This study evaluated the activity and safety of monoclonal antibody anti‐EGFR panitumumab single agent without chemotherapy in low‐risk LARC preoperative treatment. The use of anti‐EGFR monoclonal antibodies in combination with radiotherapy in preoperative treatment in patients with KRAS wild type and low‐risk LARC did not reach the pCR primary endpoint; however, this study showed a good toxicity profile and compliance to combination treatment.
Keywords: Panitumumab, Radiotherapy, KRAS, Rectal cancer
Abstract
Background.
Treatment with fluoropyrimidines and concomitant long‐course external radiotherapy (RTE) is the standard of care in locally advanced rectal cancer (LARC) preoperative chemoradiation. A randomized phase II study (RaP/STAR‐03) was conducted that aimed to evaluate the activity and safety of the monoclonal antibody anti‐epidermal growth factor receptor panitumumab as a single agent in combination with radiotherapy in low‐risk LARC preoperative treatment.
Materials and Methods.
Patients had adenocarcinoma of the mid‐low rectum, cT3N− or cT2–T3N+, KRAS wild‐type status, and negative circumferential radial margin. Panitumumab was administered concomitant to RTE. Rectal surgery was performed 6–8 weeks after the end of preoperative treatment. The adjuvant chemotherapy regimen was FOLFOX. The primary endpoint was the pathologic complete response (pCR) rate. The sample size was calculated using Simon's two‐stage design. A pCR of 16% was considered to qualify the experimental treatment for further testing.
Results.
Ninety‐eight patients were enrolled in 13 Italian centers from October 2012 to October 2015. Three panitumumab infusions were administered in 92 (93.4%) patients. The RTE compliance was median dose 50.4 Gy; ≥28 fractions in 82 (83.7%) patients. Surgical treatment was performed in 92 (93.9%) patients, and no severe intraoperative complications were observed. A pCR was observed in 10 (10.9%) patients (95% confidence interval, 4.72%–17.07%). Pathological downstaging occurred in 45 (45.9%) patients. Grade 3 toxicities were observed in 22 (22.3%) patients, and the common adverse events were skin rash in 16 (16.3%) patients. No grade 4 toxicities were reported.
Conclusion.
The pCR rate (our primary endpoint), at only 10.9%, did not reach the specified level considered suitable for further testing. However, the analysis showed a good toxicity profile and compliance to concomitant administration of panitumumab and RTE in preoperative treatment of LARC. The pCR evaluation in all wild‐type RAS is ongoing.
Implications for Practice.
The aim of the RaP/STAR‐03 study was to evaluate the activity and safety of monoclonal antibody anti‐epidermal growth factor receptor (EGFR) panitumumab as a single agent without chemotherapy in low‐risk, locally advanced rectal cancer (LARC) preoperative treatment. Nevertheless, the use of panitumumab in combination with radiotherapy in preoperative treatment in patients with KRAS wild type and low‐risk LARC did not reach the pathologic complete response primary endpoint. This study showed a good toxicity profile and compliance to combination treatment. Further analysis of NRAS and BRAF on tissue and circulating levels of the EGFR ligands and vascular factors (soluble vascular endothelial growth factor, E‐selectin) may provide insight on the potential molecular pathways involved in the anti‐EGFR response.
摘要
背景.氟尿嘧啶同步长期外部放疗(RTE)治疗是局部晚期直肠癌(LARC)术前放化疗的标准。我们开展了一项随机II期研究(RaP/STAR‐03),旨在评估单克隆抗体抗表皮生长因子受体帕尼单抗作为单药联合放疗用于低风险LARC术前治疗的活性和安全性。
材料和方法.患者患有中低段直肠腺癌,cT3N−或cT2–T3N+,KRAS野生型状态,环周切缘为阴性。RTE同步帕尼单抗给药。在术前治疗结束后6–8周时进行直肠手术。辅助化疗方案为FOLFOX。主要终点是病理完全缓解(pCR)率。使用Simon两阶段设计计算样本量。16%的pCR可使实验治疗符合进一步检测的标准。
结果.在2012年10月至2015年10月期间,13个意大利中心入组了98例患者。在92例(93.4%)患者中进行了三次帕尼单抗输注。RTE依从性:中位剂量50.4 Gy,82例(83.7%)患者≥28次分割。在92例(93.9%)患者中进行了手术治疗,未观察到严重术中并发症。在10例(10.9%)患者中观察到pCR(95%置信区间,4.72%–17.07%)。45例(45.9%)患者发生病理学降期。在22例(22.3%)患者中观察到3级毒性,常见不良事件为皮疹,见于16(16.3%)例患者。未报告4级毒性。
结论.pCR率(我们的主要终点)仅为10.9%,未达到被视为适合进一步检测的规定水平。不过,该分析显示在LARC术前治疗中同步RTE和帕尼单抗治疗具有良好的毒性特征和依从性。正在针对所有野生型RAS进行pCR评价。
对临床实践的提示:RaP/STAR‐03研究的目的是评价单克隆抗体抗表皮生长因子受体(EGFR)帕尼单抗作为单药(不联合化疗)在低风险、局部晚期直肠癌(LARC)术前治疗中的活性和安全性。不过,在存在KRAS野生型、低风险LARC患者的术前治疗中帕尼单抗与放疗联用并未达到病理学完全缓解的主要终点。本研究显示联合治疗具有良好的毒性特征和依从性。对组织以及EGFR配体和血管因子(可溶性血管内皮生长因子,E‐选择素)循环水平进行的进一步NRAS和BRAF分析可能会提供有关涉及抗EGFR应答的潜在分子途径的见解。
Background
Rectal cancer occurs in the bowel region below the peritoneal reflection, including approximately 15 cm of intestine above the anal verge, and it accounts for about 35% of the total colorectal cancer incidence in Europe [1]. Surgery is the cornerstone of rectal cancer treatment, but it still results in a high incidence of local recurrences (25%–40%) and distant metastases. Local recurrence from rectal cancer has substantially decreased over the last 30 years. The introduction of standardized surgery and total mesorectal excision have decreased the local recurrence rate to less than 10% and have increased survival [2].
Despite these substantial improvements, local control is far from optimal. First, the reduction in the local recurrence rates achieved with the introduction of total mesorectal excision (TME) technique has been particularly impressive in uncontrolled, small, single‐institutional series (mainly testing the impact of TME in comparison with historical data with more conventional surgical techniques).
The pathologic complete response (pCR) rate has been considered a prognostic factor for patients with locally advanced rectal cancer (LARC) undergoing preoperative treatment, and pCR has been associated with favorable disease‐free survival (DFS) and overall survival (OS) [3], [4].
More than 30 years ago, Moertel et al. [5] demonstrated that radiotherapy (RT) combined with fluorouracil significantly increased the OS of patients with LARC. Subsequently, several studies conducted by the European Organization for Research and Treatment of Cancer Radiotherapy Group [6] and the Fédération Francophone de Cancérologie Digestive [7] have confirmed the benefit of this combination in terms of complete pathologic tumor response (pT0) in the RT and RT combined with chemotherapy (CRT) groups (5.3% vs. 13.7%; p < .0001 and 3.6% vs. 11.4%; p < .0001), respectively.
Currently, preoperative fluoropyrimidine‐based CRT followed by TME is the standard treatment in LARC tumors to enable surgery to be more effective and provide locoregional control and increase the pCR, but an improvement in OS [7], [8], [9] has not been shown.
Several randomized phase III trials (STAR‐01, ACCORD 12/0405‐Prodige2, NSABP R‐04) evaluated the addition of oxaliplatin to preoperative fluoropyrimidine‐based CRT, and of these, the preliminary results have not shown a significant effect on early pathological response [10], [11], [12], [13], with the exception of the German CAO/ARO/AIO‐04 study [14].
Furthermore, there was a great interest in the integration into RT and CRT protocols of biological agents such as anti‐vascular endothelial growth factor (VEGF) monoclonal antibodies, considering that the inhibition of the VEGF signaling axis can act as a radiosensitizer for tumor‐associated endothelial cells, thus inhibiting tumor neoangiogenesis and reducing vascular density [15]. Moreover, anti‐VEGF agents can also lead to vascular normalization, decreasing tumor hypoxia and improving radiosensitivity. Nevertheless, the results of several phase I and II trials are not conclusive and do not definitively demonstrate a clear benefit from the addition of bevacizumab in terms of pCR or improved patient outcomes [16].
Another important membrane growth factor in several neoplasms among which rectal cancer is represented is epidermal growth factor receptor (EGFR), a transmembrane glycoprotein, which is a member of the tyrosine kinase growth factor receptor superfamily. EGFR represents an important therapeutic target in cancer and regulates cellular growth, survival, proliferation, and differentiation. In rectal cancer, EGFR is overexpressed in 50%–70% of primary tumors [17]. In patients with rectal cancer after preoperative chemoradiotherapy, EGFR overexpression is related to a decrease in the pathological response, disease‐free survival and overall survival [18], [19].
Preliminary data suggest that an EGFR‐targeted agent in combination with RT may be synergistic, as RT increases EGFR expression within tumor cells, while EGFR blockade sensitizes the cells to the effects of RT [20], [21]. In the setting of locally advanced head and neck cancer, the addition of cetuximab to RT has enhanced locoregional control and survival [22], [23]. Various mechanisms for this cetuximab synergistic activity have been proposed, including the inhibition of repopulation during the latter phase of radiotherapy [24], [25].
In our previous StarPan/STAR‐02 study [26] the addition of panitumumab to chemoradiotherapy was evaluated, showing a higher pCR rate in patients with high‐risk LARC, in comparison with the results of previous preoperative rectal cancer trials with anti‐EGFR monoclonal antibodies. However, the combination treatment was associated with a very high incidence of grade 3–4 gastrointestinal toxicity. Because the integration of anti‐EGFR monoclonal antibodies into preoperative treatments for rectal cancer is promising in terms of response rate, although there are high rates of toxicity, we conducted a randomized, phase II study to evaluate the efficacy and safety of panitumumab alone in combination with external beam radiotherapy as the preoperative regimen in low‐risk LARC.
Materials and Methods
Study Design and Patients
We conducted a multicenter phase II study approved by the local ethical committee, registered with the health authorities (EudraCT 2011‐000649‐20). The primary endpoint was pCR rate. Secondary endpoints were to assess safety, pathological downstaging, R0 (circumferential resection margin [CRM] >1 mm) resection rate, sphincter‐saving surgery, time of DFS, OS, and correlation between biological and metabolic markers and pathological response.
The eligibility criteria included histologically proven rectal adenocarcinoma of the mid‐low rectum (within 12 cm from the anal verge), wild‐type KRAS gene status, Karnofsky performance status ≥70%, stage cT3N−M0 and cT2–3N+M0 (N+ stage is defined as ≥3 lymph nodes of diameter ≥0.5 cm measured by endorectal ultrasound or ≥1 lymph node of diameter ≥1 cm measured by magnetic resonance imaging [MRI]), no previous treatment with chemotherapy or radiation therapy, neutrophil count ≥1,500/µL, platelet count ≥100.000/µL, hemoglobin ≥9.0 g/dL, serum creatinine <1.5 × upper limit of normal (ULN), alanine aminotransferase and aspartate aminotransferase ≤2.5 × ULN, total bilirubin <1.5 × ULN, and signed written informed consent. Patients with distant metastases were excluded from the study.
The baseline evaluation included history, physical examination (including digital rectal examination), recording of concomitant medication, laboratory tests (hematology and clinical chemistry, carcinoembryonic antigen, and cancer antigen 19.9), full colonoscopy, rigid rectoscopy, biopsy, endorectal ultrasound and/or pelvis magnetic resonance, and thorax and abdomen‐pelvis computed tomography.
Eligible patients were enrolled and treated with the preoperative treatment with panitumumab, in combination with external pelvic radiotherapy, followed by surgery with TME and adjuvant chemotherapy with FOLFOX4. Panitumumab was administered by intravenous (IV) infusion at a dose of 6 mg/kg once every 2 weeks for three cycles in combination with radiotherapy. RT was delivered up to a dose of 5,040 cGy in daily fractions of 1.8 Gy on 5 consecutive days per week (day 1–day 38). 5–6 weeks after the end of preoperative treatment, the patients were restaged with rectal palpation and pelvic MRI. Rectal surgery with TME was performed 6–8 weeks after the end of preoperative treatment. A radical resection (R0) was defined as the removal of all macroscopic tumor tissue, no evidence of distant metastases, the absence of microscopic residual tumor, free resection margins, and lymphoadenectomy extending beyond the involved nodes at postoperative pathologic examination. A resection was judged as nonradical if a microscopic (R1) or macroscopic (R2) residual tumor (distance between the tumor and CRM ≤1 mm) was found. Furthermore, each specimen was classified using a tumor regression grade proposed by Dworak et al. [27]. Tumor downstaging was determined by comparing the pathologic stage with the baseline clinical TNM stage; pCR was defined as the absence of viable tumor cells in the primary tumor and lymph nodes (ypT0N0).
Patients who proceeded to rectal surgery received adjuvant chemotherapy with FOLFOX performed between a minimum of 4 weeks and a maximum of 6 weeks after rectal surgery for 12 cycles. The patients were treated in the adjuvant setting until completion of the chemotherapy regimen or until withdrawal of consent or unacceptable toxicities.
Statistical Analysis
The primary endpoint was the pCR rate after preoperative treatment. A pCR of 16% was considered to qualify the experimental treatment for further testing. The sample size was calculated using Simon's two‐stage design. A pCR rate of ≤7% was ruled out as futile. The first stage required at least 3 patients out of 29 to have a confirmed pCR before proceeding to the second stage. In the second stage, 63 assessable patients could be added, and if a total of 11 or more patients achieved a confirmed pCR, then the primary endpoint would have been met. A maximum of 100 patients could be enrolled to ensure 92 treated patients. A time‐to‐event distribution of secondary endpoints was estimated with the Kaplan‐Meier method.
Collateral Studies
The pathological response and overall survival was measured in correlation with the biopathological characterization of rectal biopsy: immunohistochemistry (p53, BCL2, Ki67, TS, EGFR, ERK, PTEN, GLUT1) at baseline and after 2 weeks of treatment; cDNA microarrays of biopsy at baseline and after 2 weeks of treatment; mutational status evaluation of genes for intracellular effectors (NRAS, BRAF, PIK3CA) of rectal biopsy at baseline and the evaluation of serum biomarkers (EGF, TGF‐α, soluble EGFR, VEGF, E‐selectin) on days 1, 14, 28, and 38.
Fluorodeoxyglucose (FDG) positron emission tomography (PET) scan evaluation was performed for early prediction of response (baseline and after 2 weeks of treatment). Changes in quality of life assessment were evaluated using the EQ‐5D Health Questionnaire (EuroQol Group, Rotterdam, The Netherlands) six times: before the start of preoperative treatment, during treatment (week 4), after the end of treatment, before and after surgery, and after the end of adjuvant treatment. Questionnaire compliance rates were ascertained at each measurement time.
Results
Patients and Treatment
From October 2012 to October 2015, 98 patients were enrolled in 13 Italian centers. All 98 patients were evaluated for safety, and 92 were assessable for response.
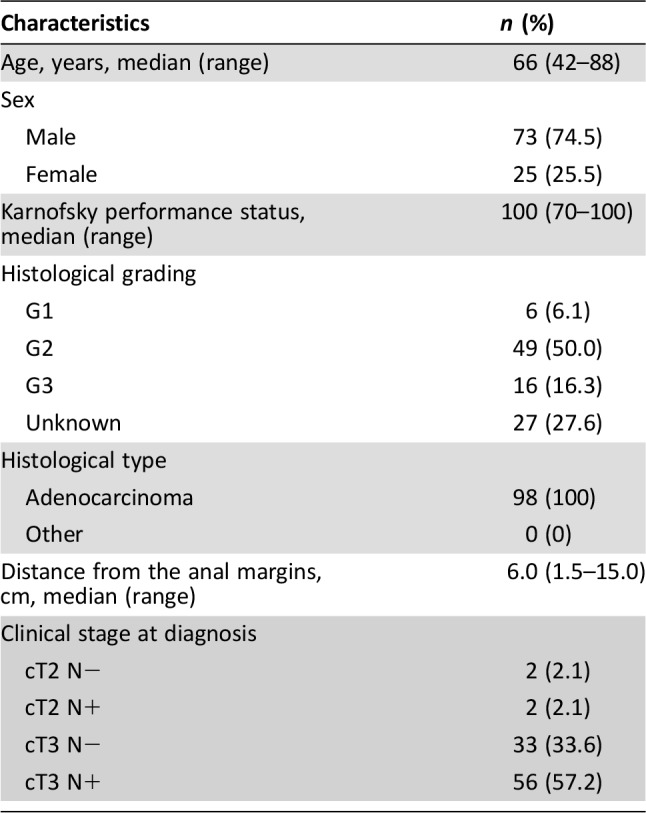
Patient characteristics are listed in Table 1. The majority of patients were male (74.5%); their median age was 66 years (range 42–88 years), and their median Karnofsky performance status was 100 (range 70–100). The median distance from the anal margin was 6 cm (range 1.5–15 cm). The clinical stage was cT2 in 4/98 (4.1%) and cT3 in 89/98 (90.8%) of patients. The majority of patients, 59/98 (60.2%), had lymph node involvement (cN+).
Table 1. Patient demographics and disease characteristics (n = 98).
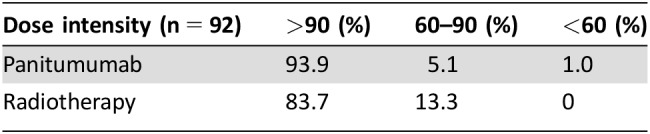
The dose intensity of panitumumab and radiotherapy is reported in Table 2. The panitumumab dose intensity >90% was delivered in 92/98 (93.9%) of patients. The full dose of planned radiotherapy was administered in 82/98 (83.7%), and >90% was administered in 83/98 (84.7%) of patients.
Table 2. Dose intensity of neoadjuvant treatment.
Surgical Procedures and Pathological Responses
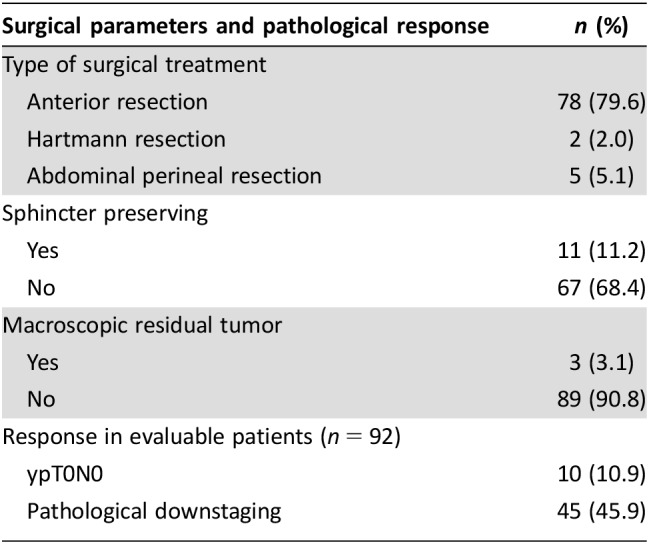
The data on the surgical procedures and the pathological responses are listed in Table 3. Six patients did not undergo surgery: two patients because disease progression during the neoadjuvant treatment, one patient because of refusal, two patients lost to follow‐up, and one for unknown reasons. Ninety‐two (93.9%) patients underwent surgery and were evaluable for pathological response. TME surgery was performed in all patients. Seventy‐eight (79.6%) were treated with a low anterior resection, two (2.0%) with a Hartmann's procedure, and five (5.1%) with an abdominoperineal resection. No postoperative deaths occurred; only in three (3.1%) patients intraoperative complication occurred, such as bleeding, organ damage, or packaging of ileostomy. Resection R0 at the primary tumor site was achieved in 89/92 (90.8%) of patients. Anal sphincter loss was performed in 67/92 (68.4%). Pathological complete response (ypT0N0) was 10.9% (95% confidence interval, 4.72%–17.07%), observed in 10/92 patients. Compared with the clinical stage before preoperative treatment, pathological downstaging (T and/or N) occurred in 45 (45.9%) patients.
Table 3. Surgical parameters and pathological response (n = 92).
Safety Analysis
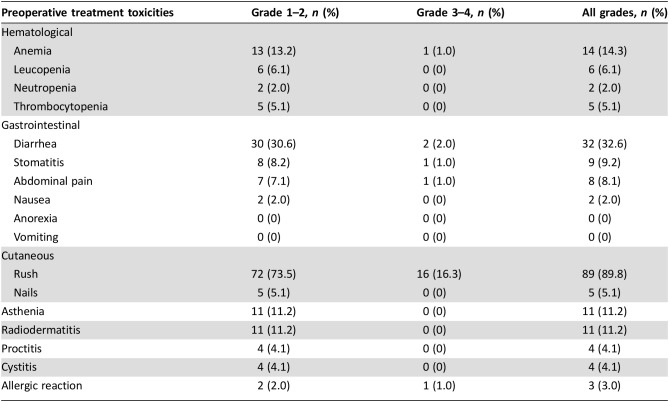
All 98 patients were evaluated for toxicity. Adverse events observed during neoadjuvant treatment are listed in Table 4. Skin rash was the most observed toxicity, with 16 (16.3%) patients developing grade 3 skin rash. Grade 3 diarrhea occurred in two (2.1%) patients. No grade 4 toxicities were observed. All grades of skin rash occurred in 89 (89.9%) patients, diarrhea in 32 (32.6%), and anemia in 14 (14.3%).
Table 4. Preoperative treatment toxicities.
Postoperative Treatment
Postoperative treatment was planned between a minimum of 4 weeks and a maximum of 6 weeks after surgery; 46 (46.9%) patients received chemotherapy according to the FOLFOX regimen for 12 cycles. Of the 65 patients known to be evaluated for safety, 16 (16.3%) developed toxicity of grade ≥3, particularly neutropenia, in 11 (11.2%) patients, and leucopenia in 5 (5.1%).
Discussion
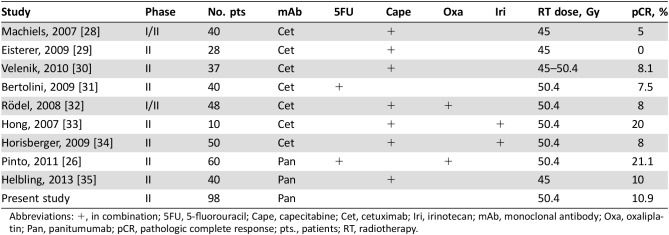
In this phase II trial, the addition of panitumumab to radiotherapy in low‐risk LARC was not shown to reach the primary endpoint pCR (10.9%). This was the first preoperative trial in LARC that did not use chemotherapy in addition to monoclonal antibody and radiotherapy. In the literature there have been several phase II studies conducted using cetuximab or panitumumab in combination with fluoropyrimidine ± oxaliplatin, some of which are summarized in Table 5.
Table 5. Phase II studies of preoperative chemoradiation using anti‐epidermal growth factor receptor monoclonal antibodies.
Abbreviations: +, in combination; 5FU, 5‐fluorouracil; Cape, capecitabine; Cet, cetuximab; Iri, irinotecan; mAb, monoclonal antibody; Oxa, oxaliplatin; Pan, panitumumab; pCR, pathologic complete response; pts., patients; RT, radiotherapy.
In the Belgian study [28], 40 patients were treated with cetuximab in combination with capecitabine 650 and 825 mg/m2 twice daily (recommended dosage) continuously for the duration of radiotherapy (45 Gy in 25 fractions) with pCR in 2 (5%) patients. No pCR was obtained in 28 patients treated with cetuximab in combination with capecitabine 825 mg/m2 twice daily on radiotherapy days (45 Gy in 25 fractions) [29]. Velenik et al. [30] treated 37 patients with cetuximab added to capecitabine 825 mg/m2 twice daily continuously for the duration of radiotherapy (45 Gy in 25 fractions), obtaining pCR in 3 (8%) patients. In another study, cetuximab and 5‐fluorouracil 225 mg/m2 per day continuous infusion concomitantly with radiotherapy (45–50.4 Gy in 25–28 fractions) were administered to 40 patients, with pCR in 3 (7.5%) patients [31]. The association of cetuximab with capecitabine 825 mg/m2 twice daily (days 1–14 and 22–35), oxaliplatin 50 mg/m2 (days 1, 8, 22, and 29), and radiotherapy (50.4 Gy in 28 fractions) was evaluated in 48 patients and a pCR was reached in 4 (8%) patients [32].
Two other phase II trials evaluated the addition of cetuximab to capecitabine plus irinotecan and radiotherapy. Cetuximab was added to capecitabine 825 mg/m2 twice daily (5 days a week), irinotecan 40 mg/m2 (days 1, 8, 15, 22, and 29), and radiotherapy (50.4 Gy in 28 fractions) in 10 patients, with pCR in 2 (20%) patients [33]. In the MARGIT study [34], 50 patients received cetuximab in combination with capecitabine 500 mg/m2 twice daily continuously, irinotecan 40 mg/m2 (days 1, 8, 15, 22, and 29), and radiotherapy (50.4 Gy in 28 fractions), obtaining pCR in 4 (8%) patients. The biological mechanisms for the disappointing results of cetuximab in combination with fluoropyrimidine‐based chemoradiotherapy in these phase II studies [28], [29], [30], [31], [32], [34] may arise because of changes in tumor cell proliferation and cell cycle distribution after cetuximab administration with cell arrest in G1 or G2‐M and failure to pass through the S phase.
These activities are lacking when 5‐fluorouracile with capecitabine is administered in monotherapy [28], [29], [30], [31] or in combination with oxaliplatin with intermittent schedules [32]. Capecitabine with 5‐fluorouracil and oxaliplatin both produced their optimal cytotoxic and radiosensitizing effect when cells proliferate into the S, G2, and M phases. The activity of fluoropyrimidines in combination with irinotecan seems to be preserved only with a full capecitabine dose [33], [34].
The use of panitumumab was evaluated in two studies. In the first, the StarPan/STAR‐02 study [26], 5‐fluorouracil 225 mg/m2 was administered continuously (without intervals), and IV oxaliplatin 60 mg/m2 was administered weekly, over a 6 week period; radiotherapy (50.4 Gy in 28 fractions) and panitumumab at an IV dose of 6 mg/kg over 1 hour was administered 2 weeks before the start of chemoradiotherapy (day −14) and then in combination with chemoradiotherapy every 2 weeks, for a total of 3 times, obtaining a higher rate of pCR in 12/60 (20%) patients, reporting a high‐grade (3–4) toxicity rate. In this study, the high pCR was probably due to the different chemotherapy schedules applied that could overcome the antagonistic effect of anti‐EGFR monoclonal antibodies.
In the second study, the SAKK 41/07 phase II trial [35], 40 patients with wild‐type KRAS LARC were randomized to receive IV panitumumab 6 mg/kg every 2 weeks for 8 weeks plus capecitabine 825 mg/m2 twice daily throughout RT (total dose of 45 Gy in 25 fractions of 1.8 Gy over 5 weeks), starting 7 days after the first panitumumab administration. The primary endpoint was pathological near‐complete (pNC) or complete tumor response (CR) rate (pNC/CR), defined as grade 3 (pNCR) or grade 4 (pCR) histological regression by Dworak classification (DC). The results obtained were translated into a high pNC/CR rate, mostly grade 3 DC. The most common toxicities were grade ≥3, such as diarrhea (10%), hand‐foot syndrome (2%), fatigue (2%), acneiform skin rash (2%), and anastomotic leakage (15%), which led to treatment discontinuation in five patients; furthermore, there were two deaths during the safety monitoring period resulting from anastomotic leakage.
In our study, no grade 4 toxicities were reported, only the specific side effects associated with panitumumab such as skin rash (16.3% grade 3 and 89.9% all grades).
The role of KRAS mutation in determining the response to EGFR‐targeted monoclonal antibodies in the preoperative setting is not very clear. In previous phase II studies in LARC that did not select patients by KRAS status, KRAS and/or BRAF mutation did not correlate with pCR after preoperative treatment containing cetuximab or panitumumab, probably because of the low numbers of patients in these studies [26], [36], [37], [38], [39], [40]. In our study, we excluded patients with tumors harboring KRAS mutation. Further mutational status evaluation of genes for intracellular effectors such as NRAS and BRAF is ongoing.
On the basis of previous results of the StarPan RaP‐02 study that showed how 18F‐FDG uptake was decreased only by panitumumab administration and markedly by combination therapy compared with the basal value, correlated with a reduction in tumor cell proliferation after panitumumab alone and later the addition of chemoradiotherapy to panitumumab increasing cytotoxic activity, in our study, earlier PET evaluation was used as a marker of cellular proliferation downregulation.
Conclusion
The use of anti‐EGFR monoclonal antibodies in combination with radiotherapy alone in preoperative treatment in patients with KRAS wild‐type, low‐risk LARC did not reach the primary endpoint, resulting in a pCR rate of 10.9%.
The analysis showed a good toxicity profile and compliance to concomitant administration of panitumumab and external radiotherapy in the preoperative treatment of LARC. The main toxicity was cutaneous without affecting treatment adherence. Further analysis of tissue NRAS and BRAF and circulating levels of the EGFR ligands (TGF‐α, EGF) and vascular factors (soluble VEGF, E‐selectin) will provide insight into the potential molecular pathways involved in the anti‐EGFR response and could serve as a predictor of tumor downstaging.
Compared with what is described in the treatment of locoregionally advanced head and neck cancer with concomitant high‐dose radiotherapy plus cetuximab that improves locoregional control and reduces mortality without increasing the common toxic effects associated with radiotherapy, in LARC there are no positive data that support the use of only anti‐EGFR with radiotherapy. Nevertheless, it is estimated that 30%–40% of rectal cancer occurs in patients aged 75 years or more. Data on adherence to preoperative CRT and its safety remain poor because of the under‐representation of older patients in randomized clinical trials and the discordance of the results from retrospective studies. A higher prevalence of comorbidities and a degradation of performance status limits the use of standard therapies in older patients. Perhaps in frail elderly patients, the use of single agent anti‐EGFR antibody in combination with radiotherapy might be a prospective strategy.
Acknowledgments
The authors thank Amgen for financial support.
This article was published online on 09 March 2018. An error was subsequently identified in an Author's affiliation. This notice is included in the online and print versions to indicate that both have been corrected 17 April 2018.
Author Contributions
Conception/design: Carmine Pinto, Maurizio Di Bisceglie, Francesca Di Fabio, Annamaria Bochicchio, Tiziana Latiano, Stefano Cordio, Gerardo Rosati, Carlo Aschele, Antonella Marino, Francesca Bergamo, Sara Bustreo, Luca Frassineti, Fortunato Ciardiello, Angela Damato, Stefania Giaquinta, Daniela Baldari, Luca Boni
Provision of study material or patients: Carmine Pinto, Maurizio Di Bisceglie, Francesca Di Fabio, Annamaria Bochicchio, Tiziana Latiano, Stefano Cordio, Gerardo Rosati, Carlo Aschele, Antonella Marino, Francesca Bergamo, Sara Bustreo, Luca Frassineti, Fortunato Ciardiello, Angela Damato, Stefania Giaquinta, Daniela Baldari, Luca Boni
Collection and/or assembly of data: Luca Boni
Data analysis and interpretation: Luca Boni
Manuscript writing: Carmine Pinto, Maurizio Di Bisceglie, Francesca Di Fabio, Annamaria Bochicchio, Tiziana Latiano, Stefano Cordio, Gerardo Rosati, Carlo Aschele, Antonella Marino, Francesca Bergamo, Sara Bustreo, Luca Frassineti, Fortunato Ciardiello, Angela Damato, Stefania Giaquinta, Daniela Baldari, Luca Boni
Final approval of manuscript: Carmine Pinto, Maurizio Di Bisceglie, Francesca Di Fabio, Annamaria Bochicchio, Tiziana Latiano, Stefano Cordio, Gerardo Rosati, Carlo Aschele, Antonella Marino, Francesca Bergamo, Sara Bustreo, Luca Frassineti, Fortunato Ciardiello, Angela Damato, Stefania Giaquinta, Daniela Baldari, Luca Boni
Disclosures
The authors indicated no financial relationships.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- 2. Kapiteijn E, Marijnen CA, Nagtegaal ID et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638–646. [DOI] [PubMed] [Google Scholar]
- 3. Rödel C, Martus P, Papadoupolos T et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688–8696. [DOI] [PubMed] [Google Scholar]
- 4. Capirci C, Valentini V, Cionini L et al. Prognostic value of pathological complete response after neoadjuvant therapy in locally advanced rectal cancer: Long‐term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 2008;72:99–107. [DOI] [PubMed] [Google Scholar]
- 5. Moertel CG, Childs DS Jr, Reitemeier RJ et al. Combined 5‐fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet 1969;2:865–867. [DOI] [PubMed] [Google Scholar]
- 6. Bosset JF, Calais G, Mineur L et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: Preliminary results–EORTC 22921. J Clin Oncol 2005;23:5620–5627. [DOI] [PubMed] [Google Scholar]
- 7. Gérard JP, Conroy T, Bonnetain F et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: Results of FFCD 9203. J Clin Oncol 2006;24:4620–4625. [DOI] [PubMed] [Google Scholar]
- 8. Sauer R, Becker H, Hohenberger W et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 9. Bosset JF, Collette L, Calais G et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–1123. [DOI] [PubMed] [Google Scholar]
- 10. Gérard JP, Azria D, Gourgou‐Bourgade S et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405‐Prodige 2. J Clin Oncol 2010;28:1638–1644. [DOI] [PubMed] [Google Scholar]
- 11. Gérard JP, Azria D, Gourgou‐Bourgade S et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol 2012;30:4558–4565. [DOI] [PubMed] [Google Scholar]
- 12. Aschele C, Cionini S, Lonardi S et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR‐01 randomized phase III study. J Clin Oncol 2011;29:2773–2780. [DOI] [PubMed] [Google Scholar]
- 13. O'Connell MJ, Colangelo LH, Beart RW et al. Captecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: Surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R‐04. J Clin Oncol 2014;32:1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rödel C, Graeven U, Fietkau R et al. Oxaliplatin added to fluorouracil‐based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO‐04 study): Final results of the multicenter, open‐label, randomized, phase 3 trial. Lancet Oncol 2015;16:979–989. [DOI] [PubMed] [Google Scholar]
- 15. Willet CG, Kozin SV, Duda DG et al. Combined vascular endothelial growth factor‐targeted therapy and radiotherapy for rectal cancer: Theory and clinical practice. Semin Oncol 2006;33(5 suppl 10):S35–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fornaro L, Caparello C, Vivaldi C et al. Bevacizumab in the pre‐operative treatment of locally advanced rectal cancer: A systematic review. World J Gastroenterol 2014;20:6081–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giralt J, de las Heras M, Cerezo L et al. The expression of epidermal growth factor receptor results in a worse prognosis for patients with rectal cancer treated with preoperative radiotherapy: A multicenter, retrospective analysis. Radiother Oncol 2005;74:101–108. [DOI] [PubMed] [Google Scholar]
- 18. Kim JS, Kim JM, Li S et al. Epidermal growth factor receptor as a predictor of tumor downstaging in locally advanced rectal cancer patients treated with preoperative chemioradiotherapy. Int J Radiat Oncol Biol Phys 2006;66:195–200. [DOI] [PubMed] [Google Scholar]
- 19. Koop R, Rothbauer E, Mueller E et al. Reduced survival of rectal cancer patients with increased tumor epidermal growth factor receptor levels. Dis Colon Rectum 2003;46:1391–1399. [DOI] [PubMed] [Google Scholar]
- 20. Bonner JA, Maihle NJ, Folven BR et al. The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivities. Int J Radiat Oncol Biol Phys 1994;29:243–247. [DOI] [PubMed] [Google Scholar]
- 21. Liang K, Ang KK, Milas L et al. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys 2003;57:246–254. [DOI] [PubMed] [Google Scholar]
- 22. Bonner JA, Harari PM, Giralt J et al. Radiotherapy plus cetuximab for squamous‐cell carcinoma of the head and neck. N Engl J Med 2006;354:567–578. [DOI] [PubMed] [Google Scholar]
- 23. Bonner JA, Harari PM, Giralt J et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5‐year survival data from a phase 3 randomised trial, and relation between cetuximab‐induced rash and survival. Lancet Oncol 2010;11:21–28. [DOI] [PubMed] [Google Scholar]
- 24. Baumann M, Krause M, Dikomey E et al. EGFR‐targeted anti‐cancer drugs in radiotherapy: Preclinical evaluation of mechanisms. Radiother Oncol 2007;83:238–248. [DOI] [PubMed] [Google Scholar]
- 25. Glynne‐Jones R, Mawdsley S, Harrison M. Cetuximab and chemoradiation for rectal cancer–Is the water getting muddy? Acta Oncol 2010;49:278–286. [DOI] [PubMed] [Google Scholar]
- 26. Pinto C, Di Fabio F, Maiello E et al. Phase II study of panitumumab, oxaliplatin, 5‐fluorouracil, and concurrent radiotherapy as preoperative treatment in high‐risk locally advanced rectal cancer patients (StarPan/STAR‐02 study). Ann Oncol 2011;22:2424–2430. [DOI] [PubMed] [Google Scholar]
- 27. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997;12:19–23. [DOI] [PubMed] [Google Scholar]
- 28. Machiels JP, Sempoux C, Scalliet P et al. Phase I/II study of preoperative cetuximab, capecitabine, and external beam radiotherapy in patients with rectal cancer. Ann Oncol 2007;18:738–744. [DOI] [PubMed] [Google Scholar]
- 29. De Eisterer WM, Vries A, Oefner D et al. Neoadjuvant chemoradiation therapy with capecitabine (X) plus cetuximab (C), and external beam radiotherapy (RT) in locally advanced rectal cancer (LARC): ABCSG trial R03. J Clin Oncol 2009;27(suppl 15):4109A. 19636001 [Google Scholar]
- 30. Velenik V, Ocvirk J, Oblak I et al. A phase II study of cetuximab, capecitabine and radiotherapy in neoadjuvant treatment of patients with locally advanced resectable rectal cancer. Eur J Surg Oncol 2010;36:244–250. [DOI] [PubMed] [Google Scholar]
- 31. Bertolini F, Chiara S, Bengala C et al. Neoadjuvant treatment with single‐agent cetuximab followed by 5‐FU, cetuximab, and pelvic radiotherapy: A phase II study in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2009;73:466–472. [DOI] [PubMed] [Google Scholar]
- 32. Rödel C, Arnold D, Hipp M et al. Phase I–II trial of cetuximab, capecitabine, oxaliplatin, and radiotherapy as preoperative treatment in rectal cancer. Int J Radiat Oncol Biol Phys 2008;70:1081–1086. [DOI] [PubMed] [Google Scholar]
- 33. Hong YS, Kim DY, Lee KS et al. Phase II study of preoperative chemoradiation (CRT) with cetuximab, irinotecan and capecitabine in patients with locally advanced resectable rectal cancer. J Clin Oncol 2007;25(suppl 18):4045A. [DOI] [PubMed] [Google Scholar]
- 34. Horisberger K, Treschl A, Mai S et al. Cetuximab in combination with capecitabine, irinotecan, and radiotherapy for patients with locally advanced rectal cancer: Results of a phase II MARGIT trial. Int J Radiat Oncol Biol Phys 2009;74:1487–1493. [DOI] [PubMed] [Google Scholar]
- 35. Helbling D, Bodoky G, Gautschi O et al. Neoadjuvant chemoradiotherapy with or without panitumumab in patients with wild‐type KRAS, locally advanced rectal cancer (LARC): A randomized, multicenter, phase II trial SAKK 41/07. Ann Oncol 2013;24:718–725. [DOI] [PubMed] [Google Scholar]
- 36. Kim SY, Hong YS, Kim DY et al. Preoperative chemoradiation with cetuximab, irinotecan, and capecitabine in patients with locally advanced resectable rectal cancer: A multicenter phase II study. Int J Radiat Oncol Biol Phys 2011;81:677–683. [DOI] [PubMed] [Google Scholar]
- 37. Bengala C, Bettelli S, Bertolini F et al. Epidermal growth factor receptor gene copy number, K‐ras mutation and pathological response to preoperative cetuximab, 5‐FU and radiation therapy in locally advanced rectal cancer. Ann Oncol 2009;20:469–474. [DOI] [PubMed] [Google Scholar]
- 38. Debucquoy A, Haustermans K, Daemen A et al. Molecular response to cetuximab and efficacy of preoperative cetuximab‐based chemoradiation in rectal cancer. J Clin Oncol 2009;27:2751–2757. [DOI] [PubMed] [Google Scholar]
- 39. Gaedcke J, Grade M, Jung K et al. KRAS and BRAF mutations in patients with rectal cancer treated with preoperative chemoradiotherapy. Radiother Oncol 2010;94:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dewdney A, Cunningham D, Tabernero J et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high‐risk rectal cancer (EXPERT‐C). J Clin Oncol 2012;30:1620–1627. [DOI] [PubMed] [Google Scholar]