Abstract
Gastroesophageal reflux (GER) is the intermittent or permanent passage of stomach content into the esophagus and gastroesophageal reflux disease (GERD) is the reflux which triggers a whole set of symptoms or complications. The study compares the 24-hours esophageal pH-metry, used for diagnosis of the GERD, with the esophagitis degree observed at the upper digestive endoscopy.
72 children were included, aged over 4 years old, admitted in a pediatric gastroenterology regional center in Northeast Romania, diagnosed with GERD by 24 hours pH-metry (with a positive Boix–Ochoa score), which also underwent the upper digestive endoscopy.
Out of the 72 children diagnosed with GERD, 47 (65.28%) had grade A esophagitis and 25 (34.72%) grade B esophagitis. In GERD associated with grade B esophagitis the Boix–Ochoa score is statistically significant higher, compared with the GERD associated with grade A esophagitis (F = 9.76, P = .0036, 95% CI).
Upper digestive endoscopy performed in patients with gastroesophageal reflux disease shows the constant presence of esophagitis at all patients. There were only grade A and B esophagitis due to the fact that they are young patients with a relative short history of the disease. The correlation tests show a perfect parallel between the pH-metry scores and the endoscopic lesion. The correlation is so accurate that the pH-metry scores can be sufficient to prove GERD and the grade of esophagitis, the upper digestive endoscopy being reserved only for the cases that does not respond to the medical treatment or have other complications.
Keywords: Boix–Ochoa score, children, esophageal pH-metry, esophagitis, gastroesophageal reflux disease, upper endoscopy
1. Introduction
Gastroesophageal reflux (GER) is define as the intermittent or permanent passage of the stomach content into the esophagus and gastroesophageal reflux disease (GERD) as the reflux which triggers a whole set of symptoms or complications.[1]
Symptoms associated with GERD are classified as digestive, respiratory, neurobehavioral symptoms, or the absence of any symptoms. Digestive conditions of GERD include postprandial regurgitation and rumination syndrome in infancy, vomiting, dysphagia and abdominal or retrosternal pain. The respiratory conditions can include chronic cough and laryngitis, wheezing in infancy, aspiration pneumonia, episodes of obstructive apnea, asthma, pharyngitis, sinusitis, and recurrent otitis media. Sleep disorders, episodes of agitation and crying, arching and rigidity, neck hyperextension, irritability, convulsions, and Sandifer syndrome in older children are possible neurobehavioral manifestation in GERD.[2–4]
The diagnostic of GERD can be shown clinically by the symptoms of patients or by the esophageal injuries found by performing upper digestive endoscopy (different degrees of esophagitis, peptic stricture, Barrett esophagus, or adenocarcinoma).[5]
Esophageal pH-metry was initially introduced in 1969 and it was considered the gold standard for the diagnosis of GERD since the 1980s.[6]
2. Material and methods
A correlational study between esophageal pH-metry and esophagitis was conducted on a group of 72 children, aged over 4 years old, admitted in a pediatric gastroenterology regional center in Northeast Romania, diagnosed with GERD by 24 hours esophageal pH-metry, which also underwent the upper digestive endoscopy. Results were interpreted using the Boix–Ochoa score. Informed consent was obtained from all patients/caregivers, and the “St. Mary” Children Emergency Hospital Ethics Committee's approval was obtained for publishing this study.
The indications for 24 hours pH monitoring involved a diagnostic uncertainty. The patients had atypical symptoms, especially respiratory, unresponsive to usual therapy, such as cough, hoarseness, sore throat, atypical chest pain and asthma.[7]
Some exclusion criteria were applied: previous therapy to eradicate Helicobacter pylori, proton pump inhibitors treatment in the last 3 months, concomitant consumption of aspirin and nonsteroidal anti-inflammatory drugs, patients with endoscopic evidence of active gastrointestinal bleeding, presence of esophageal stricture or esophagitis secondary to systemic diseases or any past history of gastric or esophageal surgery.[8]
To determine the pH, we used Medtronic Digitrapper pH 100, SN 37660, with Polygram Net TM pH Testing Application and Zinetics 24 and ComforTec by Sandhill multiuse catheters. The sensor was positioned 5 cm above the lower esophageal sphincter. Continuous pH recording was performed for 24 hours. The meals periods were excluded. Current consensuses show that the total percentage of time the pH is below 4 is the most useful single discriminator between physiologic and pathologic reflux.[7–9] The Boix–Ochoa score was used to calculate the following distal pH variables: number of acid refluxes longer than 5 minutes, longest acid reflux, fraction of total time pH below 4, fraction of upright time pH below 4, fraction of supine time pH below 4, and fraction of prone time pH below 4. The Boix–Ochoa score is developed for infant/pediatric usage.[7] A normal score is a score below 11.99.
During the study, the children took no medications that could interfere with the results and consumed an unrestricted diet.
All study patients underwent upper gastrointestinal endoscopic examinations. Intravenous sedation was given and standard upper gastrointestinal endoscopy, using the Olympus and Pentax video pediatric gastroduodenoscopes was performed to identify evidence of macroscopic abnormalities. Endoscopy was performed under general anesthesia in children aged below 10 years.[8]
The endoscopic findings of reflux esophagitis in the lower esophagus were classified according to the Los Angeles classification system. Esophagitis was graded by endoscopy: grade A, one (or more) mucosal break no longer than 5 mm, that does not extend between the tops of 2 mucosal folds; grade B, one (or more) mucosal break more than 5 mm long that does not extend between the tops of 2 mucosal folds; grade C, one (or more) mucosal break that is continuous between the tops of 2 or more mucosal folds but which involves less than 75% of the circumference; grade D, one (or more) mucosal break which involves at least 75% of the esophageal circumference.[8,10]
SPSS 20 was used for the statistical data processing. For the correlation analysis, the Pearson parametric correlation was used and the correlation coefficients were calculated for a confidence interval (CI) of 95%. The predictive power of the Boix–Ochoa score was assessed based on the ROC curve for grade A esophagitis and grade B esophagitis.
3. Results
3.1. GERD and esophagitis degree
A total of 72 children aged more than 4 years old were investigated. Upper endoscopy evaluation revealed only grade A and B esophagitis (Table 1). Grade C and D esophagitis, also Barrett esophagus were not found in any patient.
Table 1.
Esophagitis grade in GERD.
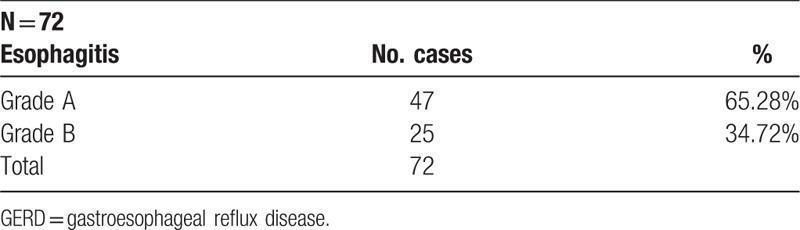
Around 47 patients (65.28%), meaning 2/3 of all, had the aspect of grade A esophagitis and, only 1/3 (34.72%) have lesions of grade B esophagitis, meaning 25 patients.
3.2. Correlation of pH-metry with the endoscopic lesion
Using the Boix–Ochoa score there is a strong correlation between the pH-metry values and the grade of esophagitis. The average score is practically double (56.75) at grade B esophagitis compared to 25.37 that is found in grade A esophagitis (Table 2); also, with a high level of confidence revealed by the ANOVA test (Table 3).
Table 2.
Statistical indicators of the Boix–Ochoa score regarding the grade of esophagitis.

Table 3.
Test for average the Boix–Ochoa score values versus grade of esophagitis.
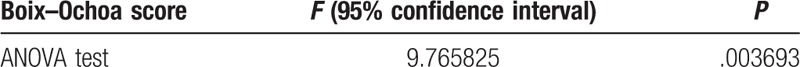
The graphical representation of the Boix–Ochoa score values regarding the grade of esophagitis, clearly proves, that in GERD with grade B esophagitis the Boix–Ochoa score is statistically significantly higher, compared to the value obtained in GERD with grade A esophagitis (F = 9.76, P = .0036, 95%CI) (Fig. 1).
Figure 1.

Statistical indicators of Boix–Ochoa score regarding the grade of esophagitis.
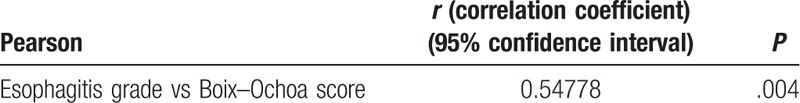
The same result is obtained using the Pearson correlation test, which shows a very strong correlation between the grade of esophagitis and the Boix–Ochoa score (Table 4).
Table 4.
Pearson correlation test—grade of esophagitis versus the Boix–Ochoa score.
The correlational analysis proves the fact that the Boix–Ochoa score values can predict, for sure, the grade of esophagitis in GERD (r = 0.547, P = .004, 95%CI) (Fig. 2).
Figure 2.
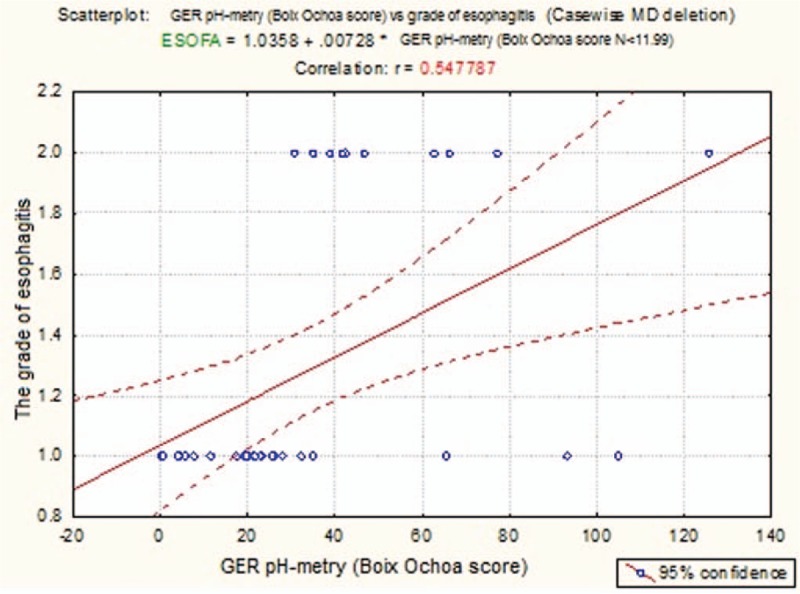
Regression line esophagitis grade versus the Boix–Ochoa score.
The large area under ROC curve (AUC—area under the ROC Curve) (AUC = 0.9111; 95% CI: 0.8692–0.9530, P < .001) shows the high diagnostic value of grade A esophagitis based on the Boix–Ochoa score (Fig. 3). The calculated reference value for Boix–Ochoa score in case of grade A esophagitis was 19.4, with a sensitivity of 93.3% and specificity of 78.6%. The positive predictive value was 81.3% and the negative predictive value 92.2% (Table 5).
Figure 3.
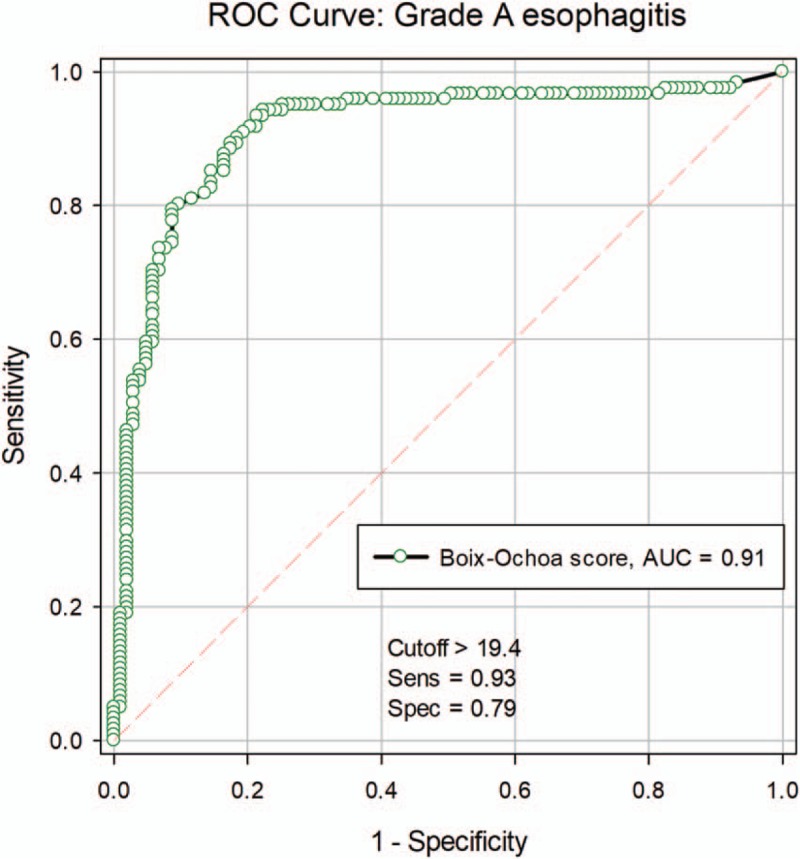
ROC curve for grade A esophagitis. ROC = receiver operating characteristics.
Table 5.
Associated parameters for grade A esophagitis.

The large area under ROC curve (AUC—area under the ROC curve) (AUC = 0.949; 95% CI: 0.9204–0.9777, P < .001) shows the high diagnostic value of grade B esophagitis based on the Boix–Ochoa score (Fig. 4). The calculated reference value for Boix–Ochoa score in case of grade B esophagitis was 53.9, with a sensitivity of 94.4% and specificity of 82.98%. The positive predictive value was 84.7% and the negative predictive value 93.7% (Table 6).
Figure 4.
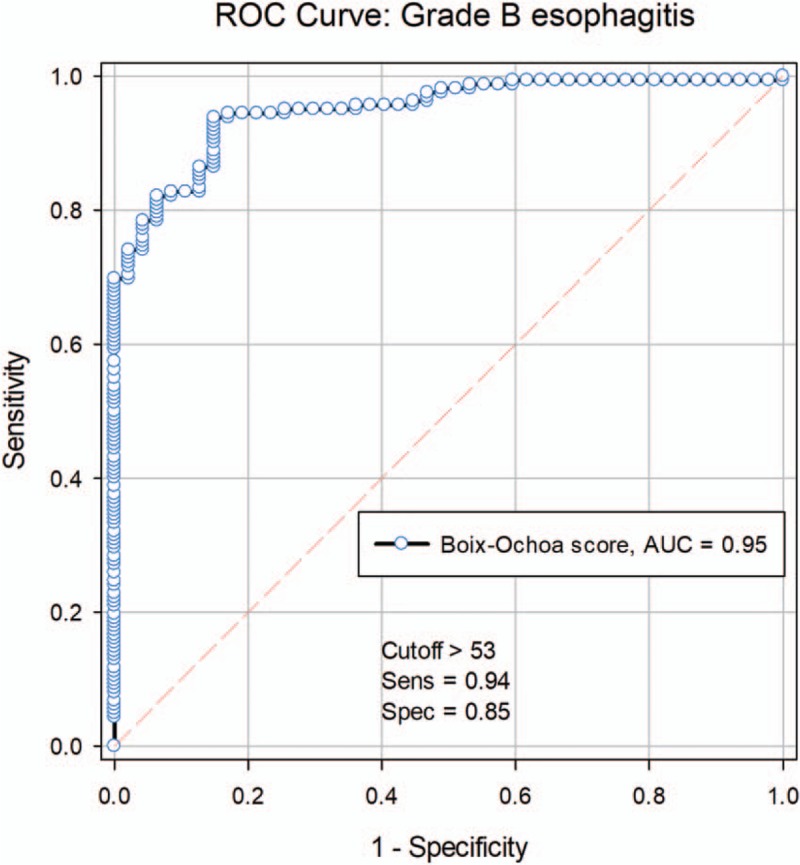
ROC curve for grade B esophagitis. ROC = receiver operating characteristics.
Table 6.
Associated parameters for grade B esophagitis.

4. Discussions
The reported prevalence of GERD in patients of all ages worldwide is increasing.[11] The current prevalence of GERD in Western Europe and North America is estimated to be 10% to 20%.[12]
The most important advantages of 24-hour esophageal pH-metry are the capacity to evaluate the correlation of acid reflux with symptoms and to measure the duration and frequency of acid reflux episodes.[13]
PH-metry is often performed, despite its major limitation, the inability to detect nonacid reflux (pH above 4). This test is not accurate for the detection of reflux episodes in patients undergoing acid suppressive treatment.[14] The results of pH-metry are affected by the ingestion of acidic foods and drinks.[14]
We performed upper digestive endoscopy in all children because this allows direct visual examination of the esophageal mucosa. Endoscopic findings in patients with GERD include esophagitis, erosions, exudates, ulcers, strictures, hiatus hernia, and adenocarcinoma. This may be a useful tool to assess GER in children with other signs and symptoms suggestive of GERD such as apnea or apparent life-threatening event, reactive airways disease, recurrent pneumonia, asthma, dental erosions or Sandifer syndrome. In our study, we found only grade A and B esophagitis, without any other type of lesions. The fact that, there were no more advanced esophagitis lesions can be hypothetically explained by the age of the patients and the duration of the disease from its debut until endoscopy. Also, the previous treatment attempts with PPIs (more than last 3 months) may have influenced this situation.
Recent consensus guidelines define reflux esophagitis as the presence of endoscopic visible breaks in the esophageal mucosa at, or immediately above the gastroesophageal junction.[15,16] The identification of esophagitis with upper gastrointestinal endoscopy has specificity 90%–95% for GERD[15] but has a poor sensitivity of around 50%.[17]
All our patients received initially home treatment for reflux esophagitis for 6–8 weeks with proton pump inhibitors (PPIs) (esomeprazole or pantoprazole). High doses of PPIs are necessary in patients with higher grades of esophagitis or when there are conditions that can determine severe GERD.[1,18] In our study, if the symptoms of children have not completely resolved, the treatment has been extended for a longer period of time (another 6–8 weeks). When patients are with high grades of esophagitis at first upper digestive endoscopy, or they have persistent symptoms after an adequate treatment, or in case of atypical symptomatology, we can follow up performing another endoscopy.[1,18]
Study limits are dictated by monitoring body position in young children in a hospital ward, which presents considerable practical difficulties, as few children maintain any position for very long. Also, pH-monitoring alone cannot diagnose alkaline reflux.[7]
5. Conclusions
Upper digestive endoscopy performed in patients with gastroesophageal reflux disease shows the constant presence of esophagitis at all patients. There were only grade A and B esophagitis due to the fact that they are young patients with a relative short history of the disease. The correlation tests show a perfect parallel between the pH-metry scores and the endoscopic lesion. The correlation is so accurate, that the pH-metry scores can be sufficient to prove the disease and the grade of esophagitis, the upper digestive endoscopy being reserved only for the cases that does not respond to the medical treatment or have other complications. Based on this correlation between the pH-metry scores and the endoscopic lesions, a correlation table can be drawn, that would be a useful tool for the practitioner in the therapeutic approach of this disease.
Acknowledgments:
We would like to thank the Endoscopy Department's staff at “St. Mary” Children's Emergency Hospital and the Vth Pediatric Clinic's staff for their help.
Author contributions
Conceptualization: Vasile Valeriu Valeriu Lupu, Marin Burlea.
Data curation: Vasile Valeriu Valeriu Lupu, Violeta Streanga, Magdalena Starcea, Gabriela Paduraru, Elena Cristina Mitrofan, Mihaela Moscalu, Ancuta Ignat.
Formal analysis: Magdalena Starcea, Dragos Catalin Ghica, Elena Cristina Mitrofan, Mihaela Moscalu.
Investigation: Vasile Valeriu Valeriu Lupu, Nicolai Nistor, Violeta Streanga, Magdalena Starcea, Gabriela Paduraru, Elena Cristina Mitrofan, Ancuta Ignat.
Methodology: Vasile Valeriu Valeriu Lupu, Dragos Catalin Ghica, Ancuta Ignat.
Software: Dragos Catalin Ghica, Mihaela Moscalu.
Supervision: Marin Burlea.
Validation: Marin Burlea, Nicolai Nistor.
Writing – original draft: Vasile Valeriu Valeriu Lupu, Ancuta Ignat.
Writing – review & editing: Vasile Valeriu Valeriu Lupu, Marin Burlea, Nicolai Nistor, Violeta Streanga, Magdalena Starcea, Gabriela Paduraru, Dragos Catalin Ghica, Elena Cristina Mitrofan, Mihaela Moscalu, Ancuta Ignat.
Footnotes
Abbreviations: GER = gastroesophageal reflux, GERD = gastroesophageal reflux disease, NPV = negative predictive value, PPV = positive predictive value, ROC = receiver operating characteristics.
All authors contributed equally to this paper.
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. North American Society for Pediatric Gastroenterology Hepatology and Nutrition, European Society for Pediatric Gastroenterology Hepatology and Nutrition. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr 2009;49:498–547. [DOI] [PubMed] [Google Scholar]
- [2].Kiljander TO, Junghard O, Beckman O, et al. Effect of esomeprazole 40 mg once or twice daily on asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 2010;181:1042–8. [DOI] [PubMed] [Google Scholar]
- [3].Chan WW, Chiou E, Obstein KL, et al. The efficacy of proton pump inhibitors for the treatment of asthma in adults: a meta-analysis. Arch Intern Med 2011;171:620–9. [DOI] [PubMed] [Google Scholar]
- [4].Chen CH, Lin CL, Kao CH. Association between gastroesophageal reflux disease and coronary heart disease: a nationwide population-based analysis. Medicine (Baltimore) 2016;95:e4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lightdale JR, Gremse DA. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics 2013;131:e1684–95. [DOI] [PubMed] [Google Scholar]
- [6].Spencer J. Prolonged pH recording in the study of gastro-oesophageal reflux. Br J Surg 1969;56:912–4. [DOI] [PubMed] [Google Scholar]
- [7].Lupu VV, Ignat A, Paduraru G, et al. Correlation between the different pH-metry scores in gastroesophageal reflux disease in children. Medicine (Baltimore) 2016;95:e3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lupu VV, Ignat A, Ciubotariu G, et al. Helicobacter pylori infection and gastroesophageal reflux in children. Dis Esophagus 2016;29:1007–12. [DOI] [PubMed] [Google Scholar]
- [9].Pandolfino JE, Vela MF. Esophageal-reflux monitoring. Gastrointest Endosc 2009;69:917–30. [DOI] [PubMed] [Google Scholar]
- [10].Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sherman PM, Hassall E, Fagundes-Neto U, et al. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. Am J Gastroenterol 2009;104:1278–95. [DOI] [PubMed] [Google Scholar]
- [12].Dent J, El-Serag HB, Wallander MA, et al. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2005;54:710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shin MS. Esophageal pH and combined impedance-pH monitoring in children. Pediatr Gastroenterol Hepatol Nutr 2014;17:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Van Wijk MP, Benninga MA, Omari TI. Role of the multichannel intraluminal impedance technique in infants and children. J Pediatr Gastroenterol Nutr 2009;48:2–12. [DOI] [PubMed] [Google Scholar]
- [15].Richter JE. Diagnostic tests for gastroesophageal reflux disease. Am J Med Sci 2003;326:300–8. [DOI] [PubMed] [Google Scholar]
- [16].Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900–20. quiz 194. [DOI] [PubMed] [Google Scholar]
- [17].Dent J, Brun J, Fendrick AM, et al. on behalf of the Genval Workshop Group. An evidence-based appraisal of reflux disease management—the Genval Workshop Report. Gut 1999;44(Suppl 2):S1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goldani HAS, Nunes DLA, Ferreira CT. Managing gastroesophageal reflux disease in children: the role of endoscopy. World J Gastrointest Endosc 2012;4:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


