This article summarizes key review findings that supported the approval of tocilizumab for treatment of severe or life‐threatening CAR T cell‐induced cytokine release syndrome.
Keywords: Tocilizumab, Chimeric antigen receptor, T cell, Cytokine release syndrome, Interleukin‐6, Immunotherapy
Abstract
On August 30, 2017, the U.S. Food and Drug Administration approved Actemra (tocilizumab, Genentech, Inc., South San Francisco, CA) for the treatment of severe or life‐threatening chimeric antigen receptor (CAR) T cell‐induced cytokine release syndrome (CRS) in adults and in pediatric patients 2 years of age and older. The approval was based on a retrospective analysis of data for patients who developed CRS after treatment with CTL019 and KTE‐C19 on prospective clinical trials. Evaluable patients had been treated with intravenous tocilizumab 8 mg/kg (12 mg/kg for patients <30 kg) for severe or life‐threatening CRS; only the first episode of CRS was included in the analysis. The efficacy population for the CTL019 cohort included 24 male and 21 female patients (total 45 patients) of median age 12 years. The median time from the start of CRS to the first dose of tocilizumab was 4 days (range, 0–18 days). Patients were considered responders if CRS resolved within 14 days of the first dose of tocilizumab, if no more than 2 doses of tocilizumab were needed, and if no drugs other than tocilizumab and corticosteroids were used for treatment. Thirty‐one patients (69%; 95% confidence interval, 53%–82%) achieved a response as defined. In an independent cohort of 15 patients with KTE‐C19‐induced CRS, 53% responded. Further study is needed to determine the optimal dose of tocilizumab and to confirm the safety of its use for treatment of patients with CAR T cell‐induced CRS.
Implications for Practice.
Severe or life‐threatening chimeric antigen receptor (CAR) T cell‐induced cytokine release syndrome (CRS) requires urgent treatment to prevent fatal outcomes. In two independent cohorts, the majority of patients with severe or life‐threatening CAR T cell‐induced CRS responded to treatment with one or two doses of tocilizumab in addition to advanced supportive care. More research is needed to determine the optimal dose and schedule of tocilizumab for treatment of CAR T cell‐induced CRS.
Introduction
Cytokine release syndrome (CRS) has been identified as a clinically significant, on‐target, off‐tumor side effect of the chimeric antigen receptor (CAR) T‐cell therapies used for treatment of malignancies [1], [2], [3]. Characteristics of CRS include fever, fatigue, headache, encephalopathy, hypotension, tachycardia, coagulopathy, nausea, capillary leak, and multiorgan dysfunction. The reported incidence of CRS after CAR T‐cell therapy ranges from 50% to 100%, with 13% to 48% of patients experiencing the severe or life‐threatening form [4], [5], [6]. Multiple approaches have been used to reduce the risk of CRS, including use of low cell doses or a split‐dosing schedule [7]. These approaches have mitigated the risk of CRS only partially.
The complex of signs and symptoms in CRS results from the effects of cytokines released by the CAR T cells once engaged with the target. Serum levels of inflammatory cytokines are elevated, particularly interleukin‐6 (IL‐6) [8]. The severity of symptoms may correlate with the serum cytokine concentrations and the duration of exposure to the inflammatory cytokines [9]. Multiple grading systems have been used to categorize the severity of CRS clinically, including the National Cancer Institute Common Terminology Criteria for Adverse Events, the Penn Grading Scale [10], and the Lee Grading Scale [4]. The criteria for severe or life‐threatening CRS differ among the grading systems with regard to the categorization at which low‐ or high‐dose vasopressors were used, but in all of these scales, severe and life‐threatening grades of CRS require advanced supportive care.
Because of the potential for fatal outcomes, severe or life‐threatening CRS is a medical emergency. Initial treatment includes antipyretics for fever, vasopressors and intravenous fluids for hypotension, and supportive care with oxygen or mechanical ventilation for respiratory distress. High‐dose corticosteroids can moderate toxicity in some cases, but corticosteroids may also reduce the clinical effectiveness of the CAR T‐cell therapy by blocking T‐cell activation, function, and proliferation [8], [11]. Even with advanced supportive care alone, the course may be protracted, requiring weeks for recovery from multiorgan injury.
Tocilizumab is a recombinant humanized monoclonal antibody directed against the interleukin‐6 receptor (IL‐6R). It binds both soluble and membrane‐bound IL‐6R and inhibits IL‐6‐mediated signaling through these receptors. Herein, we summarize key review findings that supported the approval of tocilizumab for treatment of severe or life‐threatening CAR T cell‐induced cytokine release syndrome.
Materials and Methods
Study Design
The efficacy of tocilizumab for the treatment of CRS was assessed in a retrospective analysis of pooled data from prospective clinical trials of CAR T‐cell therapies for hematological malignancies. The clinical trials included five studies of CTL019 (CCTL019A2201, CCTL019B2101J, CCTL019B2102J, CCTL019B2202, and CCTL019B2205J) and four studies of KTE‐C19 (KTE‐C19‐101, KTE‐C19‐102, KTE‐C19‐103, and KTE‐C19‐104). The cases were ascertained based on a report of CRS by the investigators on the clinical trial case report forms and the identification of tocilizumab as a concomitant medication. Only the first episode of CRS was used in the analysis.
Treatment with tocilizumab was prespecified in each protocol, but the exact dose, frequency and criteria for treatment varied. The efficacy‐evaluable population was limited to patients who had been treated using intravenous tocilizumab 8 mg/kg (12 mg/kg for patients <30 kg); this is the recommended dose approved for systemic juvenile idiopathic arthritis (SJIA), and there are substantial data available to support the safety of this dose. The target population included patients with the severe or life‐threatening grades of CRS specifically. Because the Penn Grading Scale was used for grading CRS in the CTL019 trials and the Lee Grading Scale was used in the KTE‐019 trials, the two cohorts were assessed separately.
The primary objective was to characterize resolution of CRS. Resolution of CRS was defined as the patient having a lack of fever and being off vasopressors for at least 24 hours. Patients were considered responders if (a) CRS resolved within 14 days of the first dose of tocilizumab, (b) no more than two doses of tocilizumab were used, and (c) no drugs other than tocilizumab and corticosteroids were used for treatment. Additional assessments included response at 2, 7, and 21 days from the first dose of tocilizumab. All results were reported descriptively. There was no inferential testing planned.
Clinical Pharmacology
The key clinical pharmacology review question focused on the appropriateness of the proposed dosing regimen. In study CTL019B2202, samples for analysis of tocilizumab were planned to be collected at 5–15 minutes, 24 hours ± 2 hours, and 48 hours ± 4 hours after the first and second infusions of tocilizumab. Pharmacokinetic (PK) parameters other than maximum concentration could not be generated reliably because of the sparse sampling schedule. Modeling and simulation were used to identify a recommended dosage using the PK data from study CTL019B2202 in patients with CRS and extant PK data from pediatric and young adult patients with SJIA.
In comparison with the population PK model developed using data from patients with SJIA, the observed mean maximum concentration of tocilizumab after the first dose in the patients with CRS was 41% lower than that in patients with SJIA, suggesting faster clearance of tocilizumab in patients with CRS. Therefore, the available population PK model was refined to re‐estimate linear clearance (CL) and volume of distribution in the central compartment (VC) using PK data from the patients with CRS. All other parameters, including interindividual variabilities of CL and VC as well as residual errors, were fixed to the previous PK parameters given the limited, sparse PK observations and the small sample size for patients with CRS. The goodness‐of‐fit and visual predictive check assessments, decrease in objective function value, and the precision (% coefficient variability [CV]) of the parameter estimates were used for the model comparison and evaluation. The final refined population PK model was then used in multiple scenarios to simulate median tendencies and 95% percentiles of PK‐time profiles as well as the corresponding 95% confidence intervals to provide a recommended dosage. The maximum allowable concentration was 649 μg/mL based on the observed exposure‐response relationships for safety in healthy patients.
Results
Patient Characteristics
There were 58 patients identified as having been treated with tocilizumab for CRS after infusion of CTL019 and 76 patients after infusion of KTE‐C19. After eliminating patients who had only mild or moderate CRS or who were not treated with the proposed dose of tocilizumab, the final efficacy‐evaluable populations included 45 patients with CRS after CTL019 and 15 patients with CRS after KTE‐C19. The populations included both adults and children. The baseline demographic characteristics of the patients are shown in Table 1, and the CRS characteristics are shown in Table 2. For the patients in the CTL019 series, the median time from start of CRS to first dose of tocilizumab was 4 days (range, 0–18 days); nearly all patients were treated with only one tocilizumab dose per day, and the median total number of tocilizumab doses administered was one (range, one to four). For the patients in the KTE‐C19 series, the median time from the start of CRS to the first dose of tocilizumab was 3 days (range, 0–14 days); one third of the patients received 2 or 3 doses of tocilizumab per day, and the median total number of tocilizumab doses was 2 (range, 1–13).
Table 1. Demographics.
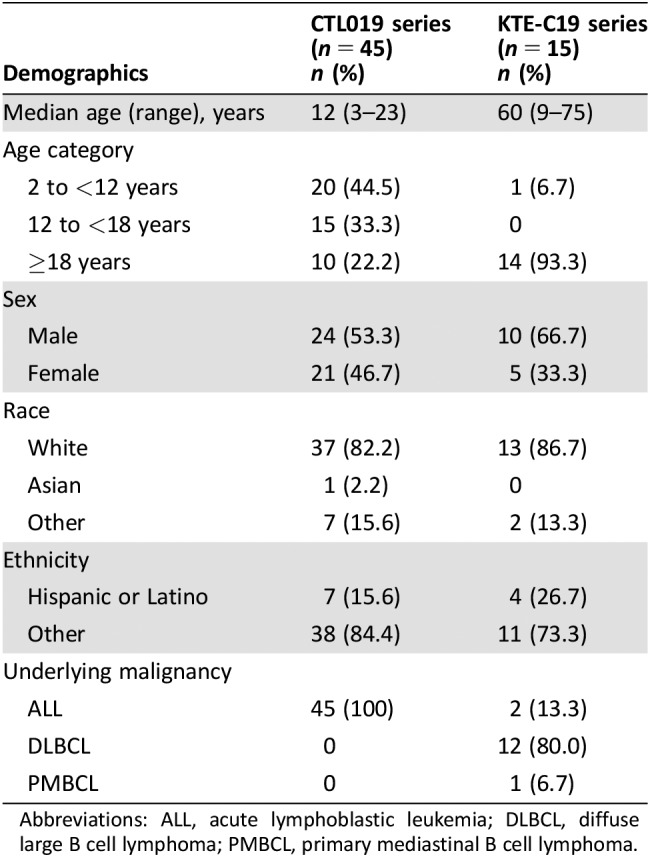
Abbreviations: ALL, acute lymphoblastic leukemia; DLBCL, diffuse large B cell lymphoma; PMBCL, primary mediastinal B cell lymphoma.
Table 2. CRS characteristics and treatment.
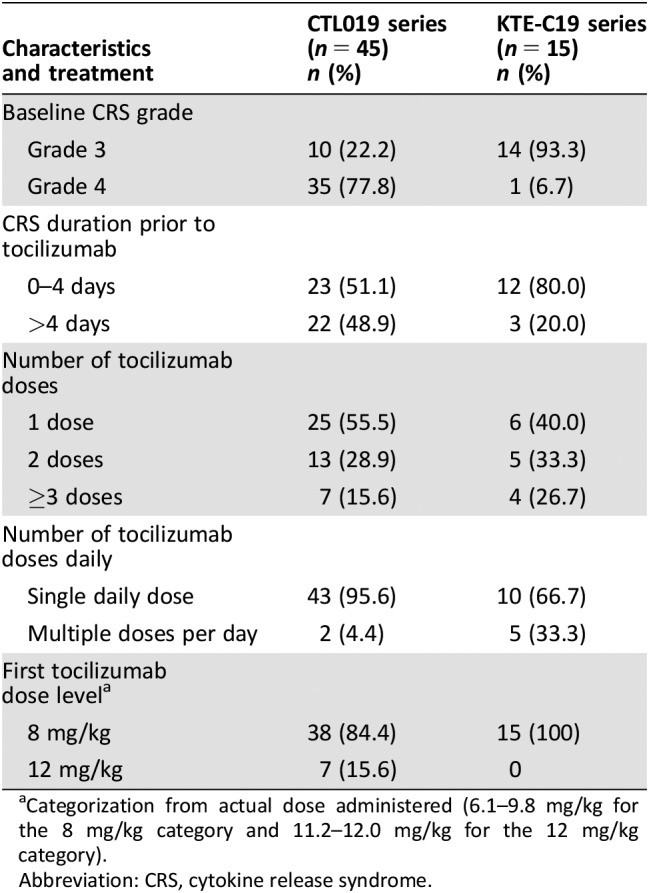
Categorization from actual dose administered (6.1–9.8 mg/kg for the 8 mg/kg category and 11.2–12.0 mg/kg for the 12 mg/kg category).
Abbreviation: CRS, cytokine release syndrome.
Efficacy
Thirty‐one patients (69%) from the CTL019 series achieved a response within 14 days of the first dose of tocilizumab, and the median time from the first dose to response was 4 days (range, 1–12 days; Table 3). Eight patients (53%) from the KTE‐C19 series achieved a response, and the median time to response was 4.5 days (range, 2–7 days). The response rates were largely consistent among subgroups such as age group, sex, race, ethnicity, grade of CRS at first dose of tocilizumab, and duration of CRS prior to treatment with tocilizumab. Response was also assessed at 2, 7, and 21 days from the first dose of tocilizumab (Table 3). In both series, the majority of patients who achieved a response did so within the first 7 days.
Table 3. Resolution of cytokine release syndrome in the efficacy populations.
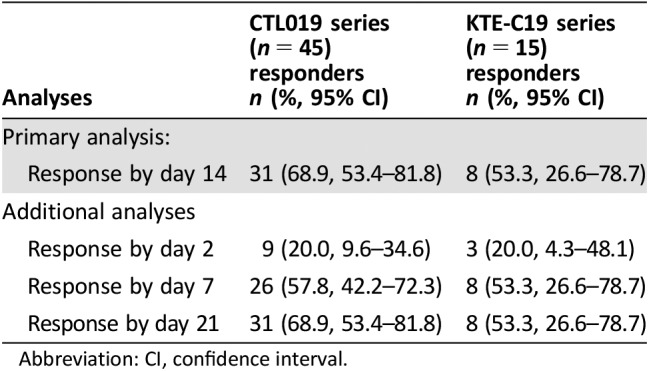
Abbreviation: CI, confidence interval.
Safety
There were no reports of adverse reactions attributable to tocilizumab. Five patients in the CTL019 series and five patients in the KTE‐C19 series died within 30 days of the first dose of tocilizumab. None of the deaths was attributed to tocilizumab.
Pharmacometrics Analysis
PK data were available for 27 patients after the first dose of tocilizumab and for 8 patients after a second dose of tocilizumab. The PK population included 15 male and 12 female patients of median age 12 years (range, 4–23 years). Based on 131 PK observations, the geometric mean (% CV) maximum concentration of tocilizumab in the patients with CAR T cell‐induced, severe or life‐threatening CRS was 99.5 μg/mL (36.8%) after the first infusion and 160.7 μg/mL (113.8%) after the second infusion. Using the refined population PK model, simulations were performed for the recommended tocilizumab dose given every 8, 12, or 24 hours. The results of the simulations suggested that tocilizumab concentrations after up to 4 doses given 8 hours apart would remain within the bounds of previously observed safe blood concentrations.
Literature Review
A review of the literature identified no prospective clinical trials of tocilizumab for treatment of CAR T cell‐induced CRS. There were numerous case reports and case series describing the activity of tocilizumab for treatment of CRS after infusion of CAR T cells directed against CD19 [2, 5, 10, 10–15], CD123 [16], CD269 [5], and mesothelin [17]. Fever and tachycardia reportedly resolved within hours after infusion of tocilizumab [2], [5], [11], [14], but resolution of hypotension and organ dysfunction was delayed [2], [14]. However, some authors noted cases of CRS refractory to tocilizumab therapy [13], [18]. Significant disease burden was identified as a potential risk factor for refractoriness to tocilizumab [18]. It was also reported that tocilizumab did not affect CAR T cell proliferation or clinical efficacy [10], [11], [14].
Discussion
Tocilizumab was approved for the treatment of severe or life‐threatening CAR T cell‐induced cytokine release syndrome in adults and in pediatric patients 2 years of age and older. Approval was based on the finding in a retrospective data analysis that 69% of patients with CTL019‐induced severe or life‐threatening CRS had resolution of fever and need for vasopressors within 14 days after receiving one or two doses of tocilizumab. In an independent cohort of 15 patients with KTE‐C19‐induced CRS, 53% of patients responded. The data reviewed were not sufficient to allow assessment of the activity of tocilizumab for treatment of CRS induced by therapies other than CAR T cells.
There is some concern that in the absence of a randomized trial or a well‐characterized historical control, it is difficult to interpret the results of a retrospective review of a treated cohort. However, the published observations of rapid effects on objective early endpoints, such as vital signs [2], [5], [11], [14], provided substantial supporting evidence. The quality of the prospectively collected data, the supporting published data, and the established clinical experience with tocilizumab in multiple other indications were considered adequate to support review for this serious disorder for which there were no available therapies.
The endpoint used to assess effectiveness was response, defined as being afebrile and off vasopressors for at least 24 hours within 14 days of the first dose of tocilizumab (maximum up to two doses) and without use of additional treatment other than corticosteroids. In both the CTL019 and KTE‐C19 series, the majority of the patients who achieved the response criteria did so within 7 days of the first dose of tocilizumab, but there were five additional responders after 7 days in the CTL019 series. It is not clear whether these additional responses were due to tocilizumab or to the natural resolution of CRS with continued supportive care.
In a study of single‐dose tocilizumab in healthy volunteers, the only significant dose‐related toxicity was a decrease in neutrophils [19]. In the healthy volunteers who received a single 20 mg/kg dose, some had neutrophil counts less than baseline for 8 weeks or longer. The mechanism of the neutropenia after treatment with tocilizumab is unclear, but based on the rapid onset and unilineage nature, it is thought to be a margination effect rather than myelosuppression [20]. The PK analysis showed that patients with CRS had a faster clearance of tocilizumab than healthy volunteers and other patient populations, and simulations showed that exposure was considered acceptable with up to four doses of tocilizumab at least 8 hours apart in patients with CRS. Nonetheless, if fewer than four doses would be effective, as was shown in our analysis, then minimizing the risk of exposure‐related prolonged neutropenia with fewer doses would seem prudent in patients already neutropenic from lymphodepleting chemotherapy and CAR T cells.
Additionally, there were no specific assessments for the safety of tocilizumab in the clinical trials of CTL019 or KTE‐C19, and the limited safety analysis did not assess for tocilizumab infusion reactions, exacerbation of CAR T cell‐induced neurotoxicity, or prolonged neutropenia after treatment with tocilizumab. However, there were no reports of adverse reactions to tocilizumab in the patients treated for CRS in the protocols included in this analysis, and the safety profile of the tocilizumab 8 mg/kg dose is well established in other intended populations down to the age of 2 years. This evidence allowed for a favorable risk‐benefit analysis in this situation. Because there remains some uncertainty about the safety of multiple doses given over a short period of time, further study to confirm the safety of tocilizumab in the intended population is a postmarketing requirement.
Of note, the optimal dose and schedule of tocilizumab for treatment of CAR T cell‐induced CRS is not known, and the dosing instructions in current tocilizumab labeling provide only general recommendations for safe dosing. Because the natural history of CRS may vary by CAR type, receptor target, baseline disease burden, or other parameters [17], labeling for each CAR T‐cell product provides specific instructions for use of tocilizumab as supported by data generated in the clinical trials for that CAR T‐cell product. More study is needed to elucidate best practices for use of tocilizumab in this population, especially to identify the cases in which tocilizumab is simply not effective [13], [18] and alternative approaches should be applied to ameliorate the cytokine effects.
Despite the unknowns, the seriousness of CRS in the setting of a potentially curative therapy for cancer warrants aggressive supportive care provided in as safe a manner as possible. Hence, the risks of using tocilizumab appear to be outweighed by the potential benefit for patients with severe or life‐threatening CAR T cell‐induced CRS.
Conclusion
The approval of tocilizumab for the treatment of CAR T cell‐induced severe or life‐threatening CRS in adults and in pediatric patients 2 years of age and older was based on a retrospective data analysis in two cohorts, showing response rates of 53%–69%. The activity of tocilizumab was supported by a literature review. The optimal dose and schedule are not well established. The available data are wholly insufficient to extend the indication to CRS induced by other immunotherapeutics. Further study is required to confirm the safety of tocilizumab in the intended population.
Acknowledgments
The authors acknowledge Novartis Pharmaceuticals Corporation and Kite Pharma, Inc., for providing the data files used for the retrospective clinical analysis. Neither Novartis Pharmaceuticals Corporation nor Kite Pharma, Inc., influenced the content of this manuscript. The authors thank Natasha Kormanik, Erica Giordano, and Mark Davidson for supportive project management.
Author Contributions
Conception/design: Robert Q. Le, Donna Przepiorka, Ann T. Farrell, Richard Pazdur
Collection and/or assembly of data: Robert Q. Le, Liang Li, Bahru A. Habtemariam, Donna Przepiorka, Ann T. Farrell
Data analysis and interpretation: Robert Q. Le, Liang Li, Weishi Yuan, Stacy S. Shord, Lei Nie, Bahru A. Habtemariam, Donna Przepiorka, Ann T. Farrell
Manuscript writing: Robert Q. Le, Liang Li, Weishi Yuan, Stacy S. Shord, Lei Nie, Bahru A. Habtemariam, Donna Przepiorka, Ann T. Farrell, Richard Pazdur
Final approval of manuscript: Robert Q. Le, Liang Li, Weishi Yuan, Stacy S. Shord, Lei Nie, Bahru A. Habtemariam, Donna Przepiorka, Ann T. Farrell, Richard Pazdur
Disclosures
The authors indicated no financial relationships.
References
- 1. Morgan RA, Yang JC, Kitano M et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fitzgerald JC, Weiss SL, Maude SL et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med 2017;45:e124–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brentjens RJ, Davila ML, Riviere I et al. CD19‐targeted T cells rapidly induce molecular remissions in adults with chemotherapy‐refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee DW, Gardner R, Porter DL et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 2016;127:3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program 2016;2016:567–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalos M, Levine BL, Porter DL et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011;3:95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teachey DT, Lacey SF, Shaw PA et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T‐cell therapy for acute lymphoblastic leukemia. Cancer Discov 2016;6:664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kochenderfer JN, Dudley ME, Feldman SA et al. B‐cell depletion and remissions of malignancy along with cytokine‐associated toxicity in a clinical trial of anti‐CD19 chimeric‐antigen‐receptor‐transduced T cells. Blood 2012;119:2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Porter DL, Hwang WT, Frey NV et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davila ML, Riviere I, Wang X et al. Efficacy and toxicity management of 19‐28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grupp SA, Kalos M, Barrett D et al. Chimeric antigen receptor‐modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kochendorfer JN, Dudley ME, Kassim SH et al. Chemotherapy‐refractory diffuse large B‐cell lymphoma and indolent B‐cell malignancies can be effectively treated with autologous T cells expressing an anti‐CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maude SL, Frey N, Shaw PA et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee Dw, Kochendorfer JN, Stetler‐Stevenson M et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose‐escalation trial. Lancet 2015;385:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luo Y, Chang LJ, Hu Y et al. First‐in‐man CD123‐specific chimeric antigen receptor‐modified T cells for the treatment of refractory acute myeloid leukemia. Blood 2015;126:3778A. [Google Scholar]
- 17. Tanyi JL, Stashwick C, Plesa G et al. Possible compartmental cytokine release syndrome in a patient with recurrent ovarian cancer after treatment with mesothelin‐targeted CAR‐T cells. J Immunother 2017;40:104–107. [DOI] [PubMed] [Google Scholar]
- 18. Frey NV, Levine BL, Lacey SF et al. Refractory cytokine release syndrome in recipients of chimeric antigen receptor (CAR) T cells. Blood 2014;124:2296A. [Google Scholar]
- 19. Grange S, Schmitt C, Banken L et al. Thorough QT/QTc study of tocilizumab after single‐dose administration at therapeutic and supratherapeutic doses in healthy subjects. Int J Clin Pharmacol Ther 2011:49:648–655. [DOI] [PubMed] [Google Scholar]
- 20. Shovman O, Shoenfeld Y, Langevitz P. Tocilizumab‐induced neutropenia in rheumatoid arthritis patients with previous history of neutropenia: Case series and review of literature. Immunol Res 2015;61:164–168. [DOI] [PubMed] [Google Scholar]


