Abstract
The current study investigated the mediating effects of body mass index (BMI), physical activity, and emotional distress on the association between short sleep duration (<7 hours per 24-hour period) and cardiovascular disease (CVD) and risk factors.
We used data from the National Health Interview Survey, an ongoing nationally representative cross-sectional study of noninstitutionalized US adults (≥18 years) from 2004 to 2013 (N = 206,049). Participants provided information about anthropometric features (height and weight), sociodemographic factors, health behaviors (smoking and physical activity), emotional distress, and physician-diagnosed health conditions, including hypertension, coronary heart disease, diabetes, heart attack, stroke, kidney disease, and cancer. Structural equation modeling was used to assess the mediating effects of physical activity, BMI, and emotional distress on the relationship between short sleep and CVDs and risk factors (coronary heart disease, hypertension, diabetes, chronic kidney disease, heart attack, and stroke).
Of the sample, 54.7% were female, 60.1% identified as white, 17.7% as Hispanic, and 15.4% as black. The mean age of the respondents was 46.75 years (SE = 0.12), with a mean BMI of 27.11 kg/m2 (SE = 0.02) and approximately 32.5% reported short sleep duration. The main relationship between short sleep and CVD and risk factors was significant (β = 0.08, P < .001), as was the mediated effect via BMI (indirect effect = 0.047, P < .001), emotional distress (indirect effect = 0.022, P < .001), and physical activity (indirect effect = -0.022, P = .035), as well as after adjustment for covariates, including age, race, sex, marital status, and income: short sleep and CVD (B = 0.15; SE = 0.01; P < .001), BMI (B = 0.05; SE = 0.00; P < .001), emotional distress (B = 0.02; SE = 0.00; P < .001), and physical activity (B = 0.01; SE = 0.00; P < .001).
Our findings indicate that short sleep is a risk factor for CVD and that the relationship between short sleep and CVD and risk factors may be mediated by emotional distress and obesity, and negatively mediated by physical activity.
Keywords: cardiovascular disease, emotional distress, obesity, physical activity, sleep
1. Introduction
There is clear and compelling evidence that pharmacotherapy and healthy behavioral and lifestyle practices are effective primary and adjunct strategies for reducing or preventing cardiovascular diseases (CVDs) risk, as well as managing the exacerbating progression of chronic CVD and its comorbidities, such as diabetes and chronic kidney disease (CKD).[1–3] However, limited access and poor adherence to medications are significant challenges in reducing CVD risk at the population level.[1,2] Approximately 90% of CVD risk factors and diseases, such as CKD, diabetes, obesity, hypertension (HTN), and heart disease, are preventable through healthy lifestyles and behaviors such as lower BMI, regular physical activity, low emotional stress, healthy sleep, and healthy diet.[4–6]
Although BMI, emotional distress/stress, and physical inactivity are well-established CVD risk factors, there is growing evidence that short sleep duration (<7 hours/24-hour period) is a novel CVD risk factor, as it is associated with HTN, obesity, diabetes,[7,8] myocardial infarctions,[9] and CVD-related mortality.[10] The association between short sleep and CVD might be explained by several direct and indirect biological and physiological mechanisms such as endothelial damage, carotid artery intima thickness, arterial calcification (markers of atherosclerosis), remodeling of the heart, cardiac load/cardiovascular stress, and hormonal dysregulation.[11–16] In general, chronic short sleep duration stresses the body's organ systems by overstimulating the sympathetic, nervous, and endocrine systems, which in turn has deleterious effects on cardiovascular health.[14] Further confirmatory evidence linking short sleep and CVDs and risk factors can be seen in recent research indicating that chronic short sleep duration may cause significant structural changes to the heart, as it is associated with plasma B-type natriuretic peptides, which are counter regulatory hormones linked to ventricular stretching and cardiovascular remodeling caused by increased blood volume.[16]
Conversely, chronic short sleep duration may also be indirectly linked to CVD through emotional distress, physical inactivity, and obesity.[17–25] The indirect effects of short sleep on CVD via emotional distress, obesity, and physical activity are buttressed by evidence indicating that weight loss[26,27]), increased physical activity,[28,29] and low levels of emotional distress[30–32] improve sleep and reduce CVD risk.
Despite the available evidence that habitual short sleep duration is directly and indirectly associated with CVD, to our knowledge, there are no studies that have examined the single and combined mediating roles of emotional distress, BMI, and physical activity on the relationship between short sleep and CVD risk factors and diseases. This study aimed to assess the mediating effects of emotional distress, BMI, and physical activity on the relationship between short sleep duration and CVD risk factors and diseases, using a large nationally representative dataset.
2. Methods
Data (N = 911,773) were obtained from the National Health Interview Survey (NHIS) for years 2004 to 2013. For the purpose of our analyses, we included only participants who provided complete data for sleep variables and CVD (N = 206,049). The NHIS is a publicly available dataset with de-identified information of noninstitutionalized US adults (> 18 years old) from all 50 states and the District of Columbia in the United States. An original informed consent was obtained by the Center for Disease Control and Prevention (CDC) from subjects, as the data are de-identified and publicly available additional consent was not necessary for the current study. Data can be accessed by visiting the IPUMS Health Survey website at nhis.ipums.org/nhis. The Ethics Review Board of the CDC and the National Center for Health Statistics provided the appropriate guidelines for this survey and is in accordance with the Declaration of Helsinki. The annual response rate for all states was approximately 80%. Participants provided information using computer-assisted personal interviewing program (CAPI).[33,34]
2.1. Variables
2.1.1. Cardiovascular diseases and risk factors
The primary outcome of the current study is a latent construct of 6 self-reported physician diagnosed CVD risk factors and diseases, which include coronary heart disease (CHD), HTN, diabetes, CKD, heart attack, and stroke.
2.1.2. Short sleep duration
Sleep duration, the independent variable, was assessed in full hour increments.[34] Participants were asked “On average, how many hours of sleep do you get in a 24-hour period?” Short sleep duration was coded as total sleep time < 7 hours. Average sleep duration was coded for 7 to 8 hours of sleep in a 24-hour period.
2.1.3. Mediators
Our mediating variables were BMI, emotional distress, and physical activity. BMI (kg/m2) was calculated on the basis of self-reported height and weight. Emotional distress was measured using the Kessler-6 (K-6) scaling system, which assesses general mood and anxiety symptoms within the last 30 days, with a score of ≥ 13 indicating significant emotional distress.[34,35] Physical activity was assessed using survey questions asking participants their self-reported minutes of moderate and vigorous physical activity per week. Physical activity was defined as a latent variable using tertiles of moderate minutes of physical activity and vigorous minutes of activity per week as indicators. Tertiles of physical activity were classified as none (0 minutes of activity per week), some (1–30 minutes of activity week), and high levels (>30 minutes of physical activity week). Vigorous physical activity was defined as activities causing “heavy sweating or large increases in breathing or heart rate.” Moderate physical activity was defined as activities that “cause light sweating or a slight to moderate increase in breathing or heart rate.”
2.1.4. Covariates
The covariates used for adjustment in our analyses included age, sex, race/ethnicity, income, education, and marital status. Age and sex were self-reported. Racial/ethnic group was divided into categories (non-Hispanic white, Hispanic, Black/African–American, Native American/Alaskan Native, and Asian/Pacific Islander). Family income was also coded dichotomously (less $35,000 per year vs ≥$35,000 per year). Education was coded using 6 levels (e.g., “never attended school,” “grade 1–4,” “grade 5–8,” “grade 9–12,” “1–4 years of college,” and “5 or more years of schooling after high school”). Marital status was coded dichotomously (married vs unmarried).
2.1.5. Statistical analysis
Analyses were based on sampling weights provided by the NHIS (from 2004 to 2013) to ensure representativeness of the sample. According to the Center for Disease Control and Prevention, the multifaceted survey analysis technique accounted for the weights, strata, and clusters in this survey design. SPSS version 22 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.)[36] and R version 3.3.0 statistical software (Vienna Austria)[37] were used to perform descriptive and inferential analyses, such as the prevalence of variables of interest (age, BMI, short sleep duration, obesity, physical activity, emotional distress, medical conditions). The analysis package “survey”[38] was used to deal with the complex sample design of NHIS and to generate P values for Chi-square and Kruskal–Wallis comparisons of participant characteristics frequencies and means between individuals with short and average sleep, excluding individuals who reported long sleep duration (>8 hrs.).
CVD risk factors and diseases, short sleep, and emotional distress were coded as dichotomous variables and BMI was coded continuously with moderate levels of skewness (skewness statistic = 0.827 in raw data and 0.835 in the clustered data). Physical activity was coded using 2 continuous variables (minutes of moderate physical activity and minutes of vigorous physical activity). These were not normally distributed but were both used as indicators of a latent physical activity variable for the mediation analysis. To remove possible bias caused by these skewed variables, moderate and physical activity were recoded by dividing the variable into tertiles (0, 1–30, and >30 minutes of activity per week).
A structural equation model (SEM) was constructed via MPLUS version 7.2 (Los Angeles, CA: Muthen & Muthen)[39] software to assess main effects of short sleep and mediating role of emotional distress, BMI, and physical activity on CVD. CVD and risk factors construct (hereon forward will be called CVD) was operationalized as a latent variable comprised of 6 conditions: CHD, HTN, diabetes, CKD, heart attack, and stroke. Fit indices for the measurement model of CVD were examined to assure that our latent CVD measure was properly specified. Next, the association between short sleep and latent CVD was examined in unadjusted, covariate-adjusted, and covariate and mediator-adjusted SEMs. We then assessed the direct effect of short sleep on CVD as well as the mediating role of short sleep via our proposed mediators (physical activity, BMI, and emotional distress). We adjusted effects of age, sex, race, family annual income, marital status, and education on all pathways of the SEM. Model was assessed using the following fit indices: Comparative Fit Index (CFI) and Root Mean Square Error of Approximation (RMSEA). Standardized root mean square of the residual (SRMR) was not used as a fit index for the current analyses, as this index is not calculated for a weighted, clustered, or stratified model. Model Chi-square (χ2) was also reported for all SEM models but was not used to evaluate model fit, as Chi-squared statistics for model fit are easily biased by sample size.[40]
3. Results
3.1. Descriptive statistics
Of the total sample, 60.1% identified as white, 17.7% as Hispanic, and 15.4% as black, and 54.7% as female. The sample had a mean age of 46.8 years (SE = 0.12), a mean BMI of 27.11 kg/m2 (SE = 0.02), and approximately 32.5% reported short sleep duration (See Table 1).
Table 1.
Characteristics of average and short sleepers.
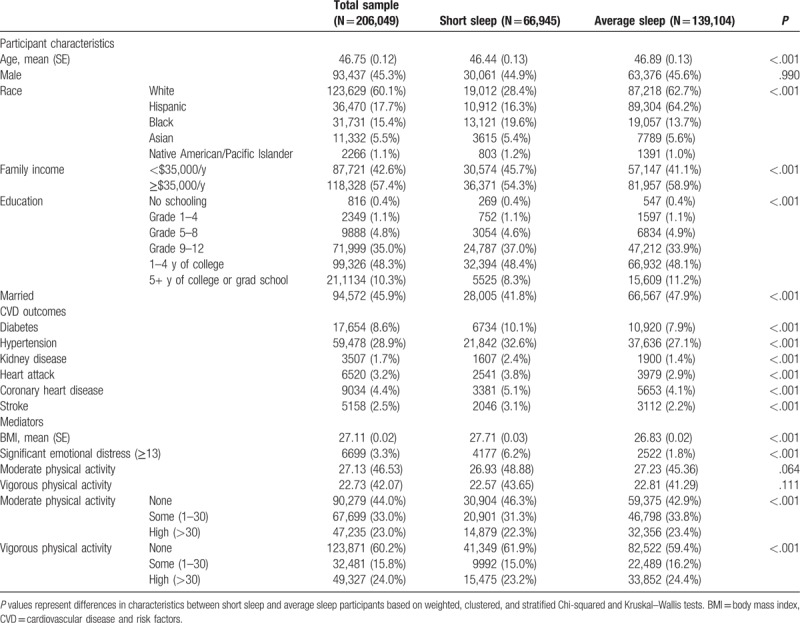
The prevalence of individual indicators of CVD risk factors and diseases (HTN, heart attack, CHD, stroke, diabetes, and kidney disease) were all significantly greater in Chi-squared analyses in the group with short sleep duration compared with the group with normal/average sleep duration (P < .001; Table 1). Similarly, short sleep was positively associated with higher BMI and levels of emotional distress (P < .001). Physical activity levels did not significantly differ between short (<7 hours) and average (7–8 hours) sleep groups in descriptive analyses (See Table 1).
3.2. Inferential statistics
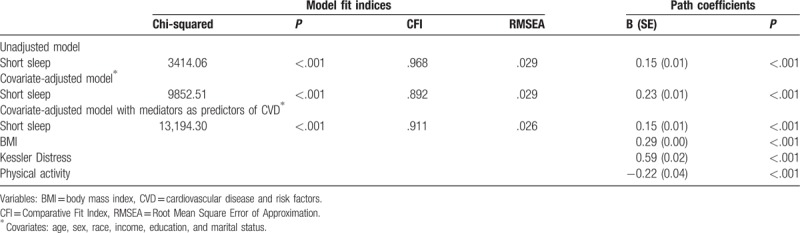
Using SEM analysis, we determined that all 6 CVDs and risk factors loaded well onto the latent construct of CVD (Fig. 1; CFI = 0.967; RMSEA = 0.042). First, we examined the association between short sleep and latent CVD (Fig. 2). Short sleep was found to be significantly and positively associated with latent CVD in unadjusted, covariate-adjusted, and covariate and mediator-adjusted models (Table 2). Second, we investigated the direct and indirect effects of BMI, emotional distress, and physical activity on the relationship between short sleep and CVD (Fig. 2). The indices of model fit were within normally accepted limits (Chi-square estimate = 8217.85, P < .001; CFI = 0.934; RMSEA = 0.029). Short sleep duration was directly associated with CVD (B = 0.08; SE = 0.02; P < .001). Mediation analyses showed that the individual indirect effects between short sleep and CVD via BMI (B = 0.04; SE = 0.00; P < .001), emotional distress (B = 0.02; SE = 0.00; P < .001), and physical activity (B = 0.02; SE = 0.00; P < .001) were all statistically significant. The total indirect relationship between short sleep via all 3 mediators on CVD was also found to be significant (B = 0.07; SE = 0.002; P < .001). After covariate adjustment for age, sex, race, income, education, and marital status on all pathways of our mediational model, indices of fit remained acceptable (Chi-square estimate = 14,710.98, P < .001; CFI = 0.874; RMSEA = 0.028). In the adjusted model, short sleep retained a significant direct relationship with latent CVD (B = 0.15; SE = 0.01; P < .001). In addition, the specific indirect relationships between short sleep and CVD mediated via BMI (B = 0.05; SE = 0.00; P < .001), emotional distress (B = 0.02; SE = 0.00; P < .001), and physical activity (B = 0.01; SE = 0.00; P < .001) were all statistically significant. The total indirect relationship between short sleep and latent CVD also remained significant after covariate adjustment (B = 0.07; SE = 0.00; P < .001).
Figure 1.
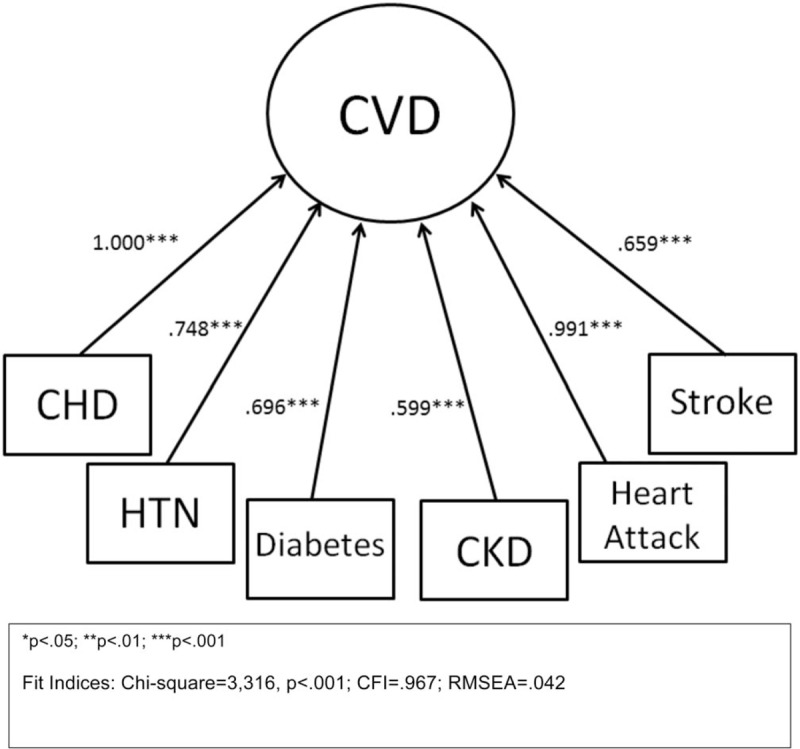
Factor loading of cardiovascular diseases and risk factors latent variable. The fit indices range from 0.599 to 1.00. CVD = cardiovascular diseases and risk factors, CHD = coronary heart disease, CKD = chronic kidney disease, HTN = hypertension.
Figure 2.
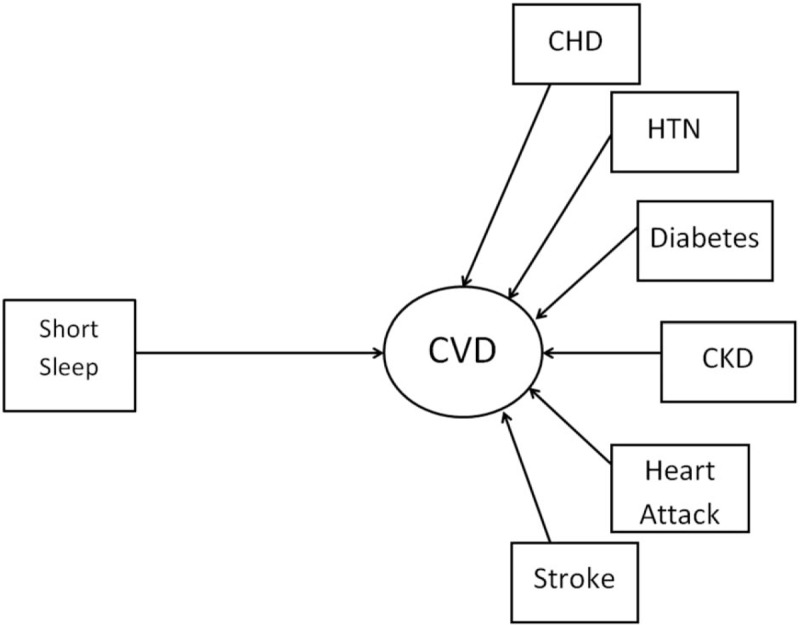
Structural equation model conceptual framework of the relationship between self-reported short sleep duration (≤6 h/24-h period) and latent variable of cardiovascular disease and risk factors (coronary heart disease, hypertension, diabetes, chronic kidney disease, heart attack, and stroke). Cardiovascular diseases and risk factors were coded dichotomously as whether the individual had a physician diagnosis of the following conditions.
Table 2.
Associations between short sleep and latent CVD: model fit indices and path coefficients.
4. Discussion
We found that compared with average sleepers, short sleepers had a higher prevalence of all CVDs and risk factors, such as diabetes, HTN, kidney disease, heart attack, CHD, and stroke. Short sleepers also had higher BMI, reduced levels of physical activity, and reported higher levels of emotional distress. Emotional distress, BMI, and physical activity all significantly mediated the relationship between short sleep duration and CVDs and risk factors (Fig. 2). However, as the relationship between short sleep duration and CVD remained significant after covariate adjustment, we infer that emotional distress, BMI, and physical activity only partially mediated the short sleep–CVD relationship, and other mediating variables remain to be identified. Overall, our findings suggest that higher BMI and emotional distress as well as lower physical activity levels are associated with an increased likelihood of cardiovascular risk factors and diseases among short sleepers (Fig. 3).
Figure 3.
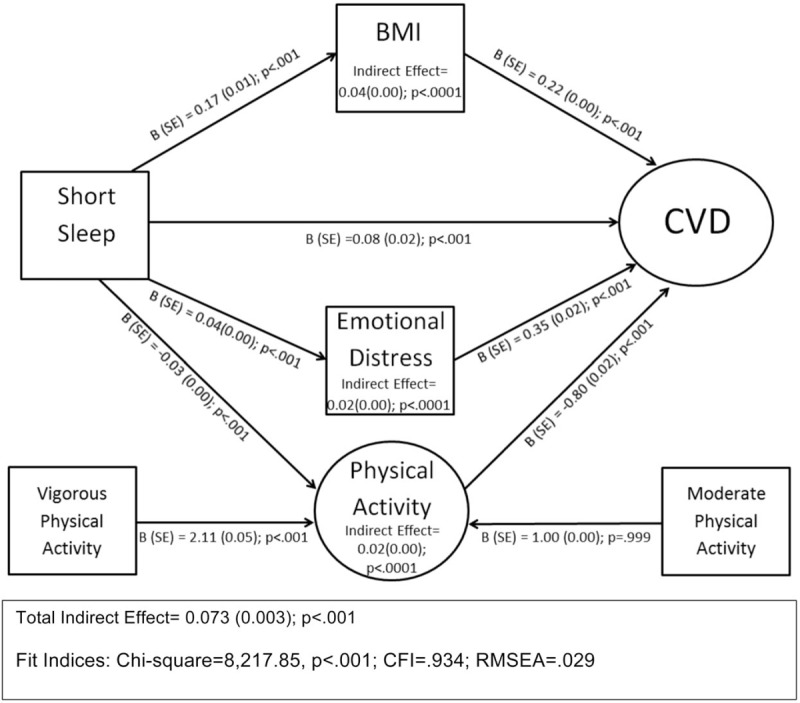
Structural equation model of the mediating effects of body mass index, emotional distress, and physical activity on the relationship between short sleep duration and cardiovascular diseases and risk factors. Physical activity was a latent variable that consisted of moderate and vigorous physical activity.
4.1. Main effects
Our findings corroborate previous work indicating that short sleepers are at a significant risk of developing CVD and corollary adverse health conditions (e.g., HTN, diabetes, obesity, CHD, stroke, and kidney disease). In our study, compared with average sleepers (7–8 hours), short sleepers had a higher prevalence of medical comorbidities (Table 1).[11–16] Our findings are supported by previous work that show short sleep disrupts endocrine and cardiometabolic functions by reducing leptin (a hormone that curbs appetite)[41,42] and decreasing glucose metabolism, which increases the risk of glucose intolerance and diabetes.[17] Spiegel et al[14] highlight that short sleepers may not receive sufficient rapid eye movement activity during sleep, which is linked to restorative sleep and is needed for healthy metabolic and endocrine functioning. Short sleepers are more likely to have elevated sympathetic and pituitary adrenal axis activity resulting in decreased cerebral glucose metabolism and elevation in growth hormone, all of which can precipitate glucose intolerance, insulin insensitivity, and subsequent development of diabetes.[14] Another possible pathophysiological explanation for the short sleep–CVD relationship is rooted in the idea that short sleep may cause carotid intima disruption, which increases the risk of HTN and carotid plaque formation, all proximal predictors of CVD.[13]
4.2. Mediating effects
In spite of epidemiological and biological evidence that habitual short sleep duration increases CVD risk, our study found that behavioral and lifestyle factors such as BMI, physical activity, and emotional distress may mediate the relationship between short sleep on CVD and its associated risk factors.
4.2.1. Negative mediating effects
In the current analyses, we found that short sleep was associated with reduced physical activity, which was associated with greater levels of CVD and risk factors (Fig. 2). Our findings are consistent with previous studies that have highlighted the health benefits of physical activity, which includes enhancing mental health well-being, reducing CVD events and other chronic diseases, and decreasing mortality risk.[43–45] Physical activity may improve cardiovascular health by buffering the adverse effects of stress on cardiovascular health.[46–48] In a randomized trial, Blumenthal et al[48] demonstrated that medically stable CHD patients who engaged in consistent physical activity and stress management had less emotional distress and less CVD risk markers compared with patients who received medical treatment alone. In addition, some studies have even suggested that consistent physical activity may reverse the negative cardiovascular effects of sleep deprivation.[23,49–53]
4.2.2. Positive mediating effects
Both BMI and emotional distress positively mediated the relationship between short sleep and CVDs and risk factors. Of the 3 mediators we considered, BMI had the strongest mediation effect on the short sleep CVD relationship.[25,54–56] However, given the cross-sectional nature of the dataset, we were unable to determine a precise causal function of BMI. It is possible that habitual short sleep increased risk of elevated BMI, which in turn increases risk for CVD.[25,55] Laboratory studies indicate that short sleep is associated with an increase in the appetite stimulating hormone called ghrelin and a decrease in the satiety hormone called leptin resulting in increased hunger, especially increased rich foods.[57] This pathophysiological argument might explain the increased BMI of short sleepers. However, the above causal chain is debunked in light of evidence indicating that not all individuals with elevated BMI are short sleepers.[58] Nevertheless, findings by Jean-Louis et al[59] and Donat et al[60] have shown that the obesity epidemic coincided with the rise in short sleep duration in the US. In addition, several prospective studies have shown that short sleep is associated with incident weight gain and/or obesity.[61–63] Despite the limitation of being unable to make causal claims, our study nevertheless makes a significant and unique contribution to the field based on our use of structural equation modeling a more robust analytic technique, relative to traditional regression analyses. The use of SEM techniques to model a latent construct for CVD risk factors and diseases reduces the problem of the correlations between BMI and any one comorbidity and allows us to test and quantify direct and indirect relationships among short sleep, CVD, and BMI, while adjusting for effects of confounders.
We also found that emotional distress positively mediated the relationship between short sleep and CVD. Emotional distress was more common among short sleepers and was positively associated with CVD. This observation was expected given that short sleep may induce emotional distress, irritability, and mood disturbances.[18,19] DRIVE, a robust Australian prospective study, concluded that self-reported short sleep duration was associated with psychological/emotional distress.[64] Similarly, in a 9-year clinical trial, Denollet and Brutsaert[32] examined the impact of treating emotional distress among male patients with CHD. The results showed that reduction in negative affect and emotional distress through a rigorous mental health rehabilitation program reduced likelihood of mortality among CHD patients. The direct link between short sleep and emotional distress is not well established in the literature; however, much can be inferred indirectly. Previous research shows that short sleep negatively affects the hypothalamic pituitary adrenal axis (HPA axis), which regulates stress in the body.[14,17,65] Activity of HPA axis is reduced during sleep onset and early stages of sleep, while it is activated during latter stages of sleep, such as rapid eye movement stage. If an individual experiences chronic short sleep, their HPA axis and cortisol levels will remain high and activated leaving the individual in a state of heightened alertness and stress. This heightened state of alertness can compromise the immune system and increase stress-related inflammation, which can lead to poor cardiovascular health. The HPA axis also plays a vital role in emotional and mental wellbeing and mediates the relationship between short sleep duration and emotional distress.[66] Evidence of this is observed in the negative effects of insomnia-related short sleep on emotional distress and depressed mood.[67–69] Prolonged sleep-related stress increases the risk of CVD and risk factors such as obesity, diabetes, and HTN[65,70] via HPA axis and other endocrine disruption.[71,72]
4.3. Limitations
Our study has several methodological and conceptual limitations. First, the cross-sectional design of the current study prevents us from inferring any casual relationships. For example, it is not clear whether a physical activity intervention could mitigate CVD risk factors and diseases associated with short sleep. Second, sleep duration was not measured objectively or prospectively, reducing precision of estimates of sleep duration. Third, BMI, physical activity, and medical conditions were not objectively determined and may be over- or underestimated. Fourth, as for the assessment of emotional distress, we were unable to confirm diagnosable mental illness. In addition, the Kessler scale is limited in assessing chronicity of emotional distress, which could affect our findings. It should be noted that the analyses in the present study did not take sleep apnea or insomnia into account, which overlap with short sleep duration[73] and are well-characterized risk factors for CVD risk factors and diseases, as well as depression, anxiety disorders, and suicide.[74–76] It is possible that the mediating effect of emotional distress on the relationship between short sleep duration and CVD risk factors and diseases may be confounded by sleep apnea or insomnia, which were not measured in the current study.
We recognize that our interpretation lacks nuance in capturing the confounding effects severity and chronicity of medical conditions may have on physical activity and sleep behaviors in this population.[55,77] Another limitation in our analyses was our inability to capture time-lagged and cumulative effect of short sleep on physical activity, which may provide greater insights on the long-term benefits of physical activity on cardiovascular health.
Another limitation is our inability to make significant generalizable inferences about all racial and ethnic groups, specifically Hispanics, as Hispanics were grouped into white race category. Current analyses did not adjust racial categories based on Hispanic ethnicity such as Hispanic and nonwhite Hispanic. Therefore, our race categories combined heterogeneous groups, such as Hispanics, which may have failed to capture actual racial differences in the covariate adjusted models. In addition, there is a lack of information on the number of dependents/children for the participants of this study. Having dependents/young children could have possibly been a confounder of short sleep status.
Additional questions remain for future work. First, the present study suggests that it is plausible that the cardioprotective benefits of physical activity may partially mitigate the detrimental effects of short sleep. Although the cross-sectional results of this study support this hypothesis, future intervention studies are needed to determine if this is the case. Second, this study cannot ascertain whether different types, levels, or categories of physical activity, emotional distress, and BMI may differentially affect the relationship between short sleep and health outcomes. More careful assessment of types of physical activity may address this issue. Third, certain factors may moderate relationships observed in our analyses; for example, individuals of differing age, sex, and race/ethnicity groups may differentially experience the cardioprotective benefits of exercise, as it relates to risks associated with sleep.
5. Conclusion
Despite these limitations, our findings provide evidence that BMI, emotional distress, and physical activity may affect the short sleep-CVD relationship. Future studies should take a personalized behavioral medicine approach to examine the nature of mediational models demonstrated in our analyses. Specifically, future studies should investigate whether specific sleep durations (short = less than 7 hours, average = 7–8 hours, or long = greater than 8 hours) when combined with BMI, emotional distress, and physical activity or sedentary behavior lead to lower and higher CVD risk. We propose that future studies should also investigate whether all short sleepers benefit equally from the mitigating effects of lower BMI, lower emotional distress, and increased physical activity.
We believe the current study adds significantly to the literature by providing a nuanced understanding of the complex interrelationships among short sleep, CVD, and behavioral and lifestyle mediators. It quantifies the direct and indirect effects of behavioral and lifestyle factors on the relationship between short sleep duration and CVD. Such insights are likely to lead to multipronged behavioral strategies to reduce the inimical effects short sleep has on CVD.
Author contributions
Conceptualization: Azizi A Seixas, Aisha T. Langford, Michael A. Grandner, Andres R. Schneeberger, Girardin Jean-Louis.
Data curation: Julian Jean Vallon, Mark Butler.
Formal analysis: Mark Butler, Azizi A. Seixas, Julian Jean Vallon, Andrea Barnes-Grant.
Funding acquisition: Azizi A. Seixas, Ferdinand Zizi, Girardin Jean-Louis.
Investigation: Azizi A. Seixas, Julian Jean Vallon, Andrea Barnes-Grant, Aisha T. Langford, Andres R. Schneeberger, Jhenelle Hutchinson.
Methodology: Azizi A Seixas, Andrea Barnes-Grant, Mark Butler.
Project administration: Azizi A. Seixas, Julian Jean Vallon, Ferdinand Zizi.
Resources: Jhenelle Hutchinson, Ferdinand Zizi.
Software: Mark Butler, Azizi A. Seixas.
Supervision: Azizi A Seixas, Julian Jean Vallon, Michael A. Grandner, Ferdinand Zizi, Girardin Jean-Louis.
Validation: Aisha T. Langford, Girardin Jean-Louis, Mark Butler.
Writing – original draft: Azizi A. Seixas, Julian Jean Vallon, Jhenelle Hutchinson, Andrea Barnes-Grant, Mark Butler
Writing – review & editing: Azizi A Seixas, Julian Jean Vallon, Mark Butler, Andrea Barnes-Grant, Aisha T. Langford, Michael A. Grandner, Andres R. Schneeberger, Jhenelle Hutchinson.
Footnotes
Abbreviations: BMI = body mass index, CDC = Center for Disease Control and Prevention, CHD = coronary heart disease, CKD = chronic kidney disease, CVD = cardiovascular diseases and risk factors, HPA axis = hypothalamic pituitary adrenal axis, HTN = hypertension, NHIS = National Health Interview Survey, PA = physical activity.
Both A. Seixas and J. Vallon are first authors.
Funding/support: This work was supported by funding from the NHLBI (K01HL13452), NIMHD (R01MD007716), and the NIA (K07AG052685).
The authors have no conflict of interest to report.
References
- [1].Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J 2008;155:772–9. [DOI] [PubMed] [Google Scholar]
- [2].Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA 2007;297:177–86. [DOI] [PubMed] [Google Scholar]
- [3].Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association nutrition committee. Circulation 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- [4].McGill HC, McMahan CA, Gidding SS. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study”. Circulation 2008;117:1216–27. [DOI] [PubMed] [Google Scholar]
- [5].Eckel RH, Krauss RM. AHA Nutrition Committee. American Heart Association call to action: obesity as a major risk factor for coronary heart disease. Circulation 1998;97:2099–100. [DOI] [PubMed] [Google Scholar]
- [6].Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The Lancet 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- [7].Buxton OM, Marcelli E. Short and long sleeps are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med 2010;71:1027–36. [DOI] [PubMed] [Google Scholar]
- [8].Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration; associations with mortality in the Whitehall II cohort. Sleep 2007;30:1659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Meisinger C, Heier M, Lowel H, et al. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep 2007;30:1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Amagai Y, Ishikawa S, Gotoh T, et al. Sleep duration and mortality in Japan: the Jichi medical school cohort study. J Epidemiol 2004;14:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 2004;43:678–83. [DOI] [PubMed] [Google Scholar]
- [12].Wolff B, Völzke H, Schwahn C, et al. Relation of self-reported sleep duration with carotid intima-media thickness in a general population sample. Atherosclerosis 2008;196:727–32. [DOI] [PubMed] [Google Scholar]
- [13].King CR, Knutson KL, Rathouz PJ, et al. Short sleep duration and incident coronary artery calcification. JAMA 2008;300:2859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol 2009;5:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hamasaki H, Katsuyama H, Sako A, Yanai H. Short sleep duration is associated with B-type natriuretic peptide levels and predicts the death of Japanese patients with type 2 diabetes. Sleep Med 2017;36:1–5. [DOI] [PubMed] [Google Scholar]
- [16].Burley DS, Hamid SA, Baxter GF. Cardioprotective actions of peptide hormones in myocardial ischemia. Heart Fail Rev 2007;12:279–91. [DOI] [PubMed] [Google Scholar]
- [17].Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev 2008;12:197–210. [DOI] [PubMed] [Google Scholar]
- [18].Vgontzas AN, Lin HM, Papaliaga M, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes (Lond) 2008;32:801–9. [DOI] [PubMed] [Google Scholar]
- [19].Seixas AA, Nunes JV, Airhihenbuwa CO, et al. Linking emotional distress to unhealthy sleep duration: analysis of the 2009 National Health Interview Survey. Neuropsychiatr Dis Treat 2015;11:2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sarchiapone M, Mandelli L, Carli V, et al. Hours of sleep in adolescents and its association with anxiety, emotional concerns, and suicidal ideation. Sleep Med 2014;15:248–54. [DOI] [PubMed] [Google Scholar]
- [21].Blasco-Fontecilla H, Alegria AA, Lopez-Castroman J, et al. Short self-reported sleep duration and suicidal behavior: a cross-sectional study. J Affect Disord 2011;133:239–46. [DOI] [PubMed] [Google Scholar]
- [22].Von Kries R, Toschke AM, Wurmser H, et al. Reduced risk for overweight and obesity in 5-and 6-y-old children by duration of sleep: a cross-sectional study. Int J Obes Relat Metab Disord 2002;26:710–6. [DOI] [PubMed] [Google Scholar]
- [23].Andersson C, Lyass A, Larson MG, et al. Physical activity measured by accelerometry and its associations with cardiac structure and vascular function in young and middle-aged adults. J Am Heart Assoc 2015;4:e001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cappuccio FP, Taggart FM, Kandala N, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008;31:619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child 2006;91:881–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the Obesity Society and the American Society of Hypertension. Obesity 2013;21:8–24. [DOI] [PubMed] [Google Scholar]
- [27].Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension new insights into mechanisms. Hypertension 2005;45:9–14. [DOI] [PubMed] [Google Scholar]
- [28].Mora S, Cook N, Buring JE, et al. Physical activity and reduced risk of cardiovascular events potential mediating mechanisms. Circulation 2007;116:2110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Harrington JM. Health effects of shift work and extended hours of work. Occup Environ Med 2001;58:68–72. [Google Scholar]
- [30].Vandekerckhove M, Cluydts R. The emotional brain and sleep: an intimate relationship. Sleep Med Rev 2010;14:219–26. [DOI] [PubMed] [Google Scholar]
- [31].Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Ann Rev Psychol 2002;53:83–107. [DOI] [PubMed] [Google Scholar]
- [32].Denollet J, Brutsaert DL. Reducing emotional distress improves prognosis in coronary heart disease 9-year mortality in a clinical trial of rehabilitation. Circulation 2001;104:2018–23. [DOI] [PubMed] [Google Scholar]
- [33].Botman SL, Moore TF, Moriarity CL, Parsons VL. Design and estimation for the National Health Interview Survey, 1995-2004. Vital Health Stat 2 2000;1–31. [PubMed] [Google Scholar]
- [34].Parsons VL, Moriarity C, Jonas K, et al. Design and estimation for the National Health Interview Survey, 2006-2015. Vital Health Stat 2 2014;165:1–53. [PubMed] [Google Scholar]
- [35].Kessler RC, Green JG, Gruber MJ, et al. Screening for serious mental illness in the general population with the K6 screening scale: results from the WHO World Mental Health (WMH) survey initiative. Int J Methods Psychiatr Res 2010;19(suppl 1):4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].IBM Corp. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp; 2013. [Google Scholar]
- [37].R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. Available at: www.R-project.org. Accessed May 19, 2018. [Google Scholar]
- [38].Lumley T. Analysis of complex survey samples. J Stat Softw 2004;9:1–9. [Google Scholar]
- [39].Muthen LK, Muthen BO. Mplus User's Guide. Seventh edition. Los Angeles, CA: Muthen & Muthen; (1998-2012). [Google Scholar]
- [40].Hooper D, Coughlan J, Mullen MR, et al. Structural equation modeling: guidelines for determining model fit. Elect J Business Res Methods 2008;6:53–60. [Google Scholar]
- [41].Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Progr Cardiovasc Dis 2009;51:285–93. [DOI] [PubMed] [Google Scholar]
- [42].Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 2004;1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. Can Med Assoc J 2006;174:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care 2004;27:2518–39. [DOI] [PubMed] [Google Scholar]
- [45].Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry 2005;18:189–93. [DOI] [PubMed] [Google Scholar]
- [46].Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev 2001;21:33–61. [DOI] [PubMed] [Google Scholar]
- [47].Bauman AE. Updating the evidence that physical activity is good for health: an epidemiological review 2000-2003. J Sci Med Sport 2004;7:6–19. [DOI] [PubMed] [Google Scholar]
- [48].Blumenthal J, Sherwood A, Babyak M, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. JAMA 2005;293:1626–34. [DOI] [PubMed] [Google Scholar]
- [49].Goto C, Higashi Y, Kimura M, et al. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans role of endothelium-dependent nitric oxide and oxidative stress. Circulation 2003;108:530–5. [DOI] [PubMed] [Google Scholar]
- [50].Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev 2007;11:163–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr 2009;90:1476–82. [DOI] [PubMed] [Google Scholar]
- [52].Garaulet M, Ortega FB, Ruiz JR, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes 2011;35:1308–17. [DOI] [PubMed] [Google Scholar]
- [53].Reid KJ, Baron KG, Lu B, et al. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med 2010;11:934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep 2004;27:661–6. [DOI] [PubMed] [Google Scholar]
- [55].Van Gaal LF, Mertens IL, Christophe E. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–80. [DOI] [PubMed] [Google Scholar]
- [56].Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes 2008;32:959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–50. [DOI] [PubMed] [Google Scholar]
- [58].Horne J. Obesity and short sleep: unlikely bedfellows? Obes Rev 2011;12:e84–94. [DOI] [PubMed] [Google Scholar]
- [59].Jean-Louis G, Williams NJ, Sarpong D, et al. Associations between inadequate sleep and obesity in the US adult population: analysis of the national health interview survey (1977-2009). BMC Public Health 2014;14:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Donat M, Brown C, Williams N, et al. Linking sleep duration and obesity among black and white US adults. Clin Pract (Lond) 2013; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chaput JP, Després JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep 2008;31:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Patel SR, Malhotra A, White DP, et al. Association between reduced sleep and weight gain in women. Am J Epidemiol 2006;164:947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep 2010;33:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Glozier N, Martiniuk A, Patton G, et al. Short sleep duration in prevalent and persistent psychological distress in young adults: the DRIVE study. Sleep 2010;33:1139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab 2005;90:3106–14. [DOI] [PubMed] [Google Scholar]
- [66].Stephens MAC, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res 2012;34:468–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sateia MJ, Doghramji K, Hauri PJ, Morin CM. Evaluation of chronic insomnia. An American Academy of Sleep Medicine review. Sleep 2000;23:243–308. [PubMed] [Google Scholar]
- [68].Harvey AG. A cognitive model of insomnia. Behav Res Ther 2002;40:869–93. [DOI] [PubMed] [Google Scholar]
- [69].Lucassen EA, Cizza G. The hypothalamic-pituitary-adrenal axis, obesity, and chronic stress exposure: sleep and the HPA axis in obesity. Curr Obes Rep 2012;1:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gathercole LL, Stewart PM. Targeting the pre-receptor metabolism of cortisol as a novel therapy in obesity and diabetes. J Steroid Biochem Mol Biol 2010;122:21–7. [DOI] [PubMed] [Google Scholar]
- [71].Denollet J. Personality, emotional distress and coronary heart disease. Eur J Person 1997;11:343–57. [Google Scholar]
- [72].Salovey P, Rothman AJ, Detweiler JB, Steward WT. Emotional states and physical health. Am Psychol 2000;55:110. [DOI] [PubMed] [Google Scholar]
- [73].Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep 2010;33:1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic insomnia and the stress system. Sleep Med Clin 2007;2:279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].National Institutes of Health. NIH state of the science statement on manifestations and management of chronic insomnia in adults. J Clin Sleep Med 2005;1:412–21. [PubMed] [Google Scholar]
- [76].Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev 2013;17:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Thomas N, Alder E, Leese GP. Barriers to physical activity in patients with diabetes. Postgrad Med J 2004;80943:287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


