously administered at a dose of 5 mg on days 4 to 5. Olanzapine was orally administered at a dose of 5 mg on days 0 to 5 (before chemotherapy) at bedtime. Metoclopramide was used as rescue therapy for breakthrough emesis. The decision to use rescue therapy was made by each individual patient.
2.3. Evaluation of parameters
The enrolled patients were hospitalized for the treatment from the day prior to and up to day 6 of chemotherapy. We recorded medical information on each patient at the time of hospitalization. Patients were given a self-recorded symptom diary to record their symptoms over the 5 day period. The 24-hour period after cisplatin administration was considered as day1, and each subsequent 24-hour period was counted as 1 day. Patients recorded their degree of nausea, the presence/absence of vomiting or retching, the presence/absence of rescue therapy, and adverse events in the symptom diary every 24 hours for the 120 hours period after cisplatin administration. The degree of nausea was evaluated by the individual patients using an 11-point (0–10) numeric rating scale (NRS). The acute phase was defined as 0 to 24 hours after cisplatin administration, the delayed phase was 25 to 120 hours after cisplatin administration, and the overall phase was 0 to 120 hours after cisplatin administration.
The primary endpoint was complete response (no vomiting and no rescue therapy) rate. The secondary endpoint was rate of patients with no nausea, and adverse events in the 3 phases. Safety including adverse events and laboratory tests were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE Version 4.0). These endpoints were evaluated during the first cycle of chemotherapy.
All patients were asked to complete the Functional Living Index-Emesis (FLIE), a self-administered questionnaire used to evaluate the impact of CINV on patients’ daily lives. The FLIE-item score was assessed at the morning of day 6 postchemotherapy. No or minimal impact on daily life (NIDL) was defined as an average FLIE item score of more than 108.
Anxiety symptoms were assessed with the Zung Self-Rating Anxiety Scale (SAS). The SAS scale consists of 20 items, and each item is answered on a 4-point Likert-type scale ranging from “never” to “always.” Higher score means more serious anxiety symptoms. Each scale contains 20 items rated on a 4-level Likert scale (“not at all or just a little of the time, ” “some of the time, ” “a good part of the time, ” and “most of the time ”). SAS has 15 positive items and 5 negative (i.e., reverse-scored) items; SDS has 10 positive items and 10 negative items. The standardized score is the total of the raw item scores (score 1–4 for each item) of the 20 items times 1.25, which results in a theoretical range of standardized scores of 25 to 100. The clinical threshold of SAS is 50; scores in the 50 to 59, 60 to 69, and 70 to 100 ranges correspond to mild, moderate and severe anxiety, respectively. For the SDS, the clinical threshold is 53; scores in the 53 to 62, 63 to 72, and 73 to 100 ranges correspond to mild, moderate, and severe depression, respectively.
2.4. Statistical analysis
Statistical analysis was performed using SPSS version 19.0 software. Descriptive statistics were used to summarize patient demographics in FLIE data analysis. One-sample (paired) t-test was used to compare the SAS and SDS scores before and after treatment for each group. And a P value of < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
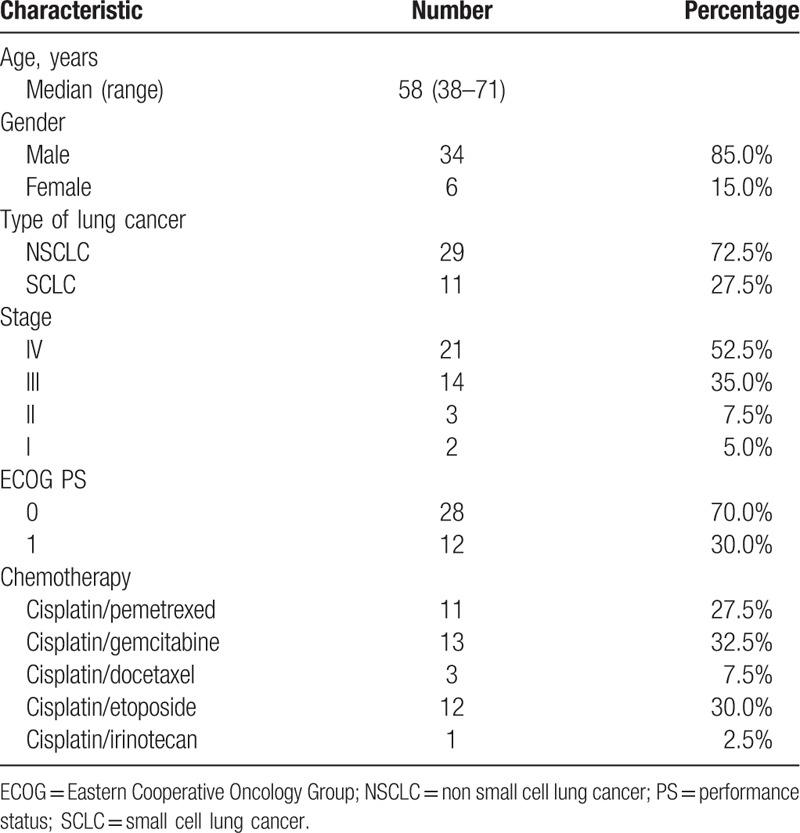
A total of 40 patients from our hospital were collected between April 2016 and April 2017. Patient characteristics are shown in Table 1. Median age was 58 years. The percentages of males and females were 85.0% and 15.0%, respectively. Around 29 patients were nonsmall cell lung cancer and 11 were small cell lung cancer.
Table 1.
Patient characteristics.
3.2. Effects
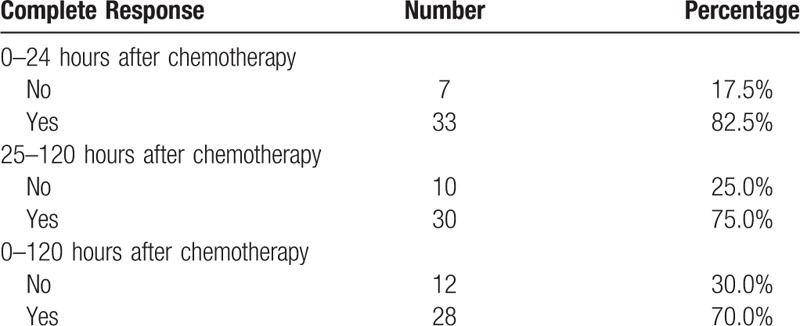
The effects of 3 drug combination are shown in Table 2. The rate of no nausea in the acute phase was 70.0% (28/40), and 62.5% (25/40) in delayed phase. Grade I of nausea in the acute phase was 75.0% (9/12) and not affect appetite. Grade I of nausea in the delayed phase was 86.7% (13/15). The rate of no vomiting in the acute phase was 85.0%, and 77.5% in delayed phase. And the rate of no nausea and no vomiting in the overall phase were 57.5% and 75.0%, respectively. The complete response rate was showed in Table 3. For the acute, delayed, and overall phases, the complete control rates were 82.5, 75.0, and 70.0%, respectively. Rescue therapy was administered for one patient on day 1 and one patient on day 5.
Table 2.
Outcomes of no nausea and no vomiting.
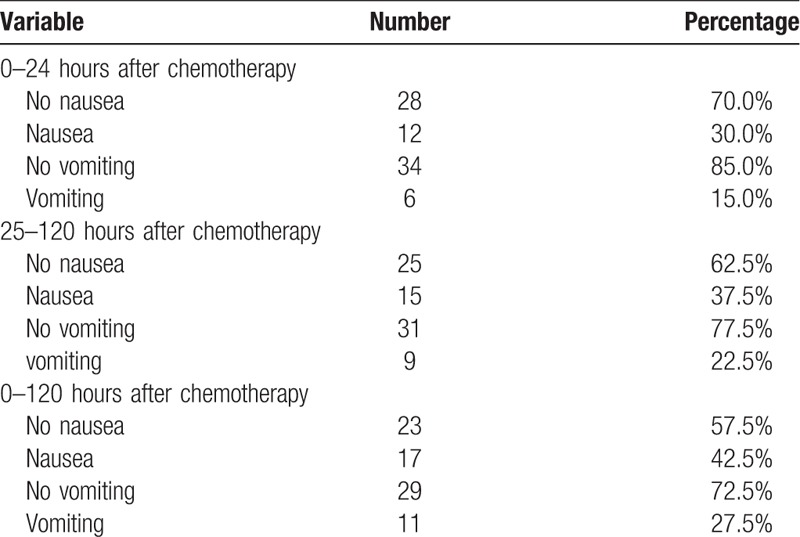
Table 3.
Outcomes of complete response.
3.3. The assessment of anxiety and depression
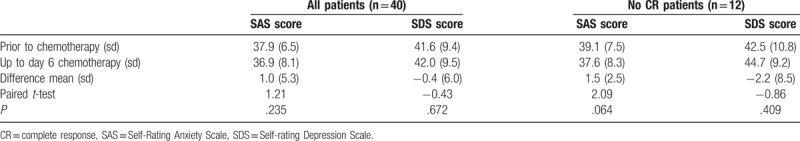
As shown in Table 4, for all patients, the median SAS score and SDS were 37.9 and 41.6 in pre-chemotherapy, respectively. Also 2 patients were defined as mid anxiety (SAS score ≥50). Two patients were mild depression and one was moderate depression. Up to day 6 after chemotherapy treatment, all patients also evaluated their moods. In addition, the median SAS score and SDS were 36.9 and 42.0, respectively. There were no significant reductions or increase in the anxiety and depressive symptoms in prior to chemotherapy treatment and up to day 6 groups. In addition, we analyzed subgroup of no CR patients (n = 12). The median SAS score and SDS were 39.1 and 42.5 in prechemotherapy group. Also one patient was mid anxiety and one patient was moderate depression. Up to day 6 after chemotherapy treatment, the median SAS score and SDS were 37.6 and 44.7, respectively. Then, one patient was mild anxiety. One patient was mild depression and the SDS score of moderate depression from 68 to 51.
Table 4.
Comparison of SAS and SDS scores before and after chemotherapy.
3.4. The assessment of FLIE
Results from all items of the FLIE obtained on day 6 are summarized in Table 5. On day 6 postchemotherapy, the median total FLIE score was 111.7. And, 67.5% of all patients reported that CINV had no or minimal impact on their daily life (NIDL) (total FLIE score>108). Among all patients, the median FLIE nausea domain score was 55.1, whereas the median FLIE vomiting domain score was 56.6. In addition, we analyzed the FLIE score of CR patients and no CR patients. In the group of CR patients, the median total FLIE score was 115.2. Also, 84.0% patients reported that CINV had NIDL. However, in the group of no CR patients, the median total FLIE score was 102.6. Then, 40.0% patients reported that CINV had NIDL. The median FLIE nausea domain score (56.8 vs 50.6) and vomiting domain score (58.3 vs 52.0) in no CR group were both higher than in the group of CR patients.
Table 5.
FLIE questionnaire on day 6 postchemotherapy.

3.5. Safety
During antiemetic treatment, the main adverse effects were mild somnolence and constipation. Grade 1 somnolence was observed in 14 patients (35.0%) and no severe to discontinue olanzapine. Grade 1 and 2 constipation was observed in 21 patients (52.5%) and they can be controlled with antidiarrhoeal medicines. And grade 1 dry mouth was in 3 patients (7.5%). The adverse effect of somnolence was thought to result from olanzapine. No patients needed to discontinue olanzapine. One patient had postural hypotension. Blood tests were conducted approximately 7 days after chemotherapy. We evaluated levels of blood glucose. Around 5 patients (12.5%) had elevated fasting plasma glucose and > 7.0 mmol/L. There were no other abnormalities observed in the biochemical profiles. No grade 3 or 4 adverse events were observed.
4. Discussion
In this study, a satisfactory antiemetic effect was demonstrated that 5 mg olanzapine was administered in combination with ondansetron and dexamethasone. Our study indicated that 5 mg olanzapine with ondansetron and dexamethasone antiemetic regimen could reduce chemotherapy-induced nausea and vomiting. In addition, olanzapine as an antipsychotic drug has some effects of decreasing anxiety and depression.
NCCN recommend patients who receive highly emetogenic chemotherapy (HEC) regimens should be offered a 3-drug combination of an NK1 RA, a 5-HT3 RA, and dexamethasone.[23] Some studies have indicated that triplet therapy prevent emesis is about 65% to 80%, with no nausea was approximately 50% to 60%.[28,29] A systematic review reported on olanzapine is effective and safe for the prevention of CINV.[12] Other some observational researches have demonstrated that olanzapine combined with other antiemetic was well effective and tolerated to treat CINV when patients receiving highly emetogenic chemotherapy regimens.[13,14,24–26] According to a study, olanzapine could effectively control CINV including acute phase, delayed and refractory CINV, especially for the delayed phase.[27] Nowadays, many studies have been conducted to confirm the effect of 10 mg olanzapine combining with the standard antiemetic regimen. Of course, it also has adverse effects including somnolence, constipation or blood glucose elevate. In a study, it conducted a comparison olanzapine at doses between 2.5 and 10 mg had a decrease in nausea and a significant improvement in quality of life in advanced cancer patients.[30] However, studies of the dose of 5 mg olanzapine are few. The optimal dose of olanzapine required to control CINV is controversial.
In our study, 5 mg olanzapine combined with ondansetron and dexamethasone was effective at controlling acute and delayed CINV. KCOG-G1301[14] conducted to investigate the efficacy and safety of triplet therapy combined with 5 mg olanzapine in gynecological cancer receiving HEC. CR rate for acute, delayed, and overall phases were 97.5%, 95.0%, and 92.5%, respectively. Total control rates were 87.5%, 67.5%, and 67.5%. For adverse effect of sedation, grade 1 somnolence was observed in 82.5%. Yanai et al[18] did a double-blind randomized phase II dose-finding study of olanzapine 10 or 5 mg combining with 3-drug antiemetic regimen for the prophylaxis of emesis induced by HEC (cisplatin-based). The results showed the CR rate in the delayed phase was 77.6% in the 10 mg group (n = 76) and 85.7% in the 5 mg group (n = 77). Both doses of 10 mg and 5 mg olanzapine provided a significant improvement in delayed emesis. Even so, it also had some studies to explore olanzapine used as an alternative to NK-1 receptor antagonist. The cost of using olanzapine is far less compared to aprepitant with potential benefit. Navari et al[31] conducted a randomized phase III trial 10 mg olanzapine versus aprepitant for the prevention of CINV. The CR was 97% for the acute period, 77% for the delayed period, and 77% for the overall period for 121 patients receiving the olanzapine. CR was 87%, 73%, and 73% for the 3 periods in 120 patients receiving the aprepitant regimen. Patients without nausea were 87% versus 87% in acute phase, 69% versus 38% in delayed phase, and 69% versus 38% overall. In our study, the rate of without nausea in the acute phase was 70.0%, and 62.5% in delayed phase. The rates of without vomiting were 85.0% versus 77.5% for the 2 periods. The CR rate of acute phase was higher than that of delayed phase in our article. We think it was due to the treatment model of cisplatin (25 mg/m2, d1-3), the dose in first day was lower than the method of one day treatment (75 mg/m2, d1). Furthermore, dizziness is the most common and cautious toxicity for olanzapine. Smaller dosage has a lower incidence of sedation than 10 mg standard dose. Therefore, 5 mg olanzapine could be effective and safe in prophylactic setting as a viable alternative to aprepitant in cisplatin-based CINV in lung cancer, particular for multiday chemotherapy regimen.
A study[32] indicated that CINV continues to adversely affect patients’ quality of life (QoL) despite antiemetic therapy and even in patients who do not experience nausea and vomiting in acute phase. On day 6 postchemotherapy, the mean total FLIE score was 105.4; 61.0% patients reported that CINV had no or minimal impact on their daily life (NIDL) (total FLIE score>108). In our research, the median total FLIE score was 111.7% and 67.5% patients had NIDL. We further analyzed the FLIE score of no CR patients. The median total FLIE score was 102.6. Also 40.0% patients had NIDL. We found nausea had a stronger negative impact on QoL than vomiting. Hence, we considered olanzapine-based antiemetic use might improve patients’ QOL to some extent.
On the other hand, CINV patients often have anxiety or depression symptoms. Olanzapine can be considered as an effective drug to decrease anxiety and depression. There were few reports to estimate the role of olanzapine to decrease anxiety and depression in CINV patients. We are the first to assess patients of anxiety and depression. Nikbakhsh et al[33] used Hospital Anxiety and Depression Scale (HADS) to evaluated symptom relief and QOL in gastric cancer patients who received olanzapine tablets (2.5–10 mg/day) a day before chemotherapy. They found olanzapine could be considered as an effective drug to increase appetite and decrease anxiety and depression. Nevertheless, there were no significant reductions or increase in the anxiety and depressive symptoms in prior to chemotherapy treatment and up to day 6 groups in our study. In addition, we analyzed subgroup of no CR patients (n = 11). The median SAS score and SDS were 39.1 and 42.5, respectively, in prechemotherapy group. Up to day 6 after chemotherapy treatment, the median SAS score and SDS were 37.6 and 44.7, respectively. Although our results were no significant reductions in the anxiety and depressive symptoms, we think olanzapine can be considered as a proper drug with potentially the effect of antianxiety with respect to other antiemetics.
However, the present study has some limitations. First, the major limitation of the study is its small sample size and it could result in statistical bias of assessment of SAS and SDS score. Second, we evaluated only one dose level of olanzapine and did not conduct a control group. These issues should be considered in future clinical trials.
Our study indicated that olanzapine (5 mg) combined with a 5-HT3 RA and dexamethasone may be effective and safe for the prevention of CINV in lung patients who receiving HEC, particularly for multiday chemotherapy regimen. The cost of olanzapine is less compared to aprepitant with potential benefits. However, further randomized and controlled studies with larger sample size are required to confirm the efficacy of olanzapine and its antianxiety and depression.
Author contributions
Conceptualization: Yiping Zhang.
Data curation: Wenxian Wang, Guangyuan Lou, Yiping Zhang.
Resources: Wenxian Wang, Guangyuan Lou, Yiping Zhang.
Software: Wenxian Wang.
Writing – original draft: Wenxian Wang.
Writing – review & editing: Guangyuan Lou, Yiping Zhang.
Footnotes
Abbreviations: 5-HT3 = 5-hydroxytryptamine type 3, CINV = chemotherapy-induced nausea and vomiting, DEX = dexamethasone, ECOG = Eastern Cooperative Oncology Group, FDA = Food and Drug Administration, FLIE = The Functional Living Index-Emesis, HEC = highly emetogenic chemotherapy, NK1 = neurokinin-1, PAL = palonosetron, SAS = Self-Rating Anxiety Scale, SDS = Self-rating Depression Scale.
The study was funded by Medical Scientific Research Foundation of Zhejiang Province (Nos. 2017KY250 and 2018KY023).
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Wiser W, Berger A. Practical management of chemotherapy-induced nausea and vomiting. Oncology 2005;5:637–45. [PubMed] [Google Scholar]
- [3].Hesketh PJ, Bohlke K, Lyman GH, et al. American Society of Clinical Oncology. Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol 2016;34:381–6. [DOI] [PubMed] [Google Scholar]
- [4].Clinical practice guidelines in oncology, version 1: anti-emesis. Fort Washington, PA: National Comprehensive Cancer Network; 2015. Available at: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed Jan 10, 2018. [Google Scholar]
- [5].MASCC/ESMO antiemetic guidelines. Hillerod, Denmark: Multinational Association of Supportive Care in Cancer 2013. Available at: http://www.mascc.org/antiemetic-guide-lines. Accessed Jan 15, 2018. [Google Scholar]
- [6].Navari RM. Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2014;722:180–6. [DOI] [PubMed] [Google Scholar]
- [7].Hocking CM, Kichenadasse G. Olanzapine for chemotherapy-induced nausea and vomiting: a systematic review. Support Care Cancer 2014;22:1143–51. [DOI] [PubMed] [Google Scholar]
- [8].Hale AS. Olanzapine Br J Hosp Med 1997;58:442–5. [PubMed] [Google Scholar]
- [9].Bymaster FP, Falcone JF, Bauzon D, et al. Potent antagonism of 5-HT3 and 5-HT6 receptors by olanzapine. Eur J Pharmacol 2001;430:341–9. [DOI] [PubMed] [Google Scholar]
- [10].Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. J Clin Psychiatry 2001;62:22–31. [PubMed] [Google Scholar]
- [11].Goldstein LE, Sporn J, Brown S, et al. New-onset diabetes mellitus and diabetic ketoacidosis associated with olanzapine treatment. Psychosomatics 1999;40:438–43. [DOI] [PubMed] [Google Scholar]
- [12].Chow R, Chiu L, Navari R, et al. Efficacy and safety of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV) as reported in phase I and II studies: a systematic review. Support Care Cancer 2016;24:1001–8. [DOI] [PubMed] [Google Scholar]
- [13].Chiu L, Chiu N, Chow R, et al. Olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a retrospective study. Ann Palliat Med 2016;5:172–8. [DOI] [PubMed] [Google Scholar]
- [14].Abe M, Hirashima Y, Kasamatsu Y, et al. Efficacy and safety of olanzapine combined with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy in gynecological cancer: KCOG-G1301 phase II trial. Support Care Cancer 2016;24:675–82. [DOI] [PubMed] [Google Scholar]
- [15].Mukhopadhyay S, Kwatra G, Alice KP, et al. Role of olanzapine in chemotherapy-induced nausea and vomiting on platinum-based chemotherapy patients: a randomized controlled study. Support Care Cancer 2017;25:145–54. [DOI] [PubMed] [Google Scholar]
- [16].Babu G, Saldanha SC, Kuntegowdanahalli Chinnagiriyappa L, et al. The efficacy, safety, and cost benefit of olanzapine versus aprepitant in highly emetogenic chemotherapy: a pilot study from South India. Chemother Res Pract 2016;2016:3439707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med 2016;374:1356–67. [DOI] [PubMed] [Google Scholar]
- [18].Yanai T, Iwasa S, Hashimoto H, et al. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol 2017;16: [DOI] [PubMed] [Google Scholar]
- [19].Zung WW. A rating instrument for anxiety disorders. Psychosomatics 1971;12:371–9. [DOI] [PubMed] [Google Scholar]
- [20].Zhang MY. Manual of Psychiatric Rating Scales. Changsha: Hunan Science Press; 2003. [Google Scholar]
- [21].Zhang Y, Muyiduli X, Wang S, et al. Prevalence and relevant factors of anxiety and depression among pregnant women in a cohort study from south-east China. J Reprod Infant Psychol 2018;9:1–1. [DOI] [PubMed] [Google Scholar]
- [22].Wang ZY, Xun YF. Self-rating Depression Scale (SDS). Shanghai Jing Shen Yi Xue 1984. 71–2. [Google Scholar]
- [23].National Comprehensive Cancer Network (2017) NCCN clinical practice guidelines in oncology: antiemesis, Version 2. Available at: http://https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed Jan 15, 2018. [Google Scholar]
- [24].Passik SD, Kirsh KL, Theobald DE, et al. A retrospective chart review of the use of olanzapine for the prevention of delayed emesis in cancer patients. J Pain Symptom Manage 2003;25:485–8. [DOI] [PubMed] [Google Scholar]
- [25].Vig S, Seibert L, Green MR. Olanzapine is effective for refractory chemotherapy-induced nausea and vomiting irrespective of chemotherapy emetogenicity. J Cancer Res Clin Oncol 2014;140:77–82. [DOI] [PubMed] [Google Scholar]
- [26].Flank J, Thackray J, Nielson D, et al. Olanzapine for treatment and prevention of acute chemotherapy-induced vomiting in children: a retrospective, multi-center review. Pediatr Blood Cancer 2015;62:496–501. [DOI] [PubMed] [Google Scholar]
- [27].Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase Trial. J Support Oncol 2011;9:188–95. [DOI] [PubMed] [Google Scholar]
- [28].Takahashi T, Hoshi E, Takagi M, et al. Multicenter, phase II, placebo-controlled, double-blind, randomized study of aprepitant in Japanese patients receiving high-dose cisplatin. Cancer Sci 2010;101:2455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pirl WF, Roth AJ. Remission of chemotherapy-induced emesis with concurrent olanzapine treatment: a case report. Psychooncology 2000;9:84–7. [DOI] [PubMed] [Google Scholar]
- [30].Passik SD, Lundberg J, Kirsh KL, et al. A pilot exploration of the antiemetic activity of olanzapine for the relief of nausea in patients with advanced cancer and pain. J Pain Symptom Manage 2002;23:526–32. [DOI] [PubMed] [Google Scholar]
- [31].Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 2011;9:188–95. [DOI] [PubMed] [Google Scholar]
- [32].Bloechl-Daum B, Deuson RR, Mavros P, et al. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 2006;20:4472–8. [DOI] [PubMed] [Google Scholar]
- [33].Nikbakhsh N, Sadeghi MV, Ramzani E, et al. Efficacy of olanzapine in symptom relief and quality of life in gastric cancer patients receiving chemotherapy. J Res Med Sci 2016;18:88. [DOI] [PMC free article] [PubMed] [Google Scholar]


