Abstract
Neutrophil–lymphocyte ratio (NLR) is a poor prognostic factor in many tumors including glioblastoma multiforme (GBM), colorectal, and prostate cancer. The aim of this study was to investigate the prognostic value of preoperative NLR in patients with craniopharyngioma.
Around 149 patients of craniopharyngioma surgically were treated at the Department of Neurosurgery, West China Hospital from January 2008 to December 2010, including 84 males and 65 females aged from 6 to 70 years were retrospectively reviewed, and preoperative NLR was analyzed. Overall survival (OS), progression free survival (PFS), and quality of life (QOL) were evaluated.
The 5-year OS and PFS rates were 81.21% and 75.84%. Preoperative NLR was significantly correlated with OS (HR = 1.44, 95% CI 1.16–1.79, P = .001) and PFS (HR = 1.46, 95% CI 1.22–1.74, P < .001). The best cut-off value of NLR was found to be 4 based on the receiver operator characteristics (ROC) curve. Patients with NLR ≥4 had a significantly worse QOL (P = .039), lower OS rate (P = .009), and PFS rate (P < .001).
Preoperative NLR may be a simple, readily available, and valid predictor of long-term outcome in craniopharyngioma. We suggest that the NLR can provide effective guidance to neurosurgeons for more information about the tumor and prognostic evaluation.
Keywords: craniopharyngioma, neutrophil–lymphocyte ratio, prognosis, survival
1. Introduction
Craniopharyngioma (CP) is a histologically benign tumor originating from remnants of Rathke's pouch and it is located in the sellar and/or parasellar area.[1,2] It accounts for 2% to 5% of all primary intracranial tumors and 5.6%–13% of intracranial neoplasms in children.[3,4] It consequently ranks as the second most frequent tumor in the hypothalamic-pituitary region regardless of age.[5] Patients may have a variety of manifestations, such as visual, neurological, and hypothalamo-pituitary dysfunction.[6] Surgical resection followed by postoperative radiation, in cases of residual tumor, is the main treatment strategy.[6] Despite its nonmalignant feature, it can be linked with a poor prognosis due to the anatomical involvement and surgical damnification of hypothalamic areas.[7–10] Prognostic predictors including sex, age, tumor size, location, and treatment were considered for possible association with recurrence and quality of life in patients suffering CP.[11] However, it is still very difficult to predict the prognosis of CP, and it is worthwhile to explore new prognostic factors.
It has been demonstrated that chronic inflammation has relationship with tumor in many ways, including cell invasion, promotion of angiogenesis and damage of DNA.[12–14] Recently, Hannahan et al. [15] emphasized the importance of uncontrolled inflammation in driving tumor proliferation. Neutrophil-lymphocyte ratio (NLR) is an easy way to evaluate inflammation that is inexpensive and readily available from the complete blood cell count. Increased pretreatment NLR has recently been shown to be a poor prognostic factor in many tumors including lung cancer, breast cancer, gastrointestinal cancers, urologic cancers, glioblastoma multiforme (GBM), gynecologic cancers, and metastatic disease.[16–20] Besides, elevated NLR has also been reported to be associated with poor outcome in non-neoplastic diseases such as stroke and coronary artery disease.[21,22] Therefore, NLR may be a promising prognostic biomarker in various diseases in clinical work.
As to CP, the histologic findings like degenerative changes and inflammation were common features.[11,23] And inflammation may cause more tumor adhesion and infiltration to adjacent brain. Even worse, this would make gross total resection (GTR) more difficult.[24] We hypothesized the prognostic role of NLR in CP, and the impact of NLR on the prognosis in CP has not been reported before. Therefore, to evaluate the prognostic value of NLR on long-term outcome in patients with CP, we retrospectively undertook a preliminary study to analyze overall survival (OS), progression free survival (PFS), weight development, and quality of life (QOL).
2. Materials and methods
2.1. Patient population and data collection
Clinical records of 176 consecutive patients with CP at the Neurosurgery Department, West China Hospital between January 2008 and December 2010 were analyzed. CP was diagnosed by preoperative computed tomography (CT) and enhanced magnetic resonance imaging (MRI) and diagnosis was confirmed by postoperative histological analysis. Patients with other diseases, including other intracranial disease, inflammatory disease, infection within 6 months, trauma, heart disease, diabetes mellitus, metabolic syndrome, severe hepatic or renal dysfunction, blood system diseases and medication usage related to inflammatory conditions that could significantly influence NLR or prognosis or those lacking complete data were excluded. Also all patients underwent craniotomy to remove the tumor under the microscope by experienced neurosurgeons. Eight patients did not accord with the inclusive criteria. In addition, 19 patients were lost at follow-up or not able to cooperate at the follow-up process. In the end, 149 patients were enrolled in this study. This study was approved by the ethics committee of our hospital.
Complete blood count was taken preoperatively before any treatment especially hormone replacement therapy. Blood samples were analyzed within 2 hours after collection using a Sysmex XN-9000 complete blood count analyzer (Sysmex, Japan). We mainly focused on blood neutrophil and lymphocyte. Also NLR was defined as the ratio between neutrophil and lymphocyte count. According to preoperative CT and enhanced MRI results provided by experienced radiologists, we recorded the location and tumor size (cm2) was calculated using the maximal tumor diameters in 2 dimensions.
Weight and height were expressed as body mass index (BMI = weight [kg]/height2 [m2]). In addition, weight development was defined as the difference between BMI at the time of follow-up and before the surgery.
QOL was assessed using the anterior skull base surgery questionnaire (ASBS-Q), and this is a specific instrument that has been validated for use in patients undergoing anterior skull base surgery.[25–28] The ASBS-Q including 35 questions was divided into 6 relevant QOL domains: performance (6 items), physical function (7 items), vitality (7 items), pain (3 items), influence on emotions (5 items), and specific symptoms (7 items). Responses were recorded on a 5-item scale, ranging from 1 to 5 points for each item. In this study we used average score of 35 questions to evaluate QOL of patients, with a higher score representing better QOL. If patient dies, his/her ASBS-Q score was considered as 1 point. Besides, for long-term outcome, we were able to analyze 5-year OS rate and 5-year PFS rate.
2.2. Statistical analysis
We used SPSS 23.0 (SPSS, Inc., Chicago, IL) for Apple's operating system to perform data analysis. Data were expressed as mean ± standard deviation for normally distributed variables and median ± interquartile range for non-normally distributed variables. The distribution of the variables was analyzed with the Kolmogorov–Smirnov test. The difference between 2 groups was tested by Independent Student's t-tests for normally distributed variables and Mann–Whitney U test for nonparametrically distributed variables. The chi-square test or Fisher exact test was used to compare categorical variables. The bivariate relationship between 2 continuous variables was assessed using the Spearman's correlation coefficient. Univariate and multivariate Cox proportional hazard regression models were constructed to explore the association of NLR and other clinical factors with PFS and OS. OS and PFS were estimated using the Kaplan–Meier method, and differences in PFS and OS were examined with the log-rank test. All tests were 2-sided and P values of ≤ .05 were considered as being statistically significant.
3. Results
3.1. Patient characteristics
One hundred and forty-nine patients (female 65, male 84; median age 36, range 6–70) with CP who underwent surgery between January 2008 and December 2010 were finally enrolled in the study. The distributions of clinical and prognostic factors were shown in Table 1. The postoperative stay was 14 ± 17 days. A total of 101 (67.79%) patients achieved gross total resection. Eleven patients died during perioperative period. The tumor size was 4.17 ± 4.01 cm2. Tumors were mainly located supra- and intrasellar (91) and supra-sellar (51). Patients have different endocrinopathies, such as growth hormone deficiency, hypogonadism, hypothyroidism, hypoadrenalism, and diabetes insipidus. Thirty patients received radiation therapy. The mean NLR was 3.10 ± 1.59.
Table 1.
Distribution of clinical and prognostic factors, and univariate and multivariate analysis of their association with 5-year progression free survival and overall survival.
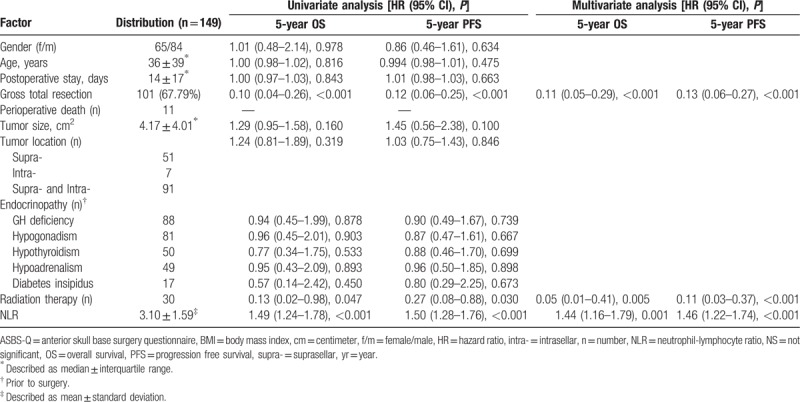
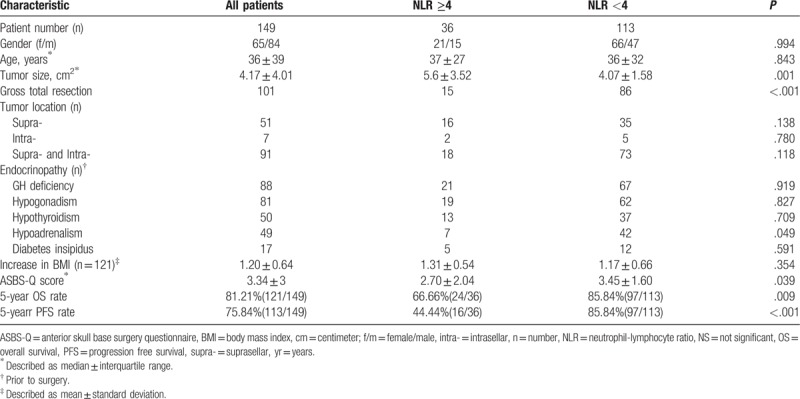
Thirty-six of 149 (24.16%) had elevated NLR (≥4) at baseline. The distributions of clinical factors and long-term outcome between patients with preoperative NLR ≥4 versus <4 were shown in Table 2. Median follow-up time was 80 months (71.79 ± 27.04, range 1–102). Average increase in BMI was 1.20 ± 0.64 in living individuals. Median ASBS-Q score was 3.34 ± 3. Median progression free survival was 82 months (83.00 ± 9.72, range 66–102). Median overall survival was 83 months (83.00 ± 10.00, range 66–102). Among the entire cohort, 5-year PFS rate was 75.84% while 5-year OS rate was 81.21%, respectively.
Table 2.
Distribution of clinical factors and long-term outcome stratified by NLR.
3.2. Univariate and multivariate analysis of factors associated with OS and PFS (Table 1)
In the univariate analysis, gross total resection of the tumor (HR = 0.10, 95%CI 0.04–0.26, P < .001), radiation therapy (HR = 0.13, 95%CI 0.02–0.98, P = .047) and NLR (HR = 1.49, 95% CI 1.24–1.78, P < .001) were associated with OS. Also gross total resection of the tumor (HR = 0.12, 95%CI 0.06–0.25, P < .001), radiation therapy (HR = 0.27, 95%CI 0.08–0.88, P = .03), and NLR (HR = 1.50, 95%CI 1.28–1.76, P < .001) were associated with PFS. Other factors were not significantly associated with OS or PFS.
In the multivariate analysis, factors associated with OS were NLR (HR = 1.44, 95%CI 1.16–1.79, P = .001), radiation therapy (HR = 0.05, 95%CI 0.01–0.41, P = .005) and gross total resection (HR = 0.11, 95%CI 0.05–0.29, P < .001). Besides, NLR (HR = 1.46, 95%CI 1.22–1.74, P < .001), radiation therapy (HR = 0.11, 95%CI 0.03–0.37, P < .001) and gross total resection (HR = 0.13, 95%CI 0.06–0.27, P < .001) were associated with PFS.
3.3. NLR ≥4 versus <4 of factors and long-term outcome (Table 2)
Based on the receiver operator characteristics (ROC) curve, the best cut-off value of NLR was found to be 4 (Fig. 1). Moreover, median OS was 77 versus 80 months in patients with NLR ≥4 versus <4 (Fig. 2). The median PFS was 73 versus 78 months in patients with NLR ≥4 versus <4 (Fig. 3).
Figure 1.
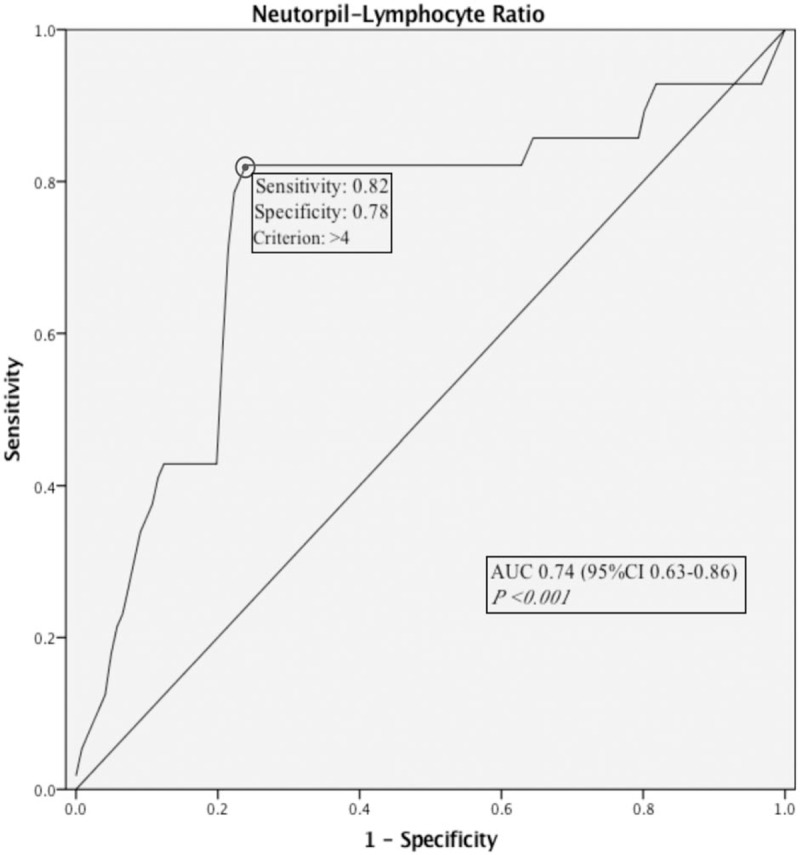
Receiver operator characteristics curve of neutrophil-lymphocyte ratio to predict mortality.
Figure 2.
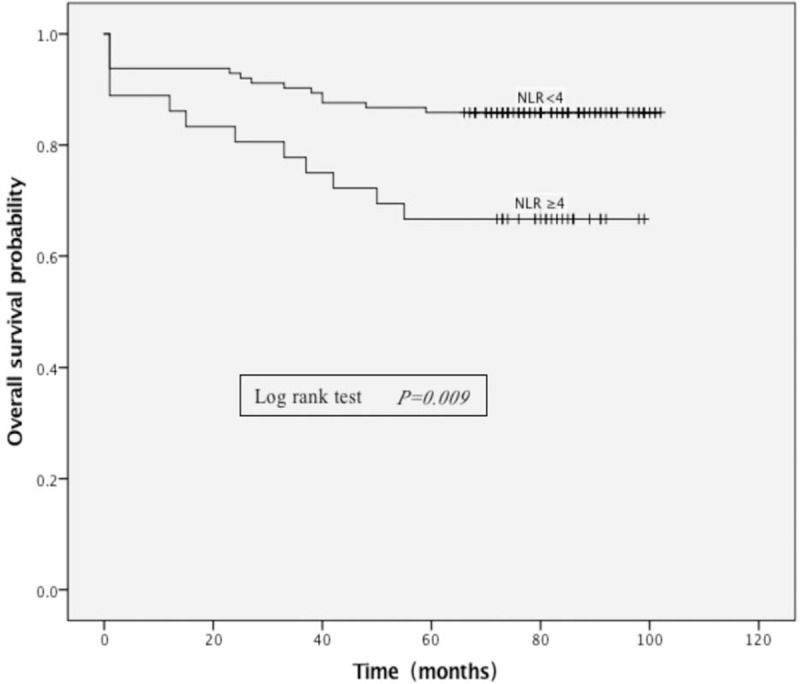
Kaplan–Meier curves showing overall survival, stratified by neutrophil-lymphocyte ratio (NLR). The median OS was 77 versus 80 months in patients with NLR ≥4 versus <4. NLR = neutrophil-lymphocyte ratio.
Figure 3.
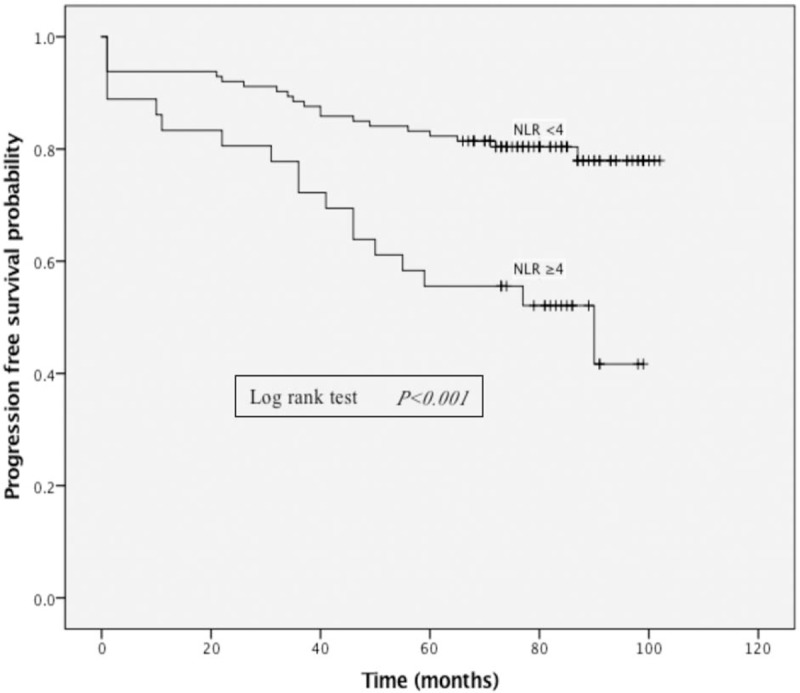
Kaplan–Meier curves showing progression free survival, stratified by neutrophil-lymphocyte ratio (NLR). The median PFS was 73 versus 78 months in patients with NLR ≥4 versus <4. NLR = neutrophil-lymphocyte ratio.
Tumor size was 5.6 ± 3.52 cm2 versus 4.07 ± 1.58 cm2 (P = .001) and gross total resection of the tumor was achieved in 15 versus 86 individuals (P < .001) in patients with NLR ≥4 versus <4. More individuals had hypoadrenalism in patients with NLR <4 (P = .049).
Patients with NLR ≥4 had an average ASBS-Q score of 2.70 ± 2.04, while those with NLR <4 had an average ASBS-Q score of 3.45 ± 1.60 (P = .039). Patients with NLR ≥4 had a 5-year OS rate of 66.66% (24/36) whereas patients with NLR <4 had a 5-year OS rate of 85.84% (97/113) in Kaplan–Meier curve (P = .009) (Fig. 2). The 5-year PFS rate was 44.44% (16/36) versus 85.84% (97/113) in patients with NLR ≥4 versus <4 (P < .001) (Fig. 3). No significant difference was found among other factors between patients with NLR ≥4 and <4.
4. Discussion
Craniopharyngiomas are difficult intracranial lesions to treat due to its deep location and extremely variable growth pattern.[29,30] Although histologically benign, the tumor's potential adhesion to adjacent vital brain structures makes gross total resection and follow-up treatment difficult. In recent studies, about 18% to 84% [3,6,29,31–37] of patients had gross total resection and the 5-year OS rates ranged from 54% to 96%.[29,36–43] In the present study, the 5-year OS rate was 81.21% and 67.79% (101/149) of patients had gross total resection.
The present study assessed the prognostic value of preoperative NLR in surgically treated patients with CP. According to the data we obtained from this study, preoperative NLR may be associated with QOL, OS, and PFS. In addition, NLR value of 4 was determined as cut-off value with a good sensitivity and acceptable specificity. In this retrospective study, patients with preoperative NLR <4 had a better long-term outcome and NLR ≥4 was an alarm signal of increased mortality risk. To our knowledge, this is the first study to assess the prognostic role of preoperative NLR in patients with CP.
Composed of 2 major component of immune system, NLR can be applied to clinical work as marker of a patient's immune state prior to surgery. In the present study, higher NLR (≥4) was associated with larger tumor and worse long-term outcome. Clinical data from recent studies suggest that immune response plays a role in tumorigenesis.[12,13] Tumor and tumor-related neutrophils produce cytokines like vascular endothelial growth factor (VEGF), which promote angiogenesis, provoke tumor cells to proliferate, therefore, to further promote invasive growth, metastasis, and recurrence.[11,44–47] All the above mentioned are associated with worse QOL after surgical treatment and lower PFS rate.[3,33,36,48–51]
In neoplastic processes, neutrophil is consequence of tumor-related inflammatory cytokines including tumor necrosis factor (TNF), interleukin-6 (IL-6), and granulocyte-colony stimulating factor (G-CSF) by tumor cells.[52–57] Thus, a high level of neutrophil in peripheral blood may indicate tumor-associated inflammation. In our study, patients with NLR ≥4 had lower gross total resection rate. This phenomenon could be explained by studies indicating a possible involvement of inflammation in the process of cyst formation and adhesion to adjacent brain tissue of craniopharyngioma.[56–58] And as mentioned before, such adhesion could make gross total resection more difficult. Besides, microscopically, craniopharyngioma tissue are closely connected with gliosis area induced by inflammatory in the adjacent brain tissues, which may result in recurrence even the patient had received gross total resection of the tumor.[57,59,60]
Gross total resection is associated with a favorable long progression free survival, better QOL and even cure. About 10%–15% of totally removed CP relapsed and nearly 80% of patients were asymptomatic after total tumor resection during a long follow-up period. In the present study, gross total resection was related to better long-term outcome.
It is controversial whether age at diagnosis is associated with long-term outcome. Previous studies have shown that the younger patients had better outcome.[61] On the contrary, some studies have found longer survival in older patients.[43,62] Our result did not present any difference in long-term outcome with respect to age. The prognostic value of sex has not been conclusively established. Some researchers found a higher mortality in females,[39,62] while the others suggested there were no differences between the sexes.[36,38,63] In the present study, our data did not show any difference between the sexes. Besides, tumor size did not relate to 5-year OS and PFS in our research, and the same finding was reported in an article by Yosef et al.[64].
In some patients, gross total resection cannot be achieved without injury to vital structures of hypothalamus. Subtotal resection and radiation therapy are safe and effective approaches to control the tumor and improve long-term outcome of the patients.[65–67] In our cohort, radiation therapy after subtotal resection was linked to OS and PFS.
The present study had several limitations. The first was that the study was a single-center retrospective study and more reliable findings should be confirmed in multi-center prospective cohort. Secondly, in 48 patients who underwent subtotal resection, only 30 of them received radiation therapy for various reasons, so data from radiation therapy is limited.
5. Conclusion
Preoperative NLR may be a simple, readily available, and valid predictor of long-term outcome in craniopharyngioma. It could not only reflect the local inflammatory information of the tumor and provide effective guidance to neurosurgeons for prognostic evaluation, but also suggest further exploration on tumor therapies based upon modulating host immune response. Patients with NLR ≥4 have worse long-term outcome and they are a more reasonable group to receive radiation therapy. However, more studies are warranted to verify our findings and address the underlying mechanisms.
Acknowledgments
We would like to thank the reviewers for their constructive comments.
Author contributions
Conceptualization: Jing Zhang, Min He, Jianguo Xu.
Data curation: Jing Zhang, Min He, Yanlin Song.
Formal analysis: Jing Zhang, Zhiyong Liu, Yanlin Song, Yuelong Wang, Ruichao Liang.
Investigation: Jing Zhang.
Methodology: Jing Zhang, Min He, Zhiyong Liu, Ruichao Liang, Hongxu Chen.
Project administration: Jing Zhang, Jianguo Xu.
Resources: Jing Zhang.
Software: Jing Zhang, Min He, Yanlin Song.
Supervision: Jianguo Xu.
Validation: Jing Zhang.
Visualization: Jing Zhang.
Writing – original draft: Jing Zhang, Min He, Zhiyong Liu, Yanlin Song, Yuelong Wang, Ruichao Liang, Hongxu Chen, Jianguo Xu.
Writing – review & editing: Jing Zhang, Min He, Zhiyong Liu, Yanlin Song, Yuelong Wang, Ruichao Liang, Hongxu Chen, Jianguo Xu.
Footnotes
Abbreviations: ASBS-Q = anterior skull base surgery questionnaire, CP = craniopharyngioma, CT = computed tomography, GBM = glioblastoma multiforme, G-CSF = granulocyte-colony stimulating factor, GTR = gross total resection, HR = hazard ratio, IL-6 = interleukin-6, MRI = magnetic resonance imaging, NLR = neutrophil-lymphocyte ratio, OS = overall survival, PFS = progression free survival, QOL = quality of life, ROC = receiver operator characteristics, TNF = tumor necrosis factor, VEGF = vascular endothelial growth factor.
The authors have no conflicts of interest to disclose.
References
- [1].Muller HL. Craniopharyngioma. Endocr Rev 2014;35:513–43. [DOI] [PubMed] [Google Scholar]
- [2].Muller HL. Craniopharyngioma. Handbook Clin Neurol 2014;124:235–53. [DOI] [PubMed] [Google Scholar]
- [3].Mortini P, Losa M, Pozzobon G, et al. Neurosurgical treatment of craniopharyngioma in adults and children: early and long-term results in a large case series. J Neurosurg 2011;114:1350–9. [DOI] [PubMed] [Google Scholar]
- [4].Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst 2001;17:503–11. [DOI] [PubMed] [Google Scholar]
- [5].Bunin GR, Surawicz TS, Witman PA, et al. The descriptive epidemiology of craniopharyngioma. J Neurosurg 1998;89:547–51. [DOI] [PubMed] [Google Scholar]
- [6].Karavitaki N, Cudlip S, Adams CB, et al. Craniopharyngiomas. Endocrine Rev 2006;27:371–97. [DOI] [PubMed] [Google Scholar]
- [7].Muller HL, Gebhardt U, Teske C, et al. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. Eur J Endocrinol 2011;165:17–24. [DOI] [PubMed] [Google Scholar]
- [8].Muller HL, Gebhardt U, Faldum A, et al. Xanthogranuloma, Rathke's cyst, and childhood craniopharyngioma: results of prospective multinational studies of children and adolescents with rare sellar malformations. J Clin Endocrinol Metab 2012;97:3935–43. [DOI] [PubMed] [Google Scholar]
- [9].Bereket A, Kiess W, Lustig RH, et al. Hypothalamic obesity in children. Obes Rev 2012;13:780–98. [DOI] [PubMed] [Google Scholar]
- [10].Muller HL, Gebhardt U, Etavard-Gorris N, et al. Prognosis and sequela in patients with childhood craniopharyngioma—results of HIT-ENDO and update on KRANIOPHARYNGEOM 2000. Klinische Padiatrie 2004;216:343–8. [DOI] [PubMed] [Google Scholar]
- [11].Vidal S, Kovacs K, Lloyd RV, et al. Angiogenesis in patients with craniopharyngiomas: correlation with treatment and outcome. Cancer 2002;94:738–45. [DOI] [PubMed] [Google Scholar]
- [12].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [13].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [16].Bambury RM, Teo MY, Power DG, et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol 2013;114:149–54. [DOI] [PubMed] [Google Scholar]
- [17].Han S, Liu Y, Li Q, et al. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer 2015;15:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181–4. [DOI] [PubMed] [Google Scholar]
- [19].Keizman D, Gottfried M, Ish-Shalom M, et al. Pretreatment neutrophil-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with ketoconazole: association with outcome and predictive nomogram. Oncologist 2012;17:1508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- [21].Gokhan S, Ozhasenekler A, Mansur Durgun H, et al. Neutrophil lymphocyte ratios in stroke subtypes and transient ischemic attack. Eur Rev Med Pharmacol Sci 2013;17:653–7. [PubMed] [Google Scholar]
- [22].Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis 2012;225:456–60. [DOI] [PubMed] [Google Scholar]
- [23].Eldevik OP, Blaivas M, Gabrielsen TO, et al. Craniopharyngioma: radiologic and histologic findings and recurrence. AJNR Am J Neuroradiol 1996;17:1427–39. [PMC free article] [PubMed] [Google Scholar]
- [24].Petito CK. Craniopharyngioma: prognostic importance of histologic features. AJNR Am J Neuroradiol 1996;17:1441–2. [PMC free article] [PubMed] [Google Scholar]
- [25].Gil Z, Fliss DM. Quality of life in patients with skull base tumors: current status and future challenges. Skull Base 2010;20:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gil Z, Abergel A, Spektor S, et al. Development of a cancer-specific anterior skull base quality-of-life questionnaire. J Neurosurg 2004;100:813–9. [DOI] [PubMed] [Google Scholar]
- [27].Gil Z, Abergel A, Spektor S, et al. Patient, caregiver, and surgeon perceptions of quality of life following anterior skull base surgery. Arch Otolaryngol Head Neck Surg 2004;130:1276–81. [DOI] [PubMed] [Google Scholar]
- [28].Gil Z, Abergel A, Spektor S, et al. Quality of life following surgery for anterior skull base tumors. Arch Otolaryngol Head Neck Surg 2003;129:1303–9. [DOI] [PubMed] [Google Scholar]
- [29].Fahlbusch R, Honegger J, Paulus W, et al. Surgical treatment of craniopharyngiomas: experience with 168 patients. J Neurosurg 1999;90:237–50. [DOI] [PubMed] [Google Scholar]
- [30].Garnett MR, Puget S, Grill J, et al. Craniopharyngioma. Orphanet J Rare Dis 2007;2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Campbell PG, McGettigan B, Luginbuhl A, et al. Endocrinological and ophthalmological consequences of an initial endonasal endoscopic approach for resection of craniopharyngiomas. Neurosurg Focus 2010;28:E8. [DOI] [PubMed] [Google Scholar]
- [32].Cavallo LM, Prevedello DM, Solari D, et al. Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. J Neurosurg 2009;111:578–89. [DOI] [PubMed] [Google Scholar]
- [33].de Divitiis E, Cappabianca P, Cavallo LM, et al. Extended endoscopic transsphenoidal approach for extrasellar craniopharyngiomas. Neurosurgery 2007;61(5 suppl 2):219–27. discussion 228. [DOI] [PubMed] [Google Scholar]
- [34].Dehdashti AR, Ganna A, Witterick I, et al. Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: indications and limitations. Neurosurgery 2009;64:677–87. discussion 687-679. [DOI] [PubMed] [Google Scholar]
- [35].Duff J, Meyer FB, Ilstrup DM, et al. Long-term outcomes for surgically resected craniopharyngiomas. Neurosurgery 2000;46:291–302. discussion 302-295. [DOI] [PubMed] [Google Scholar]
- [36].Karavitaki N, Brufani C, Warner JT, et al. Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf) 2005;62:397–409. [DOI] [PubMed] [Google Scholar]
- [37].Van Effenterre R, Boch AL. Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg 2002;97:3–11. [DOI] [PubMed] [Google Scholar]
- [38].Stripp DC, Maity A, Janss AJ, et al. Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys 2004;58:714–20. [DOI] [PubMed] [Google Scholar]
- [39].Pereira AM, Schmid EM, Schutte PJ, et al. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin Endocrinol (Oxf) 2005;62:197–204. [DOI] [PubMed] [Google Scholar]
- [40].Regine WF, Mohiuddin M, Kramer S. Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol 1993;27:13–21. [DOI] [PubMed] [Google Scholar]
- [41].Bartlett JR. Craniopharyngiomas—a summary of 85 cases. J Neurol Neurosurg Psychiatry 1971;34:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pemberton LS, Dougal M, Magee B, et al. Experience of external beam radiotherapy given adjuvantly or at relapse following surgery for craniopharyngioma. Radiother Oncol 2005;77:99–104. [DOI] [PubMed] [Google Scholar]
- [43].Rajan B, Ashley S, Gorman C, et al. Craniopharyngioma—a long-term results following limited surgery and radiotherapy. Radiother Oncol 1993;26:1–0. [DOI] [PubMed] [Google Scholar]
- [44].Spolverato G, Maqsood H, Kim Y, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol 2015;111:868–74. [DOI] [PubMed] [Google Scholar]
- [45].Xia Z, Liu W, Li S, et al. Expression of matrix metalloproteinase-9, type IV collagen and vascular endothelial growth factor in adamantinous craniopharyngioma. Neurochem Res 2011;36:2346–51. [DOI] [PubMed] [Google Scholar]
- [46].Vaquero J, Zurita M, de Oya S, et al. Expression of vascular permeability factor in craniopharyngioma. J Neurosurg 1999;91:831–4. [DOI] [PubMed] [Google Scholar]
- [47].Elmaci L, Kurtkaya-Yapicier O, Ekinci G, et al. Metastatic papillary craniopharyngioma: case study and study of tumor angiogenesis. Neuro Oncol 2002;4:123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].De Vile CJ, Grant DB, Kendall BE, et al. Management of childhood craniopharyngioma: can the morbidity of radical surgery be predicted? J Neurosurg 1996;85:73–81. [DOI] [PubMed] [Google Scholar]
- [49].Frank G, Pasquini E, Doglietto F, et al. The endoscopic extended transsphenoidal approach for craniopharyngiomas. Neurosurgery 2006;59(1 suppl 1): ONS75-83; discussion ONS75-83. [DOI] [PubMed] [Google Scholar]
- [50].Laws ER, Weiss MH, White WL. Craniopharyngioma. Skull Base 2003;13:55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Minamida Y, Mikami T, Hashi K, et al. Surgical management of the recurrence and regrowth of craniopharyngiomas. J Neurosurg 2005;103:224–32. [DOI] [PubMed] [Google Scholar]
- [52].Ulich TR, del Castillo J, Guo KZ. In vivo hematologic effects of recombinant interleukin-6 on hematopoiesis and circulating numbers of RBCs and WBCs. Blood 1989;73:108–10. [PubMed] [Google Scholar]
- [53].Ulich TR, del Castillo J, Guo K, et al. The hematologic effects of chronic administration of the monokines tumor necrosis factor, interleukin-1, and granulocyte-colony stimulating factor on bone marrow and circulation. Am J Pathol 1989;134:149–59. [PMC free article] [PubMed] [Google Scholar]
- [54].Lord BI, Bronchud MH, Owens S, et al. The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc Natl Acad Sci U S A 1989;86:9499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ulich TR, del Castillo J, Keys M, et al. Kinetics and mechanisms of recombinant human interleukin 1 and tumor necrosis factor-alpha-induced changes in circulating numbers of neutrophils and lymphocytes. J Immunol 1987;139:3406–15. [PubMed] [Google Scholar]
- [56].Pettorini BL, Inzitari R, Massimi L, et al. The role of inflammation in the genesis of the cystic component of craniopharyngiomas. Childs Nerv Syst 2010;26:1779–84. [DOI] [PubMed] [Google Scholar]
- [57].Mori M, Takeshima H, Kuratsu J. Expression of interleukin-6 in human craniopharyngiomas: a possible inducer of tumor-associated inflammation. Int J Mol Med 2004;14:505–9. [PubMed] [Google Scholar]
- [58].Zhou J, Qi ST, Chen LG, et al. Expression pattern of inflammatory cytokines at various inflammatory levels of adamantinomatous craniopharyngioma. Zhonghua Yi Xue Za Zhi 2013;93:2499–501. [PubMed] [Google Scholar]
- [59].Kawamata T, Kubo O, Hori T. Histological findings at the boundary of craniopharyngiomas. Brain Tumor Pathol 2005;22:75–8. [DOI] [PubMed] [Google Scholar]
- [60].Kasai H, Hirano A, Llena JF, et al. A histopathological study of craniopharyngioma with special reference to its stroma and surrounding tissue. Brain Tumor Pathol 1997;14:41–5. [DOI] [PubMed] [Google Scholar]
- [61].Bulow B, Attewell R, Hagmar L, et al. Postoperative prognosis in craniopharyngioma with respect to cardiovascular mortality, survival, and tumor recurrence. J Clin Endocrinol Metab 1998;83:3897–904. [DOI] [PubMed] [Google Scholar]
- [62].Yasargil MG, Curcic M, Kis M, et al. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg 1990;73:3–11. [DOI] [PubMed] [Google Scholar]
- [63].Tomita T, Bowman RM. Craniopharyngiomas in children: surgical experience at Children's Memorial Hospital. Childs Nerv Syst 2005;21:729–46. [DOI] [PubMed] [Google Scholar]
- [64].Yosef L, Ekkehard KM, Shalom M. Giant craniopharyngiomas in children: short- and long-term implications. Childs Nerv Syst 2016;32:79–88. [DOI] [PubMed] [Google Scholar]
- [65].Harrabi SB, Adeberg S, Welzel T, et al. Long term results after fractionated stereotactic radiotherapy (FSRT) in patients with craniopharyngioma: maximal tumor control with minimal side effects. Rad Oncol (London, England) 2014;9:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Greenfield BJ, Okcu MF, Baxter PA, et al. Long-term disease control and toxicity outcomes following surgery and intensity modulated radiation therapy (IMRT) in pediatric craniopharyngioma. Radiother Oncol 2015;114:224–9. [DOI] [PubMed] [Google Scholar]
- [67].Scott RM, Hetelekidis S, Barnes PD, et al. Surgery, radiation, and combination therapy in the treatment of childhood craniopharyngioma—a 20-year experience. Pediatr Neurosurg 1994;21(suppl 1):75–81. [DOI] [PubMed] [Google Scholar]


