Abstract
Background
Oesophagectomy has a high risk of postoperative morbidity. The impact of postoperative complications on overall survival of oesophageal cancer remains unclear. This meta‐analysis addressed the impact of complications on long‐term survival following oesophagectomy.
Methods
A search of PubMed and Cochrane Library databases was undertaken for systematic review of papers published between January 1995 and August 2016 that analysed the relation between postoperative complications and long‐term survival. In the meta‐analysis, data were pooled. The main outcome was overall survival (OS). Secondary endpoints included disease‐free (DFS) and cancer‐specific (CSS) survival.
Results
A total of 357 citations was reviewed; 21 studies comprising 11 368 patients were included in the analyses. Overall, postoperative complications were associated with significantly decreased 5‐year OS (hazard ratio (HR) 1·16, 95 per cent c.i. 1·06 to 1·26; P = 0·001) and 5‐year CSS (HR 1·27, 1·09 to 1·47; P = 0·002). Pulmonary complications were associated with decreased 5‐year OS (HR 1·37, 1·16 to 1·62; P < 0·001), CSS (HR 1·60, 1·35 to 1·89; P < 0·001) and 5‐year DFS (HR 1·16, 1·00 to 1·33; P = 0·05). Patients with anastomotic leakage had significantly decreased 5‐year OS (HR 1·20, 1·10 to 1·30; P < 0·001), 5‐year CSS (HR 1·81, 1·11 to 2·95; P = 0·02) and 5‐year DFS (HR 1·13, 1·02 to 1·25; P = 0·01).
Conclusion
Postoperative complications after oesophagectomy, including pulmonary complications and anastomotic leakage, decreased long‐term survival.
Introduction
Worldwide, oesophageal cancer is the fifth most common cause of cancer‐related death in men, and the eighth in women1. The postoperative 5‐year survival rate in patients with AJCC stage I oesophageal cancer is approximately 90 per cent, and decreases to 45, 20 and 10 per cent in patients with stage II, III and IV disease respectively2 3. For most patients without distant metastases, oesophagectomy is still the mainstay of cancer treatment with or without chemoradiotherapy4. Despite advances in surgical techniques and perioperative management5, oesophagectomy is a highly invasive procedure associated with serious postoperative complications3. In a Japanese national database comprising 5354 patients who underwent oesophagectomy in 2011 in 713 hospitals, the overall morbidity rate was 41·9 per cent, and 30‐day and surgery‐related mortality rates were 1·2 and 3·4 per cent respectively6.
The impact of postoperative complications on long‐term survival has been investigated for many cancers3 7, 8. In some studies3 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, a negative impact of complications following oesophagectomy on long‐term survival was reported. In other studies3 18, 19 22, 23, 24, 25, 26, 27, 28, 29, complications did not affect long‐term survival. Meta‐analyses focusing on the long‐term impact of postoperative complications are not available. A systematic review and meta‐analysis was therefore performed to assess the impact of postoperative complications on long‐term survival after oesophagectomy.
Methods
A systematic review and meta‐analysis was carried out in accordance with the MOOSE criteria30. The key clinical question was: ‘Do postoperative complications after oesophagectomy for oesophageal cancer impact survival?’. A systematic literature search of studies describing clinical trials published from January 1995 to August 2016 was conducted. Literature searches of the PubMed and Cochrane Library databases were conducted using the search formula: (‘esophageal cancer’ OR ‘esophageal neoplasms’ OR ‘esophageal squamous cell carcinoma’) AND (‘esophagectomy’ OR ‘resection’ OR ‘surgery’) AND (‘anastomotic leakage’ OR ‘lung disease’ OR ‘pneumonia’ OR ‘postoperative complications’ OR ‘postoperative morbidity’ OR ‘pulmonary complications’ OR ‘respiratory tract disease’) AND (‘survival’ OR ‘disease free survival’ OR ‘mortality’ OR ‘prognosis’ OR ‘hospital mortality’ OR ‘neoplasm recurrence’).
Eligibility criteria
RCTs and observational studies, including all types of operation (such as salvage surgery) and all types of neoadjuvant or adjuvant therapy, comparing the long‐term survival of patients with or without postoperative oesophagectomy complications were eligible for inclusion. Postoperative pulmonary complications, anastomotic leakage and the total number of postoperative oesophagectomy complications were included in the analysis. Other complications such as recurrent laryngeal nerve paralysis or atrial fibrillation were excluded. Articles for which the full text was not available in English were excluded.
Data extraction
Data were extracted by one author and one reviewer from the Japan Medical Library Association. Any discrepancies were dealt with by discussion among all authors until consensus was reached. The primary outcome was 5‐year overall survival (OS) and secondary outcomes included disease‐free (DFS) and cancer‐specific (CSS) survival rates, which were extracted from the Kaplan–Meier curves in each study. The GRADE guidelines31 were used to evaluate the quality of individual studies, considering risk of bias, inconsistency, indirectness, imprecision, publication bias, size of effect, dose‐dependent gradient and plausible confounders. Studies assessed as of high quality in GRADE were included in the qualitative synthesis. It was expected that some studies would and others would not have included postoperative mortality. If some studies including postoperative mortality were excluded from the meta‐analysis, the sample size for each comparison would have been smaller, and the results would have been meaningless; these studies were therefore included in the meta‐analysis.
Statistical analysis
Analyses were performed using Review Manager® version 5.3 software (The Cochrane Collaboration, Oxford, UK). Pooled analysis was performed using a Mantel–Haenszel model, and the values were reported as hazard ratios (HRs) with 95 per cent confidence intervals. The significance of pooled HRs was determined by the Z test. P < 0·050 was considered statistically significant.
Statistical heterogeneity for each pooled estimate was assessed using Cochran's χ2 statistic and quantified with the I 2 statistic. An I 2 value exceeding 50 per cent was considered to indicate heterogeneity. When heterogeneity was detected, a random‐effects model was adopted; when heterogeneity was not observed, a fixed‐effect model was used.
Results
Search results
A total of 357 potentially relevant studies were identified, of which 32 were eligible for full‐text review (Fig. 1). Twenty‐one studies3 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 23, 24, 25, 26, 27, 28, 29 met the eligibility criteria for qualitative synthesis (Table 1), and 203,9,10,12–21,23–29 were eventually included for quantitative synthesis. The study that did not report 5‐year outcome results was excluded from the quantitative synthesis11. One study19 was a randomized trial; the others were observational studies.
Figure 1.
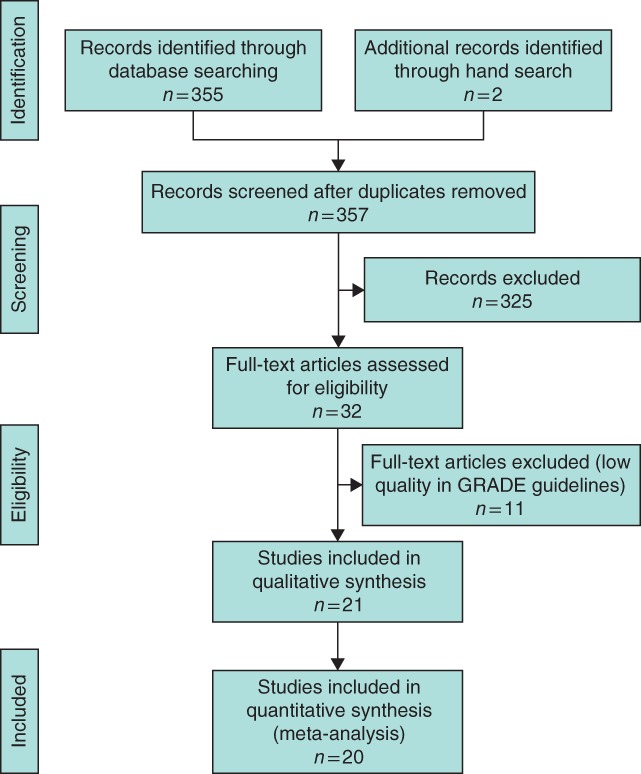
Flow diagram of inclusion and exclusion criteria for the study
Table 1.
Characteristics of included studies
| Reference | Year | Complication | No. with complications | No. without complications |
|---|---|---|---|---|
| Hirai et al. 12 | 1998 | Any | 47 | 100 |
| Kinugasa et al. 9 | 2004 | Pulmonary | 38 | 80 |
| Rizk et al. 10 | 2004 | Any | 138 | 372 |
| Abou‐Jawde et al. 13 | 2005 | Pulmonary | 18 | 123 |
| Junemann‐Ramirez et al. 23 | 2005 | Anastomotic leak | 9 | 251 |
| Martin et al. 28 | 2005 | Anastomotic leak | 30 | 446 |
| Ancona et al. 24 | 2006 | Pulmonary | 110 | 327 |
| Any | 85 | 437 | ||
| Ferri et al. 26 | 2006 | Any | 98 | 336 |
| Lerut et al. 11 | 2009 | Any | 97 | 41 |
| Hu et al. 14 | 2010 | Any | 90 | 271 |
| D'Annoville et al. 25 | 2012 | Pulmonary | 118 | 223 |
| Xia et al. 29 | 2013 | Any | 72 | 99 |
| Lindner et al. 27 | 2014 | |||
| Adenocarcinoma | Any | 14 | 49 | |
| Squamous cell carcinoma | Any | 7 | 15 | |
| Booka et al. 3 | 2015 | Pulmonary | 64 | 220 |
| Anastomotic leak | 55 | 229 | ||
| Markar et al. 15 | 2015 | Anastomotic leak | 208 | 2231 |
| Doorakkers et al. 16 | 2015 | Any | 75 | 221 |
| Luc et al. 17 | 2015 | Any | 16 | 95 |
| Baba et al. 18 | 2016 | Pulmonary | 99 | 403 |
| Any | 217 | 285 | ||
| Yamashita et al. 21 | 2016 | Pulmonary | 22 | 233 |
| Anastomotic leak | 6 | 249 | ||
| Any | 104 | 151 | ||
| Kataoka et al. 19 | 2017 | Pulmonary | 22 | 130 |
| Anastomotic leak | 21 | 131 | ||
| Saeki et al. 20 | 2017 | |||
| Stage 0–2 | Pulmonary | 44 | 360 | |
| Anastomotic leak | 88 | 316 | ||
| Stage 3–4 | Pulmonary | 15 | 161 | |
| Anastomotic leak | 26 | 150 | ||
| Stage 0–4 | Any | 154 | 426 |
The severity of postoperative complications was based on each study, and there was variability on grading of the severities. Almost all studies categorized the severity of postoperative complications using the Clavien–Dindo classification32.
The range in 1‐, 3‐ and 5‐year OS rates for patients with complications was 47–84 per cent27 29, 18–84 per cent27 29 and 8–84 per cent27 29 respectively. For patients without complications, the respective rates were 70–90 per cent18 29, 30–71 per cent18 29 and 10–66 per cent27 29. The range in 1‐, 3‐, and 5‐year OS rates for patients with pulmonary complications was 28–87 per cent13 19, 22–59 per cent13 19 and 6–41 per cent13 20 respectively, compared with 58–96 per cent13 19, 36–78 per cent13 20 and 29–65·7 per cent13 20 for those without pulmonary complications. The range in 1‐, 3‐ and 5‐year OS rates for patients with anastomotic leakage was 66–86 per cent19 23, 20–58 per cent20 and 15–57 per cent19 20 respectively, compared with 64–94 per cent19 23, 30–79 per cent20 23 and 24–68 per cent20 23 for those without anastomotic leakage. Of the 20 studies included in the quantitative synthesis, seven15, 16, 17 20, 23 25, 27 excluded and 133,9,10,12–14,18,19,21,24,26,28,29 included perioperative mortality.
Impact of pulmonary complications on survival
The impact of pulmonary complication on OS, CSS and DFS was evaluated in seven studies3 9, 13 18, 19, 20 24 including 2214 patients (Fig. 2 a), three studies18 20, 21 including 1337 patients (Fig. 3 a) and three studies3 19, 25 including 777 patients (Fig. 4 a) respectively. Patients with pulmonary complications had significantly decreased 5‐year OS (HR 1·37, 95 per cent c.i. 1·16 to 1·62; P < 0·001), 5‐year CSS (HR 1·60, 1·35 to 1·89; P < 0·001) and 5‐year DFS (HR 1·16, 1·00 to 1·33; P = 0·05).
Figure 2.
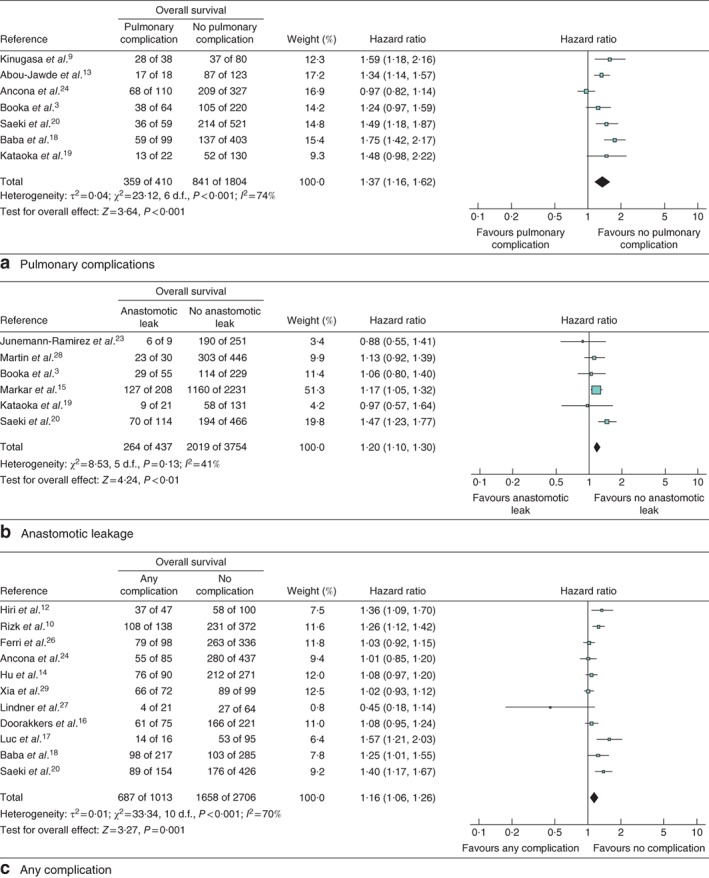
Forest plot comparing overall survival following oesophagectomy in patients with and without a pulmonary complications, b anastomotic leakage and c any complication. Mantel–Haenszel random‐effects (a,c) or fixed‐effect (b) models were used for meta‐analysis. Hazard ratios are shown with 95 per cent confidence intervals
Figure 3.
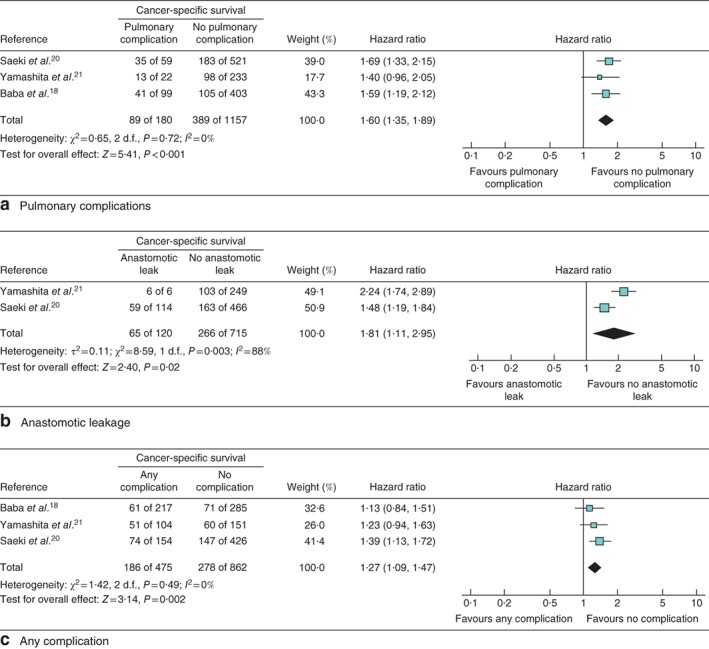
Forest plot comparing cancer‐specific survival following oesophagectomy in patients with and without a pulmonary complications, b anastomotic leakage and c any complication. Mantel–Haenszel fixed‐effect (a,c) or random‐effects (b) models were used for meta‐analysis. Hazard ratios are shown with 95 per cent confidence intervals
Figure 4.
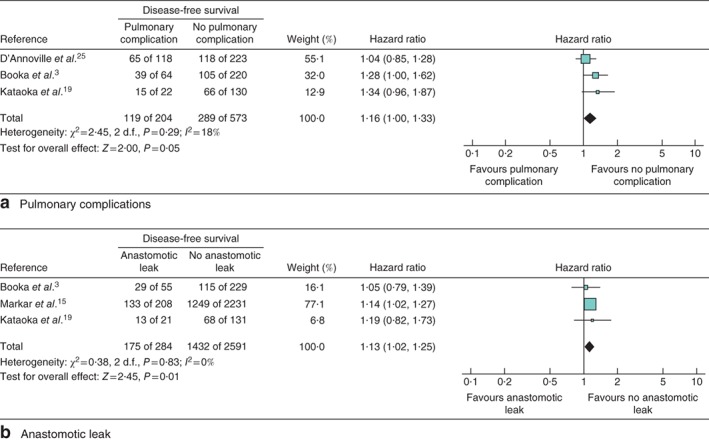
Forest plot comparing disease‐free survival following oesophagectomy in patients with and without a pulmonary complications and b anastomotic leakage. A Mantel–Haenszel fixed‐effect model was used for meta‐analysis. Hazard ratios are shown with 95 per cent confidence intervals
Impact of anastomotic leakage on survival
The impact of anastomotic leakage on OS, CSS and DFS was evaluated in six studies3 15, 19 20, 23 28 including 4191 patients (Fig. 2 b), two studies20 21 including 835 patients (Fig. 3 b) and three studies3 15, 19 including 2875 patients (Fig. 4 b) respectively. Patients with anastomotic leakage had significantly decreased 5‐year OS (HR 1·20, 95 per cent c.i. 1·10 to 1·30; P < 0·001), 5‐year CSS (HR 1·81, 1·11 to 2·95; P = 0·02) and 5‐year DFS (HR 1·13, 1·02 to 1·25; P = 0·01).
Impact of overall complications on survival
The impact of postoperative complications in general on OS and CSS was evaluated in 11 studies10 12, 14 16, 17, 18 20, 24 26, 27 29 including 3719 patients (Fig. 2 c) and three studies18 20, 21 including 1337 patients (Fig. 3 c) respectively. There was no study investigating the impact of overall postoperative complications on DFS. Patients with more complications had significantly worse 5‐year OS (HR 1·16, 95 per cent c.i. 1·06 to 1·26; P = 0·001) and 5‐year CSS (HR 1·27, 1·09 to 1·47; P = 0·002).
Impact of postoperative oesophagectomy complications on type of recurrence
Of the 21 eligible studies, four12 15, 17 21 investigated the impact of postoperative complications on recurrence type. One15 of these studies investigated the impact of anastomotic leakage on recurrence type and found it to be independently associated with locoregional and mixed recurrence (simultaneous local and distant recurrence) but not distant recurrence. The other three studies12 17, 21 investigated the impact of overall complications on recurrence pattern. Meta‐analysis revealed that postoperative oesophagectomy complications did not specifically influence the site of recurrence (Fig. S1, supporting information).
Risk of bias
Only studies that were assessed as high quality in GRADE31 were included. The I 2 statistic detected heterogeneities in the studies analysed in Figs 2 a, 2 c and 3 b; however, the forest plots showed that the direction of the point estimates was, in general, similar for all of the figures. As the heterogeneity was not significant, a random‐effects analysis was used, which resolved the heterogeneity that could not readily be explained, leading to reliable results.
Discussion
In this meta‐analysis, postoperative complications after oesophagectomy had a significant negative impact on survival. Previous reports3 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 of the impact of postoperative complications on long‐term survival have been inconsistent.
Long‐term survival was influenced in two ways. Some complications resulted in perioperative mortality, and there was an incremental effect of any type of complication on long‐term survival. Deterioration of the general condition may have affected long‐term OS and may have increased deaths unrelated to the oesophageal cancer3 19. Moreover, worsening of the general condition may have led to delay or cessation of additional therapy after oesophagectomy, resulting in oesophageal cancer recurrence and having a negative impact on CSS and DFS3 19.
Specific complications studied included pulmonary complications and anastomotic leakage. These often lead to generalized infection, which impacts significantly on the immunological system and in turn may lead to oesophageal cancer recurrence3. It was reported previously33 that infectious postoperative oesophagectomy complications significantly increased the levels of inflammatory cytokines such as interleukin (IL) 6 and IL‐8. Increased expression of both IL‐8 and its receptor CXCR‐2 have been correlated with tumour progression after oesophagectomy34. Anastomotic leakage may result in the local spread of viable tumour cells from stapled or sutured anastomoses. Locoregional recurrence after anastomotic leakage may be associated with a proinflammatory response that promotes tumour growth15.
Preventing postoperative complications may improve long‐term survival after oesophagectomy. High‐volume institutions with appropriate infrastructure are more able to deliver high‐quality outcomes35, 36, 37. Recently, minimally invasive oesophagectomy has become widespread, and may reduce the number of postoperative complications38. Moreover, better selection for surgery using risk models may improve outcomes6. Previously it was reported3 that oesophagectomy was not recommended for patients over 65 years of age or those with stage I if they were smokers. Definitive chemoradiotherapy may be recommended as an effective treatment for patients at high risk of postoperative surgical morbidity4 39.
Survival was clearly affected by a higher mortality rate in patients with postoperative complications compared with that in patients without complications. The exclusion of postoperative mortality could have avoided this bias, but the sample size would have been significantly smaller. In the 13 studies3 9, 10 12, 13, 14 18, 19 21, 24 26, 28 29 that included postoperative mortality, however, the postoperative mortality rate was low. Thus the impact of postoperative mortality on long‐term survival was relatively limited and a superimposed effect of complications in the long‐term is clear.
This meta‐analysis had some limitations. Nearly all studies were retrospective and only one19 evaluated prospectively collected data. However, only the high‐quality observational studies were included in the meta‐analysis, and heterogeneity was overcome by using a random‐effects analysis. The severity of postoperative complications, neoadjuvant chemotherapy regimen and surgical procedure differed between studies. Some studies included neoadjuvant chemoradiotherapy for more advanced cancer stages or salvage surgery: factors known to be related to respiratory and gastric tube complications, and associated with poor survival40. The extent of confounding as a result of co‐morbidity and (neo)adjuvant treatments means that the present results should be interpreted with caution.
Supporting information
Fig. S1. Forest plot comparing A locoregional, B lymphatic and C disseminated recurrence following oesophagectomy in patients with (+) and without (−) any complication. Mantel–Haenszel fixed‐effect (A) and random‐effects (B,C) models were used for meta‐analysis. Hazard ratios are shown with 95 per cent confidence intervals
Acknowledgements
The authors thank M. Sonohara of the Japan Medical Library Association for assisting in the systematic literature search.
Disclosure: The authors declare no conflict of interest.
Funding information
No funding information provided
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Ando N, Ozawa S, Kitagawa Y, Shinozawa Y, Kitajima M. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg 2000; 232: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Booka E, Takeuchi H, Nishi T, Matsuda S, Kaburagi T, Fukuda K et al The impact of postoperative complications on survivals after esophagectomy for esophageal cancer. Medicine (Baltimore) 2015; 94: e1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al‐Sarraf M et al Chemoradiotherapy of locally advanced esophageal cancer: long‐term follow‐up of a prospective randomized trial (RTOG 85‐01). Radiation Therapy Oncology Group. JAMA 1999; 281: 1623–1627. [DOI] [PubMed] [Google Scholar]
- 5. Takeuchi H, Saikawa Y, Oyama T, Ozawa S, Suda K, Wada N et al Factors influencing the long‐term survival in patients with esophageal cancer who underwent esophagectomy after chemoradiotherapy. World J Surg 2010; 34: 277–284. [DOI] [PubMed] [Google Scholar]
- 6. Takeuchi H, Miyata H, Gotoh M, Kitagawa Y, Baba H, Kimura W et al A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web‐based database. Ann Surg 2014; 260: 259–266. [DOI] [PubMed] [Google Scholar]
- 7. Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra‐abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol 2013; 20: 1575–1583. [DOI] [PubMed] [Google Scholar]
- 8. McSorley ST, Horgan PG, McMillan DC. The impact of the type and severity of postoperative complications on long‐term outcomes following surgery for colorectal cancer: a systematic review and meta‐analysis. Crit Rev Oncol Hematol 2016; 97: 168–177. [DOI] [PubMed] [Google Scholar]
- 9. Kinugasa S, Tachibana M, Yoshimura H, Ueda S, Fujii T, Dhar DK et al Postoperative pulmonary complications are associated with worse short‐ and long‐term outcomes after extended esophagectomy. J Surg Oncol 2004; 88: 71–77. [DOI] [PubMed] [Google Scholar]
- 10. Rizk NP, Bach PB, Schrag D, Bains MS, Turnbull AD, Karpeh M et al The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg 2004; 198: 42–50. [DOI] [PubMed] [Google Scholar]
- 11. Lerut T, Moons J, Coosemans W, Van Raemdonck D, De Leyn P, Decaluwé H et al Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg 2009; 250: 798–807. [DOI] [PubMed] [Google Scholar]
- 12. Hirai T, Yamashita Y, Mukaida H, Kuwahara M, Inoue H, Toge T. Poor prognosis in esophageal cancer patients with postoperative complications. Surg Today 1998; 28: 576–579. [DOI] [PubMed] [Google Scholar]
- 13. Abou‐Jawde RM, Mekhail T, Adelstein DJ, Rybicki LA, Mazzone PJ, Caroll MA et al Impact of induction concurrent chemoradiotherapy on pulmonary function and postoperative acute respiratory complications in esophageal cancer. Chest 2005; 128: 250–255. [DOI] [PubMed] [Google Scholar]
- 14. Hu Y, Zheng B, Rong TH, Fu JH, Zhu ZH, Yang H et al Prognostic analysis of the patients with stage‐III esophageal squamous cell carcinoma after radical esophagectomy. Chin J Cancer 2010; 29: 178–183. [DOI] [PubMed] [Google Scholar]
- 15. Markar S, Gronnier C, Duhamel A, Mabrut JY, Bail JP, Carrere N et al; FREGAT (French Eso‐Gastric Tumors) working group, FRENCH (Fédération de Recherche EN CHirurgie), and AFC (Association Française de Chirurgie). The impact of severe anastomotic leak on long‐term survival and cancer recurrence after surgical resection for esophageal malignancy. Ann Surg 2015; 262: 972–980. [DOI] [PubMed] [Google Scholar]
- 16. Doorakkers E, Konings P, Mattsson F, Lagergren J, Brusselaers N. Early complications following oesophagectomy for cancer in relation to long‐term healthcare utilisation: a prospective population‐based cohort study. PLoS One 2015; 10: e0121080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luc G, Durand M, Chiche L, Collet D. Major post‐operative complications predict long‐term survival after esophagectomy in patients with adenocarcinoma of the esophagus. World J Surg 2015; 39: 216–222. [DOI] [PubMed] [Google Scholar]
- 18. Baba Y, Yoshida N, Shigaki H, Iwatsuki M, Miyamoto Y, Sakamoto Y et al Prognostic impact of postoperative complications in 502 patients with surgically resected esophageal squamous cell carcinoma: a retrospective single‐institution study. Ann Surg 2016; 264: 305–311. [DOI] [PubMed] [Google Scholar]
- 19. Kataoka K, Takeuchi H, Mizusawa J, Igaki H, Ozawa S, Abe T et al Prognostic impact of postoperative morbidity after esophagectomy for esophageal cancer: exploratory analysis of JCOG9907. Ann Surg 2017; 265: 1152–1157. [DOI] [PubMed] [Google Scholar]
- 20. Saeki H, Tsutsumi S, Tajiri H, Yukaya T, Tsutsumi R, Nishimura S et al Prognostic significance of postoperative complications after curative resection for patients with esophageal squamous cell carcinoma. Ann Surg 2017; 265: 527–533. [DOI] [PubMed] [Google Scholar]
- 21. Yamashita K, Makino T, Miyata H, Miyazaki Y, Takahashi T, Kurokawa Y et al Postoperative infectious complications are associated with adverse oncologic outcomes in esophageal cancer patients undergoing preoperative chemotherapy. Ann Surg Oncol 2016; 23: 2106–2114. [DOI] [PubMed] [Google Scholar]
- 22. Karl RC, Schreiber R, Boulware D, Baker S, Coppola D. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg 2000; 231: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Junemann‐Ramirez M, Awan MY, Khan ZM, Rahamim JS. Anastomotic leakage post‐esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume centre. Eur J Cardiothorac Surg 2005; 27: 3–7. [DOI] [PubMed] [Google Scholar]
- 24. Ancona E, Cagol M, Epifani M, Cavallin F, Zaninotto G, Castoro C et al Surgical complications do not affect longterm survival after esophagectomy for carcinoma of the thoracic esophagus and cardia. J Am Coll Surg 2006; 203: 661–669. [DOI] [PubMed] [Google Scholar]
- 25. D'Annoville T, D'Journo XB, Trousse D, Brioude G, Dahan L, Seitz JF et al Respiratory complications after oesophagectomy for cancer do not affect disease‐free survival. Eur J Cardiothorac Surg 2012; 41: e66–e73. [DOI] [PubMed] [Google Scholar]
- 26. Ferri LE, Law S, Wong KH, Kwok KF, Wong J. The influence of technical complications on postoperative outcome and survival after esophagectomy. Ann Surg Oncol 2006; 13: 557–564. [DOI] [PubMed] [Google Scholar]
- 27. Lindner K, Fritz M, Haane C, Senninger N, Palmes D, Hummel R. Postoperative complications do not affect long‐term outcome in esophageal cancer patients. World J Surg 2014; 38: 2652–2661. [DOI] [PubMed] [Google Scholar]
- 28. Martin LW, Swisher SG, Hofstetter W, Correa AM, Mehran RJ, Rice DC et al Intrathoracic leaks following esophagectomy are no longer associated with increased mortality. Ann Surg 2005; 242: 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xia BT, Rosato EL, Chojnacki KA, Crawford AG, Weksler B, Berger AC. Major perioperative morbidity does not affect long‐term survival in patients undergoing esophagectomy for cancer of the esophagus or gastroesophageal junction. World J Surg 2013; 37: 408–415. [DOI] [PubMed] [Google Scholar]
- 30. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 31. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J et al GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–406. [DOI] [PubMed] [Google Scholar]
- 32. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al The Clavien–Dindo classification of surgical complications: five‐year experience. Ann Surg 2009; 250: 187–196. [DOI] [PubMed] [Google Scholar]
- 33. Okamura A, Takeuchi H, Matsuda S, Ogura M, Miyasho T, Nakamura R et al Factors affecting cytokine change after esophagectomy for esophageal cancer. Ann Surg Oncol 2015; 22: 3130–3135. [DOI] [PubMed] [Google Scholar]
- 34. Ogura M, Takeuchi H, Kawakubo H, Nishi T, Fukuda K, Nakamura R et al Clinical significance of CXCL‐8/CXCR‐2 network in esophageal squamous cell carcinoma. Surgery 2013; 154: 512–520. [DOI] [PubMed] [Google Scholar]
- 35. Anderson O, Ni Z, Møller H, Coupland VH, Davies EA, Allum WH et al Hospital volume and survival in oesophagectomy and gastrectomy for cancer. Eur J Cancer 2011; 47: 2408–2414. [DOI] [PubMed] [Google Scholar]
- 36. Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg 2007; 245: 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Markar SR, Karthikesalingam A, Thrumurthy S, Low DE. Volume–outcome relationship in surgery for esophageal malignancy: systematic review and meta‐analysis 2000–2011. J Gastrointest Surg 2012; 16: 1055–1063. [DOI] [PubMed] [Google Scholar]
- 38. Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR et al Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open‐label, randomised controlled trial. Lancet 2012; 379: 1887–1892. [DOI] [PubMed] [Google Scholar]
- 39. Kato H, Sato A, Fukuda H, Kagami Y, Udagawa H, Togo A et al A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn J Clin Oncol 2009; 39: 638–643. [DOI] [PubMed] [Google Scholar]
- 40. Kumagai K, Rouvelas I, Tsai JA, Mariosa D, Klevebro F, Lindblad M et al Meta‐analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro‐oesophageal junctional cancers. Br J Surg 2014; 101: 321–338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Forest plot comparing A locoregional, B lymphatic and C disseminated recurrence following oesophagectomy in patients with (+) and without (−) any complication. Mantel–Haenszel fixed‐effect (A) and random‐effects (B,C) models were used for meta‐analysis. Hazard ratios are shown with 95 per cent confidence intervals


