There is good evidence of the benefits of early detection of cancer and removal of adenomas in asymptomatic individuals; however, the widespread implementation of colorectal screening programs is resource‐dependent. Such programs have been widely implemented in high‐income regions and countries in North America, Europe, Japan, and South Korea, but such programs are few in low and middle‐income countries [35]. This article describes the implementation of a comprehensive approach to colorectal cancer screening in Shanghai, China.
Keywords: Cancer screening, Colorectal cancer, Public health
Abstract
Background.
The incidence and mortality rate of colorectal cancer (CRC) have increased dramatically over the past 3 decades in China due to changes in lifestyle factors. Early detection and treatment guidelines for asymptomatic cases have shown to improve CRC control in developed countries. In response to these challenges, the Shanghai Municipal Government launched a community‐based CRC screening program in 2012.
Materials and Methods.
Free initial screening, inclusive of immunochemical fecal occult blood and risk assessment (questionnaire), was provided by community health centers in Shanghai. Participants with positive results were referred to a specialist for a colonoscopy.
Results.
In 2013, 828,302 Shanghai residents were registered; 97.7% (809,528) of the registrants completed initial screening. Among 180,094 initial screening‐positive participants, 71,733 underwent colonoscopy. The proportion of compliance to colonoscopy was 39.8%; the proportion decreased with age and educational level. A total of 6,668 adenomas were detected, and 1,630 CRC cases were diagnosed. The CRC detection rate of the program was 201.35/100,000; among the detected CRCs, 51.6% were in stage 0–I.
Conclusion.
The screening program achieved great progress, especially on initial screening completion and CRC early stage rate, although particular intervention is still needed to improve the compliance of colonoscopy.
Implications for Practice.
Due to socioeconomic transitions and lifestyle changes, colorectal cancer is now becoming one of the most common cancers in developing countries, as it is in developed countries. While most developed countries have now initiated national colorectal cancer screening programs based on recommended country‐specific colorectal cancer screening guidelines, colonoscopy has become the most commonly used screening method. This is a challenge in developing countries due to limited resources. Based on the analysis of the Shanghai colorectal cancer screening program, with immunological fecal occult blood test and risk assessment as initial screening, followed by a diagnostic testing of colonoscopy for individuals with positive results, this article provides the basis and suggestion for similar program in other regions of China and other developing countries.
摘要
背景.中国由于生活方式改变,过去30年结直肠癌(CRC)的发生率和死亡率显著升高。在发达国家,无症状病例的早期检测和治疗已被证明可改善CRC控制。为应对这些挑战,2012年上海市政府发起了一项社区性CRC筛查计划。
材料和方法.上海社区卫生服务中心提供免费初步筛查,包括免疫化学粪便潜血检测和风险评估(问卷)。将结果为阳性的受试者转诊给专科医生行结肠镜检查。
结果.2013年,有828 302名上海居民进行注册;97.7%(809 528)的居民完成了初步筛查。在180 094名初步筛查结果为阳性的参与者中,71 733名进行了结肠镜检查。结肠镜检查依从性比例为39.8%;该比例随年龄和教育水平降低而降低。共计检出6 668例腺瘤,诊断出1 630例CRC。此计划的CRC检出率为201.35/100 000;在检出的CRC中,51.6%为0–I期。
结论.筛查计划取得了巨大进展,特别是在初步筛查完成度和CRC早期诊断率方面,不过仍然需要采取特别干预措施以提高结肠镜检查依从率。
对临床实践的提示:由于社会经济变革和生活方式发生变化,结直肠癌现已成为发展中国家最常见的癌症之一,此情况与发达国家一致。多数发达国家现在已根据推荐的国家特定结直肠癌筛查指南启动了全国性结直肠癌筛查计划,结肠镜检查已成为最常使用的筛查方法。由于资源有限,此检查在发展中国家是一项挑战。基于上海结直肠癌筛查计划的分析,使用免疫学粪便潜血检测和风险评估作为初步筛查,随后对阳性结果个体进行结肠镜检查诊断性检测,本文为中国其他地区以及其他发展中国家的类似计划提供了基础和建议。
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world [1]. The highest CRC incidence rates were mostly observed in high‐income countries such as the U.S., New Zealand, Slovakia, Japan, and South Korea. Deaths from CRC accounted for 8% of all cancer mortality, which ranked as the fourth most common cause of death from cancer [2]. However, trends in incidence and mortality rates of CRC have differed between developed and developing countries. Whereas CRC incidence and mortality rates have decreased in the U.S. and most European countries [3], [4], [5], a dramatic increase in CRC incidence and mortality rates has been observed in middle‐ and low‐income countries such as Brazil, China, Mexico, and Thailand [6], [7], [8]. Furthermore, the 5‐year survival rate of CRC is only 28%–42% in developing countries, compared with that of more than 60% in developed countries [9], [10], [11]. The reduction in CRC incidence and mortality rates in developed countries can largely be attributed to the establishment of national‐level colorectal cancer screening guidelines, as well as the utilization and improvement of CRC screening programs [12]. In China, CRC is the fourth most common cancer among men and the fifth most common cancer among women; its incidence and mortality rates have increased by 37% and 71% from 1991 to 2005 [13].
In Shanghai, one of the most developed cities in China, the incidence and mortality of CRC have increased dramatically over the past 3 decades and are now among the highest in the nation [14]. According to the Shanghai Cancer Registry (SCR), the incidence of CRC has doubled in the past 20 years and has almost reached the incidence level in developed countries [15]. The incidence of CRC increased by 4.2% annually in Shanghai over the past 30 years; only 12% of the newly diagnosed cases per year were in the early stages [16]. In addition, overall 5‐year survival rates of CRC in Shanghai were 10%–15% lower than those of developed countries [17]. According to the Shanghai Behavior Risk Factor Surveillance System, test rates for CRC screening tests, such as colonoscopy and fecal occult blood test, remained low in the past decade, at 4.01% and 11.01% [18]. Therefore, there was strong evidence of the necessity and urgency to set up a CRC screening program in Shanghai.
Although there is now good evidence of the benefits of early detection of cancer cases and removal of adenomas in asymptomatic individuals [19], [20], [21], [22], [23], [24], [25], [26], [27], the widespread implementation of colorectal screening programs is very resource‐dependent [28], [29]. Although such programs have now been widely implemented in high‐income regions and countries in North America, Europe, Japan, and South Korea [30], [31], [32], [33], [34], there are few examples of large‐scale screening programs in low‐ and middle‐income countries [35]. This paper describes the implementation of a comprehensive approach to CRC screening in Shanghai, China.
Materials and Methods
Health System and Infrastructure
The Shanghai Municipal Commission of Health and Family Planning, Shanghai Municipal Finance Bureau, and Shanghai Municipal Human Resources and Social Security Bureau released a document in 2012 outlining an approach to CRC screening. This was the first municipal‐level CRC screening approach in Shanghai. The whole screening service contained two steps—initial screening and colonoscopy. The initial screening service was facilitated by the community health centers (CHCs). Fifty officially designated hospitals were responsible for colonoscopy and further treatment of participants. The hospitals were selected by the District Health Bureau, with capacity of colonoscopy and multidisciplinary team of CRC. Of 50 designated hospitals, 35 (70%) were tier‐2 hospitals, and the rest were tier‐3 hospitals. The Shanghai Center for Disease Control and Prevention was responsible for program management, data management, training, quality control, and program evaluation. Immunological fecal occult blood test (FIT) package, provided by the Shanghai Government, was free to participants; it costs 1.5 Yuan RMB for each test, and each participant would take two tests in initial screening. The cost of colonoscopy was covered by each participant's personal health insurance account when the participant was referred to a designated hospital. About 95% of Shanghai residents were covered by different kinds of health insurance, including Basic Medical Insurance for Employees and Urban & Rural Residents Basic Medical Insurance; both official Shanghai residents and migrant populations could be covered.
Staff Training and Quality Control
Health workers from CHCs, including general practitioners and nurses, staff from district CDCs, and physicians from designated hospitals were recruited for training. The training contents contained CRC screening protocol, registration and system management instructions, standard CRC health education courses, CRC diagnosis and treatment guidelines, and colonoscopy criteria. A total of 733 staff members, from 223 CHCs, 50 officially designated hospitals, and 17 district CDCs, completed the training in 2013. Training would be provided to program staff annually to enhance the capacity of implementation.
The Shanghai Municipal Center for Disease Control and Prevention organized specialists from surgery, endoscopy, and pathology to check the quality of the diagnosis and treatment of CRCs and lesions.
Screening Protocol
The screening has two steps: (a) initial screening, including FIT and risk assessment, followed by (b) diagnostic testing of colonoscopy for individuals with positive results.
FIT.
In the first step of initial screening, each participant was evaluated by FIT and a risk‐assessment questionnaire during an in‐person survey. For FIT, the minimal detective level was 100 ng/mL; each participant was given two sample containers to collect fecal samples at home, within an interval of 7 days, and was asked to return each sample to a CHC within 48 hours after collection. A 60‐second video was made to explain how to collect the fecal samples at home and was played on repeat in CHCs. A participant was identified as FIT‐positive if either one or both of the tests were positive. Participants who failed to return samples within 2 weeks would be reminded twice through telephone by CHCs and were recorded as nonresponders after the failure of the second attempt.
Risk Assessment.
A face‐to‐face interview for risk assessment was conducted by questionnaire either before or after fecal sample collection. There were nine questions in this questionnaire, including anorectal symptoms, related diseases such as polyps and appendicitis, CRC family history, personal cancer history, etc. Questions had different weights [36], and the result would be given by computer after the data were entered into the system.
Colonoscopy.
A participant who had either a positive FIT result or a positive risk assessment questionnaire result would be identified to be at a high risk for CRC and referred to officially designated hospitals for colonoscopy examination. In colonoscopy examination, the entire colon was examined [37]. If the condition of participants permitted, the scope should reach to ileocecus, and returning of scope should last for longer than 20 minutes.
Data Management.
A 12‐number barcode was assigned to each participant after registration to follow further screening results. The barcode would appear on the fecal collect tube, and when participants returned the tube, the FIT result could be entered into the system easily by scanning the barcode, the same as entering colonoscopy data in designated hospitals. The barcode was coded by year of screening, district of residence, community, and a random digit.
The Shanghai CRC screening registry and management system was an internet‐based system that was created for the Shanghai CRC screening program. All screening data, including personal information, risk‐assessment questionnaire, FIT result, colonoscopy result, diagnosis and treatment information, and initial screening result, would be provided by the system through comprehensive analysis of the questionnaire and FIT result. The system could also remind the staff to contact participants who did not return the FIT tube on time. The system would print initial screening result notification letters for participants, and positive‐result letters could be used as referral letters when participants went for colonoscopies in designated hospitals. The system could remind the staff to contact participants who did not go for colonoscopies on time.
Target Population.
Official Shanghai residents and migrant populations aged 50–74 years with no history of CRC were identified by CHCs and were invited to participate in the screening. CHCs mobilized target populations with the collaboration of neighborhood committees and village committees. A variety of mass media outlets were used to raise advocacy and awareness on CRC prevention and early detection and to increase enrollment. Promotion campaigns, aimed at increasing CRC screening, were launched on radio, television, and posters in public places. Health information brochures were distributed to individuals in target age groups. All participants were fully informed of the risks and benefits of CRC screening, and all participants provided written informed consent prior to participation. As a public health service, some residents older than 75 years took the initiative to participate in the program but were excluded in this analysis.
Data for Analysis.
Based on the national census in 2010, the size of the target population between 50 and 74 years of age was 4 million; both official Shanghai residents and migrant populations were included. According to the official document released in 2012, each round of the Shanghai CRC screening program would last for 3 years. By comprehensive evaluation of the capacity of health resources, about 1 million participants from the target population could be covered in one round (3 years); therefore, it was estimated that most of the participants who were willing to join the screening could be enrolled within three rounds. Initially, the first round of screening should have started in 2011 and completed in 2013; however, because of the preparation of the program, the implementation of screening was carried out from January to December in 2013. Minhang District is one of the 17 districts in Shanghai; however, the screening data in Minhang was not submitted to Shanghai CDC because of the different information‐management system used. Thus, the data of Minhang District was excluded in this analysis.
Because of the age range of the target population, occupation in this paper was the occupation before retired, and urban and rural areas were defined by Administrative Divisions Codes from the National Bureau of Statistics of China.
Statistical Analysis.
SPSS 22.0 (IBM, Armonk, NY) was used for statistical analysis. Chi‐square test was used for comparison between groups; α < .05 was statistically significant.
Results
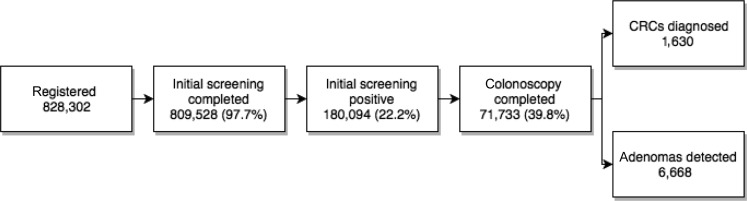
By the end of 2013, a total of 828,302 individuals were registered; they signed the informed consent, and their personal information was recorded by the screening system. Among the registered populations, 809,528 (97.7%) participants completed both FIT and risk assessment (Fig. 1).
Figure 1.
Number of screened population and outcomes in screening process.
Abbreviation: CRC, colorectal cancer.
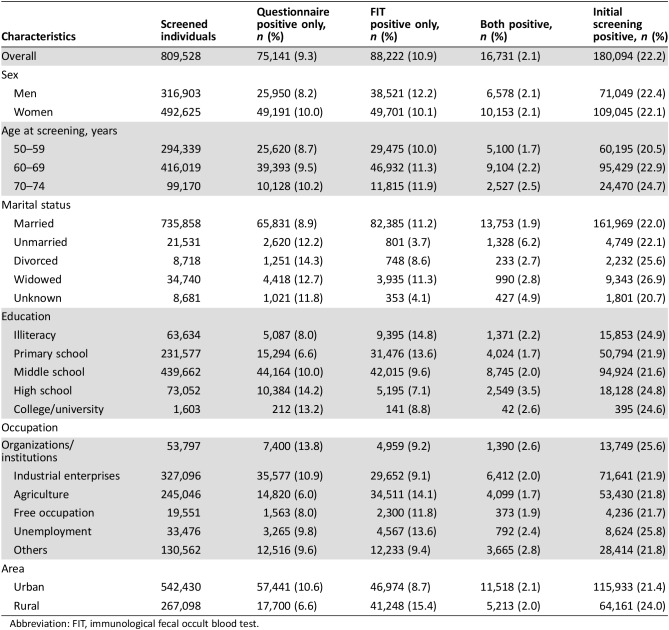
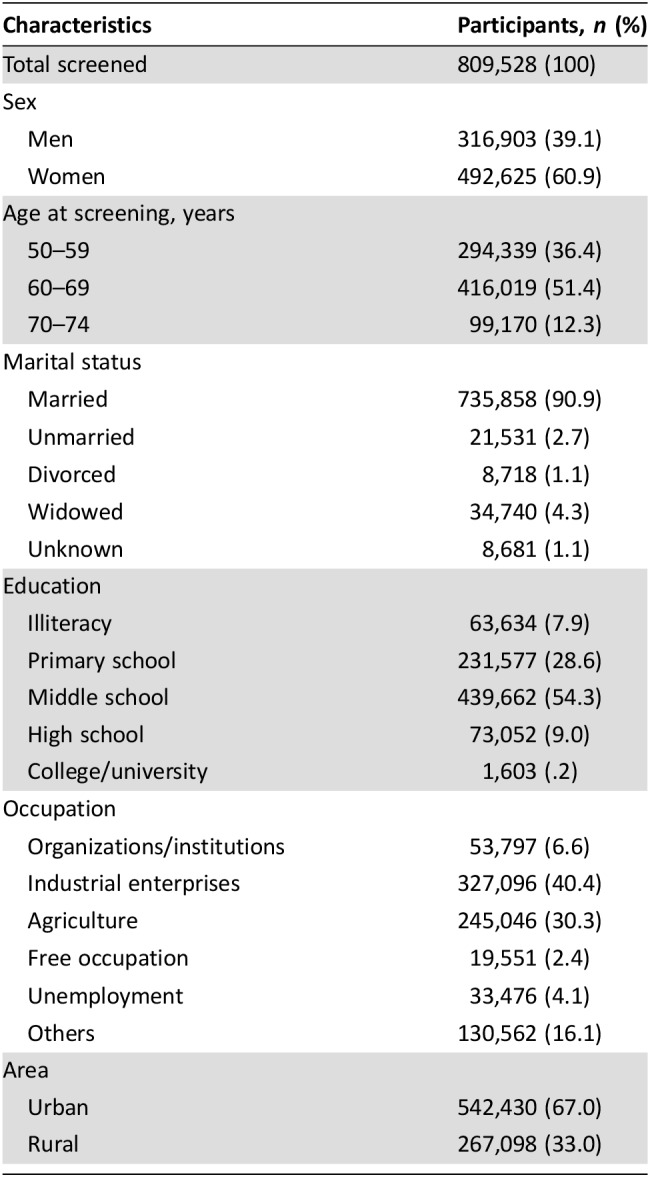
Of the screened participants, 39.1% were men and 60.9% were women, the majority of the participants were in age group 50–59 (36.4%) and 60–69 (51.4%), almost all the participants were married, and the major education level was middle school or lower. For the occupation of participants, 40.4% were workers and 30.3% were in agriculture, and 67.0% were in urban and 33.0% in rural areas (Table 1).
Table 1. Characteristics of participants who completed initial screening.
Among the participants who completed the initial screening, 180,094 (22.2%) tested positive. Similar proportions of men and women had positive results: 71,049 (22.4%) for men and 109,045 (22.1%) for women. The positive rate of only risk assessment was 9.4%, only FOBT was 10.9%, and both positive was 2.2%. The positive rates were different among age groups and were higher in rural than in urban areas (Table 2).
Table 2. Proportion of positive for initial screening.
Abbreviation: FIT, immunological fecal occult blood test.
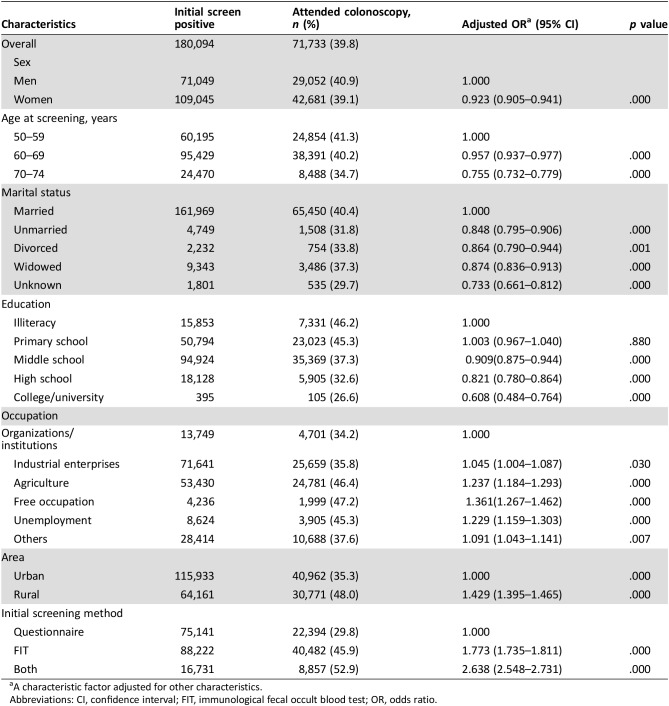
All the participants with a positive initial screening result received a referral letter for colonoscopy. By the end of the first round, 71,733 (39.8%) participants with a positive initial screening result completed a colonoscopy test. Women had a lower proportion than men (odds ratio [OR] = 0.923, p = .0001). For different age groups, the proportion decreased with the increasing of age. Married people had the highest participation rate in all marital status. Participation rate decreased with the increasing of education level from middle school. Participation rate in rural areas was higher than in urban areas (OR = 1.429, p = .000). For initial screening method, the participants with only questionnaire positive showed the lowest proportion of colonoscopy, whereas both questionnaire and FIT positive showed the highest proportion of colonoscopy in different screening method (OR = 2.638, p = .000; Table 3).
Table 3. Proportion of colonoscopy among initial screening‐positive participants.
A characteristic factor adjusted for other characteristics.
Abbreviations: CI, confidence interval; FIT, immunological fecal occult blood test; OR, odds ratio.
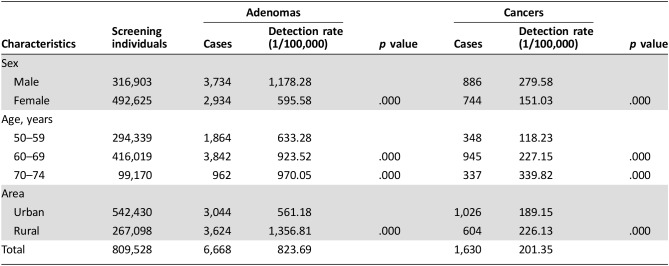
Among the 71,733 participants who underwent colonoscopy, 6,668 had adenoma (4,472 among males and 3,439 among females). The overall detection rate for adenomas is 847.91 per 100,000 people. A total of 1,630 CRC cases were diagnosed. The detection rate of CRC was 201.35/100,000. The detection rate of adenomas was much higher in males than in females (p = .000); 70–74 years showed the highest detection rate in different age groups; and rural areas had higher detection rates of adenomas than urban areas. For CRC, the detection rate showed a similar situation with adenomas (Table 4).
Table 4. Detection of adenomas and cancers.
According to the information collected from the Shanghai CRC screening registry and management system, and data linkage with the Shanghai Cancer Registry, detailed staging information has been identified for 1,205 (73.9%) cases. There were 622 (51.6%) early‐stage (0–I) cases. The proportion of later‐stage (II–IV) cases in the screened population was lower than that in the Shanghai Cancer Registry 2012 (Table 5).
Table 5. Comparison of colorectal cancer staging with Shanghai Cancer Registry 2012.
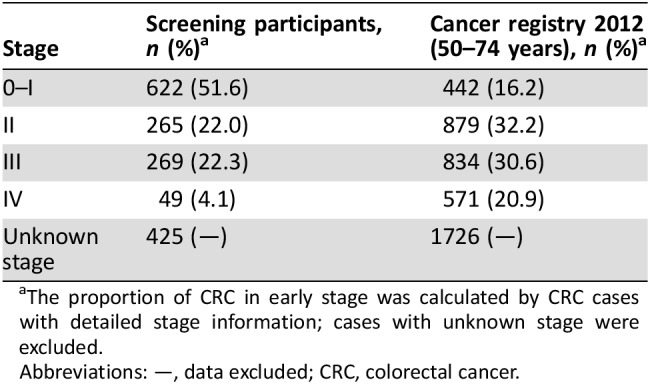
The proportion of CRC in early stage was calculated by CRC cases with detailed stage information; cases with unknown stage were excluded.
Abbreviations: —, data excluded; CRC, colorectal cancer.
Discussion
During the program implementation in 2013, there were a total of 828,302 individuals registered; 97.7% (809,528) of the registered individuals completed initial screening. Overall initial screening‐positive rate was 22.2%; 180,094 participants tested positive. A total of 71,733 initial screening‐positive participants underwent colonoscopy. Adenomas were detected in 6,668 participants, and 1,630 participants were diagnosed with CRC; among 1,205 CRC cases with detailed stage information, 51.6% were in early stage, more than four times higher than the data of the Shanghai Cancer Registry 2012. A total of 6,668 adenoma cases were detected by this program; the detection rate of adenomas was underestimated because of the compliance of colonoscopy and information collection. The early diagnosis of CRC could improve prognosis and quality of life of patients and ultimately reduce the mortality of CRC in the future.
In the Shanghai CRC screening program, the completion rate of initial screening was high, compared with a similar screening program in Germany during 2014 (97.7% vs. 71.3%) [38]. The main explanation is the effect of the reminder system, in which participants who did not return fecal samples on time would be reminded twice within the interval of 7 days through telephone by CHC staff. Another explanation may be that participants in Germany received FIT kit passively, whereas participants in Shanghai were registered initiatively [39].
When prioritizing the CRC screening program, the data from SCR provided strong evidence that the accessibility of data in a screening program is important so that the public and policy makers are able to understand the real situation. Local statistics help to show variation in risk factor prevalence and hence cancer burden and outcomes [40]. The census should be used to determine the size of the target population and expected number of participants.
For the consideration of introducing a population‐based screening program, regarding its public service characteristics, the capacity of the current medical system must be considered. All the hospitals with colonoscopy capabilities allocate the facilities and capacity according to medical needs. A screening program will inevitably bring more colonoscopy examinations than usual [41]. It was necessary to carefully examine the gap between the current capacity of colonoscopy and the need produced by the proposed screening program. A survey was conducted to collect information on the current colonoscopy capacity, including number of colonoscopies and trained physicians and the actual number and expected number of examinations. It was found that there was around 25% spare capacity, which meant the workload space to undertake the colonoscopy of the program. Most of the spare capacity existed in the tier‐2 hospitals. It was also found that the spare capacity accounted for over 80% of the needs for the colonoscopy examination from the screening program. The obstacle can be overcome by well‐designed referral systems between the CHCs and the hospitals.
Limitations and Disadvantages
Although great progress has been achieved, as a large‐scale public health service program for millions of the target population, there were several disadvantages that still need to be improved. The main weakness of the screening program was the low compliance rate of colonoscopy among initial screening‐positive participants. The overall proportion of compliance to colonoscopy was 39.8%; the low compliance rate might affect the effect of CRC screening, because initial screening‐positive participants who did not attend colonoscopy might have never received a diagnosis of CRC or adenomas.
The compliance to colonoscopy in the Shanghai CRC screening program was lower than that in the U.S. (53%) [42] and Australian (68%) programs [43]; however, the proportion was higher than the programs without colonoscopy subsidy in other cities in similar CRC screening program in China, which was 2.8% in Hangzhou [44], 11.8% in Beijing [45], and 20.5% [46] in Tianjing, but lower than most of the programs with colonoscopy subsidy, which was 76.2% in Haining [47] and 78.7% in Jingzhou [48]. The result showed that the proportion of compliance to colonoscopy decreased with the increasing of age, which may be caused by the fear of colonoscopy examination risk in elder people. Elder people have higher risk of undergoing colonoscopy. The U.S. Preventive Services Task Force recommends that the decision to screen for colorectal cancer in adults aged >75 years should be an individual one, depending on personal health and prior screening history [49]. As the cost of anesthesia is not covered by health insurance, elder people were less likely to tolerate the discomfort of colonoscopy. Populations with higher education levels showed lower participation rates of colonoscopy. Higher education level commonly means higher income, which could allow for more resources for medical examination. Similar results could be found in different occupation groups and different areas: agriculture, free occupation, and unemployed people were more likely to attend colonoscopy because no employer can provide additional medical examination for that population.
To compare the characteristics of populations with and without colonoscopy, it was found that there were significant differences between the two populations. Factors included sex, age, marital status, education level, areas, and initial screening methods. More detailed investigations will be needed to confirm the factor of colonoscopy compliance. The question of how to improve the participation rate of colonoscopy among initial screening‐positive populations, and especially promote particular populations such as high education level or single/unmarried to attend colonoscopy, may have great significance in improving early‐stage rate of CRC and the effect of screening.
The collection of colonoscopy, diagnosis, and treatment information was limited. There were 50 official designated hospitals in the Shanghai CRC screening program, which had the responsibility to collect information. However, 70% of the designated hospitals were tier‐2 hospitals, and some participants chose higher‐level, but nondesignated, hospitals for further examinations. Among all the participants who attended colonoscopy, 34.8% chose nondesignated hospitals that lacked corresponding information collection mechanisms, so the information could only be obtained from community CHCs by follow‐up. Diagnosis information of CRC cases could be collected by data linkage with SCR, but the detection of adenomas was not registered, which means the number of adenoma cases might be underestimated. To comprehensively evaluate the effectiveness of the screening program, more efficient mechanisms are needed to improve the collection of diagnosis information.
For sustainability of the CRC screening program, it is necessary to improve the compliance of colonoscopy. The monitoring of adverse events is a relevant negative performance indicator that should be followed in further implementation, and the performance of colonoscopy, such as adenoma detection rate, will be evaluated to ensure the quality of colonoscopy.
Launching the colorectal cancer screening program in Shanghai is a good example of transforming a mature population intervention technique into a health service, by careful design and organization. The experience and methods detailed in this program will help to scale up the program.
Conclusion
This paper focuses on the first round of CRC screening in 2013. A series of studies, such as detection rate of different initial screening methods, compliance of colonoscopy, and change of mortality, will follow. The next report of screening outcomes of the second round should be released in 2019; we are going to pay more attention to indicators of colonoscopy performance and cost‐effectiveness of screening.
Acknowledgments
This work was supported by Three‐year Action Plan on Public Health, Phase IV, Shanghai, China (15GWZK0801), and The Fourth Round of Shanghai Three‐year Action Plan on Public Health Discipline and Talent Program: Evidence‐based Public Health and Health Economics (15GWZK0901). Y.G. and P.P. are co‐first authors.
Footnotes
Editor's Note: See the related commentary, “Important Role of Health Surveillance Systems in Community‐Based Colorectal Cancer Screening,” by Ann Chao and Sudha Sivaram, on p. 871 of this issue.
Author Contributions
Conception/design: Pingping Bao, Weijian Zhong, Yan Shi, Ying Zheng, Sanjun Cai, Ye Xu, Jun Sheng, Fan Wua
Collection and/or assembly of data: Yangming Gong, Peng Peng, Kai Gu, Chunxiao Wu
Data analysis and interpretation: Yangming Gong, Peng Peng, Pingping Bao, Kai Gu
Manuscript writing: Yangming Gong
Final approval of manuscript: Yangming Gong, Peng Peng, Pingping Bao, Weijian Zhong, Yan Shi, Kai Gu, Ying Zheng, Chunxiao Wu, Sanjun Cai, Ye Xu, Jun Sheng, Fan Wu
Disclosures
The authors indicated no financial relationships.
References
- 1.World Health Organization. World Cancer Report 2014. International Agency for Research on Cancer. 2014. ISBN 978‐92‐832‐0432‐9.
- 2.World Health Organization. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Lyon, France: International Agency for Research on Cancer, 2014.
- 3. Majek O, Gondos A, Jansen L et al. Survival from colorectal cancer in Germany in the early 21st century. Br J Cancer 2012;106:1875–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bosetti C, Levi F, Rosato V et al. Recent trends in colorectal cancer mortality in Europe. Int J Cancer 2011;129:180–191. [DOI] [PubMed] [Google Scholar]
- 5.The potential for prevention of colorectal cancer in the UK. Eur J Cancer Prev 2009;18:179–190. [DOI] [PubMed] [Google Scholar]
- 6. Yee YK, Gu Q, Hung I et al. Trend of colorectal cancer in Hong Kong: 1983–2006. J Gastroenterol Hepatol 2010;25:923–927. [DOI] [PubMed] [Google Scholar]
- 7. Khuhaprema T, Srivatanakul P. Colon and rectum cancer in Thailand: An overview. Jpn J Clin Oncol 2008;38:237–243. [DOI] [PubMed] [Google Scholar]
- 8. Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev 2009;18:1688–1694. [DOI] [PubMed] [Google Scholar]
- 9.Global Burden of Disease Cancer Collaboration . Cancer survival in developing countries. JAMA Oncol 2015;1:505–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coleman MP, Quaresma M, Berrino F et al. Cancer survival in five continents: A worldwide population‐based study (CONCORD). Lancet Oncol 2008;9:730–756. [DOI] [PubMed] [Google Scholar]
- 11. Allemani C, Weir HK, Carreira H et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population‐based registries in 67 countries (CONCORD‐2). Lancet 2015;385:977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Center MM, Jemal A, Smith RA et al. Worldwide variations in colorectal cancer. CA Cancer J Clin 2009;59:366–378. [DOI] [PubMed] [Google Scholar]
- 13.Zheng S, ed. Colorectal Tumor. Basic Research and Clinical Practice. Beijing: People's Medical Publishing House, 2006. [Google Scholar]
- 14. Zhang S, Chen W, Kong L et al. An analysis of cancer incidence and mortality from 30 cancer registries in China, 1998–2002. Bull Chin Cancer 2006;7:430–448. [Google Scholar]
- 15. Ying Z, Yangming G. Research and practice of screening for colorectal cancer in population of Shanghai. Chin Cancer 2013;22:86–89. [Google Scholar]
- 16. Wan DS. Epidemiologic trend of and strategies for colorectal cancer. Chin J Cancer 2009;28:897–902. [DOI] [PubMed] [Google Scholar]
- 17. Kuipers EJ, Rösch T, Bretthauer M. Colorectal cancer screening–Optimizing current strategies and new directions. Nat Rev Clin Oncol 2013;10:130–142. [DOI] [PubMed] [Google Scholar]
- 18. Goss PE, Strasser‐Weippl K, Lee‐Bychkovsky BL et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol 2014;15:489–538. [DOI] [PubMed] [Google Scholar]
- 19. Stock C, Knudsen AB, Lansdorp‐Vogelaar I. Colorectal cancer mortality prevented by use and attributable to nonuse of colonoscopy. Gastrointest Endosc 2011;73:435–443. [DOI] [PubMed] [Google Scholar]
- 20. Petryszyn PW, Kempiński R, Michałowicz J. Non‐medical costs of colonoscopy. Prz Gastroenterol 2014;9:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zauber AG, Winawer SJ, O'Brien MJ. Colonoscopic polypectomy and long‐term prevention of colorectal‐cancer deaths. N Engl J Med 2012;366:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lansdorp‐Vogelaar, van Ballegooijen M, Zauber AG. Effect of rising chemotherapy costs on the cost savings of colorectal cancer screening. J Natl Cancer Inst 2009;101:1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacob BJ, Moineddin R, Sutradhar R. Effect of colonoscopy on colorectal cancer incidence and mortality: An instrumental variable analysis. Gastrointest Endosc 2012;76:355–364. [DOI] [PubMed] [Google Scholar]
- 24. Bode JG, Nitschmann S. Colonoscopic polypectomy for prevention of colorectal cancer. Follow‐up investigation of the National Polyp Study [in German]. Internist (Berl) 2013;54:263–264. [DOI] [PubMed] [Google Scholar]
- 25. Heresbach D, Manfredi S, D'halluin PN et al. Review in depth and meta‐analysis of controlled trials on colorectal cancer screening by faecal occult blood test 2006. Eur J Gastroenterol Hepatol 2006;18:427–433. [DOI] [PubMed] [Google Scholar]
- 26. Hewitson P, Glasziou P, Watson E et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): An update. Am J Gastroenterol 2008;103:1541–1549. [DOI] [PubMed] [Google Scholar]
- 27. Pignone MP, Flitcroft KL, Howard K et al. Costs and cost‐effectiveness of full implementation of a biennial faecal occult blood test screening program for bowel cancer in Australia. Med J Aust 2011;194:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lertkhachonsuk AA, Yip CH, Khuhaprema T et al. Cancer prevention in Asia: Resource‐stratified guidelines from the Asian Oncology Summit 2013. Lancet Oncol 2013;14:e497–e507. [DOI] [PubMed] [Google Scholar]
- 29. Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2015;51:1201–1202. [DOI] [PubMed] [Google Scholar]
- 30. Edwards BK, Ward E, Kohler BA et al. Annual report to the nation on the status of cancer, 1975–2006: Featuring colorectal cancer trends and impact of interventions. Cancer 2010;116:544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Altobelli E, Lattanzi A, Paduano R et al. Colorectal cancer prevention in Europe: Burden of disease and status of screening programs. Prev Med 2014;62:132–141. [DOI] [PubMed] [Google Scholar]
- 32. Kuriki K, Tajima K. The increasing incidence of colorectal cancer and the preventive strategy in Japan. Asian Pac J Cancer Prev 2006;7:495–501. [PubMed] [Google Scholar]
- 33. Sekiguchi M, Matsuda T, Tamai N et al. Cost‐effectiveness of total colonoscopy in screening of colorectal cancer in Japan. Gastroenterol Res Pract 2012;2012:728454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoo KY. Cancer control activities in the Republic of Korea. Jpn J Clin Oncol 2008;38:327–333. [DOI] [PubMed] [Google Scholar]
- 35. Lambert R, Sauvaget C, Sankaranarayanan R. Mass screening for colorectal cancer is not justified in most developing countries. Int J Cancer 2009;15;125:253–256. [DOI] [PubMed] [Google Scholar]
- 36. Huang YQ, Zheng S. Past, present and prospect with high incidence spots of prevention and treatment for colorectal cancer in Zhejiang Province. Chin Cancer 2013;22:83–85. [Google Scholar]
- 37.Disease Prevention and Control Bureau, Ministry of Health . Proposal of cancer early diagnosis and treatment. Beijing: People's Medical Publishing House, 2011. [Google Scholar]
- 38. Toes‐Zoutendijk E, van Leerdam ME, Dekker E et al. Real‐time monitoring of results during first year of Dutch colorectal cancer screening program and optimization by altering fecal immunochemical test cut‐off levels. Gastroenterology 2017;152:767–775. [DOI] [PubMed] [Google Scholar]
- 39. de Visser M, van Ballegooijen M, Bloemers SM et al. Report on the Dutch consensus development meeting for implementation and further development of population screening for colorectal cancer based on FOBT. Cell Oncol 2005;27:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shanghai Municipal Center for Disease Control & Prevention. The Shanghai Non‐communicable and Chronic Disease Surveillance Report 2013. Shanghai, 2014.
- 41.Australian Institute of Health and Welfare. National Bowel Cancer Screening Program monitoring report: July 2011–June 2012. Cancer series no. 75 Cat. no. CAN 71. 2013. Available at https://www.aihw.gov.au/getmedia/de7f3a9b-659c-4fa3-8066-1fb6f8b1b860/15799.pdf.aspx?inline=true. Accessed November 30, 2013.
- 42.Centers for Disease Control and Prevention (CDC) . Vital signs: Colorectal cancer screening test use–United States, 2012. MMWR Morb Mortal Wkly Rep 2013;62:881–888. [PMC free article] [PubMed] [Google Scholar]
- 43.AIHW . 2015 National Bowel Cancer Screening Program: Monitoring report 2013–14, Australian Institute of Health and Welfare Canberra.
- 44. Shanrong C, Shuren Z, Lun Z et al. Screening of colorectal cancer in Hangzhou urban community. J Pract Oncol 2006,21:177–178. [Google Scholar]
- 45. Yadong W, Liyuan W, Lizheng G et al. Screening of colorectal cancer in urban community of Beijing. Analysis of results and problems. Chin Gen Pract 2007;10:1586–1588. [Google Scholar]
- 46. Zhao LZ, Wei H et al. Preliminary results of colorectal cancer screening in Tianjin. Clinic 2015;15:760–764. [Google Scholar]
- 47. Chen YZ, Qian J, Feng HE et al. Analysis of colorectal cancer early‐diagnosis and early‐treatment screening in Haining from 2007 to 2008. Chin J Cancer 2009;18:728–730. [Google Scholar]
- 48. Zhimin X, Quan J. 312 cases of community residents' colorectal cancer screening analysis. Chin J Trauma Disabil Med 2014;22:297. [Google Scholar]
- 49. Preventive Service Task Force US, Bibbins‐Domingo K, Grossman DC et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation statement. JAMA 2016;315:2564–2575. [DOI] [PubMed] [Google Scholar]