This article explores the potential role of seriated troponin assessments during treatment with nivolumab for advanced non‐small cell lung cancer as a biomarker of nivolumab‐related cardiac toxicity.
Keywords: Cardiotoxicity, Troponin, Nivolumab, Immunotherapy, Lung cancer
Abstract
Background.
Rare cases of severe myocarditis are reported during treatment with nivolumab. Troponin, a biomarker of cardiac damage, is a key component of the diagnostic workup of many cardiac disorders, including myocarditis. This study investigates the role of troponin to assess cardiac involvement during nivolumab therapy for non‐small cell lung cancer (NSCLC).
Materials and Methods.
We evaluated 59 NSCLC patients, analyzing serum samples collected within a translational research study. Troponin above the upper normal limit (0.046 ng/mL) was defined as Tn+, whereas normal but detectable troponin (0.015–0.045) was defined as Tndet. Troponin alterations were interpreted on the grounds of the following elements: peak values and time curve, cardiac comorbidities, signs and symptoms coincident to troponin elevation, ECG, echocardiography, and disease progression.
Results.
No patient had cardiovascular events. Among 362 available blood samples, Tn+ (max 0.317 ng/mL) was found in 13 determinations belonging to 6 patients. Seven other patients had isolated Tndet. In five patients, Tn+ was attributed to cardiac comorbidities, disease progression, or worsening clinical status. One patient without cardiac history and in good clinical condition had a sustained troponin increase—soon after the start of therapy—and after careful evaluation of all relevant elements, it was interpreted as a marker of nivolumab‐related subclinical myocarditis.
Conclusion.
Tn+ may occur in NSCLC patients treated with nivolumab, but in most cases it does not indicate nivolumab cardiotoxicity. In some cases, however, a careful interpretation of troponin alteration, especially at the beginning of therapy, enables identification of subclinical myocarditis, thus allowing early cardiac treatment.
Implications for Practice.
Myocarditis is a rare but serious adverse event of immune checkpoint blockade with nivolumab, which needs to be recognized as soon as possible. This article suggests that troponin, a user‐friendly biomarker of myocardial cytotoxicity, might be useful for early detection of immune‐mediated myocarditis. However, because troponin abnormalities might also be related to a number of conditions capable of causing myocardial oxygen demand‐supply mismatch, a careful cardiac assessment should be performed in non‐small cell lung cancer patients in order to properly interpret any troponin increase. According to the available evidence, monitoring troponin during the first weeks of treatment can be considered reasonable.
摘要
背景. 在使用Nivolumab治疗期间报告了罕见的重症心肌炎病例。肌钙蛋白是心脏损伤的生物标志物,是诊断多种心脏疾病(包括心肌炎)的关键组成部分。本研究探讨肌钙蛋白在非小细胞肺癌(NSCLC)治疗中评估心脏受累程度所起的作用。
材料与方法.我们评估了59例NSCLC患者,分析了转化研究中采集的血清样本。高于正常上限的肌钙蛋白(0.046 ng/mL)定义为Tn+,而正常但可检测到的肌钙蛋白(0.015‐0.045)定义为 Tndet。肌钙蛋白的变化根据以下因素解释:峰值和时间曲线、心脏合并症、与肌钙蛋白升高相一致的症状和体征、ECG、超声心动图、疾病进展情况。
结果.患者未出现心血管事件。362份有效血样中,6例患者的13份血样测出Tn+(最高0.317 ng/mL)。另外7例患者仅检出Tndet。在5例患者中,Tn+被认为是心脏并发症、疾病进展或临床状况恶化所致。1例无心脏病史的患者,临床状况良好,治疗开始后不久肌钙蛋白持续升高,经仔细评估所有相关因素后,持续升高的肌钙蛋白解释为与Nivolumab相关的亚临床心肌炎的标志物。
结论.NSCLC患者使用Nivolumab治疗时可能出现Tn+,但在大多数情况下,并不代表Nivolumab有心脏毒性。然而,在某些情况下,仔细解释肌钙蛋白的变化,特别是在治疗开始时,可以识别亚临床心肌炎,从而使早期心脏治疗成为可能。
实践启示:心肌炎是一种罕见,但严重的免疫检查点阻断剂Nivolumab治疗的不良事件,需要尽快识别。本文认为肌钙蛋白是一种识别心肌细胞毒性有用的生物标志物,可用于早期发现免疫介导的心肌炎。然而,由于肌钙蛋白异常也可能与一些可能导致心肌氧供需不匹配的情况有关,因此应在非小细胞肺癌患者中仔细评估,正确解释任何肌钙蛋白增加的情况。根据现有证据,在治疗头几周监测肌钙蛋白被认为是合理的。
Introduction
Monoclonal antibodies targeting programmed cell death protein 1 (PD‐1) have demonstrated impressive clinical efficacy against cancer, and several of these compounds are currently approved for the treatment of many forms of malignant tumors including melanoma, renal cell carcinoma [1], urothelial carcinoma [2], head and neck cancer [3], non‐small cell lung cancer (NSCLC) [4], [5], [6], and Hodgkin lymphoma after transplant [7]. In addition, ongoing clinical studies are now evaluating extending their clinical indications to other solid and hematologic malignancies. There is a widespread consensus [8], [9] that this therapy marks a turning point in the fight against cancer. This belief arises not only from the proven efficacy of these medications, but also from the conviction that anti‐PD‐1 treatment has a great safety window. In fact, the PD‐1 blockade has been viewed as an intervention that restores antitumor activity to a desirable level without triggering excessive autoimmune response [10]. Nevertheless, it has been recognized that immune‐related adverse events (irAEs) may occur during anti‐PD‐1 therapy, mainly due to gastrointestinal, dermatologic, hepatic, endocrine, and pulmonary toxicities, which can be severe and lead to treatment discontinuation [11], [12].
Very recently, there have been several reports of severe, sometimes fatal myocarditis [13], [14], [15], [16]. The review of Bristol‐Myers Squibb corporate safety databases suggest that these myocarditis events are serious, but uncommon, at least with nivolumab alone [15], [17]. Among 17,620 patients treated with nivolumab, only 10 drug‐related severe adverse events consistent with myocarditis were found (0.06%). However, we do not have a full understanding of the true incidence of immune therapy‐related myocarditis, and we do not even know whether the severity of these adverse events is a distinctive feature of immune checkpoint inhibitor‐related myocarditis. Published papers on nivolumab‐related cardiac adverse effects were case reports and retrospective studies, which by their nature mainly focus on events that are clinically relevant. In clinical trials involving nivolumab, there was no routine testing for myocarditis by means of either biochemical analysis or cardiac imaging. Furthermore, adverse event recording from oncologists is impacted by the likelihood that the event was expected and treatment related. Unexpected events for which the investigators have limited experience—such as myocarditis—can be undiagnosed or unreported.
Cardiac troponin is a protein present in the cardiac myocytes, and in no other tissue, which is released into the bloodstream when the heart suffers from all sorts of damage. For this reason, troponin testing plays an important role in the management of many cardiac diseases. Troponin is primarily the biomarker of choice in acute coronary syndromes and is pivotal for the diagnosis of acute myocardial infarction. Troponin may also be found in a number of other heart conditions, such as myocarditis, heart failure, cardiomyopathies including takotsubo cardiomyopathy, aortic valve stenosis, tachyarrhythmias, pulmonary embolism, and aortic dissection. In all the situations listed above, serum troponin is a marker of myocardial injury, even when it is present at very low serum concentrations, and brings important diagnostic and/or prognostic information [18], [19], [20]. Practical guidelines state that troponin testing is a key component of the diagnostic workup whenever myocarditis is suspected [21].
The aim of the present study was to explore the potential role of seriated troponin assessments during treatment with nivolumab for advanced NSCLC as a biomarker of nivolumab‐related cardiac toxicity.
Materials and Methods
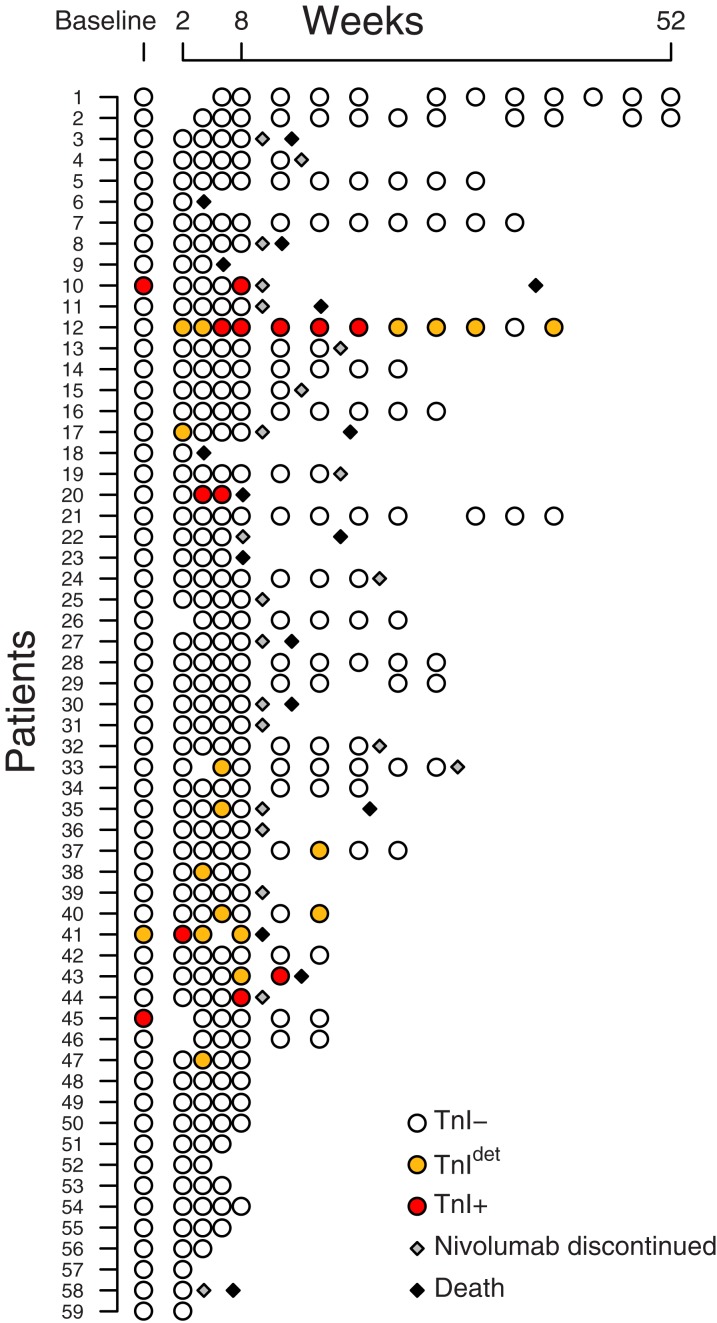
From April 2015 to June 2016, 74 patients with advanced pretreated NSCLC were enrolled within a single‐institutional translational research study at Lung Cancer Unit of IRCCS San Martino‐IST, which was aimed at investigating predictive factors of response and toxicity during nivolumab treatment. The main inclusion criteria were as follows: age ≥18 years, histologically or cytologically confirmed NSCLC, clinical stage IIIb or IV (according to tumor‐Node‐Metastases classification (TNM) v7.0), at least one previous line of systemic therapy for advanced NSCLC, at least one measurable lesion by RECIST v.1.1, previously treated or stable brain metastases from at least 2 weeks before the treatment with nivolumab, and no steroids treatment with more than 10 mg/day of prednisone or equivalent was allowed. Nivolumab was provided by Bristol‐Myers Squibb within the Italian expanded access program in NSCLC and was administered at the dose of 3 mg/kg every 14 days. Blood samples were collected at the time of the first five nivolumab administrations, just before the drug infusion, and only at odd administrations thereafter (Fig. 1). Serum and plasma were stored at −80°C to allow subsequent determinations.
Figure 1.
Time point of troponin evaluation in the 59 enrolled patients. Each circle represents a blood sample (and troponin determination); nivolumab was administered every 2 weeks. Patients are ordered by date of enrollment.
Abbreviations: TnI−, troponin I negative; TnI+, troponin I positive; TnIdet, troponin I detectable.
Heart and lung physical examination, blood pressure measurement, and ECG had been performed at baseline: all patients were in stable cardiologic condition, suitable for nivolumab treatment by oncologist's judgment. An expert cardiologic consult was not planned because neither the product label nor medical literature requested it.
The design of the study was approved by the local Institutional Review Board. All the patients gave a written informed consent to participate in this study.
Troponin I (TnI) was determined on frozen serum aliquots by a homogeneous sandwich chemiluminescent immunoassay based on the Luminescent Oxygen Channeling Immunoassay technology [22]. Assay reagents consist of two synthetic bead reagents and a biotinylated anti‐cardiac TnI monoclonal antibody fragment (Siemens Healthineers, Milan, Italy). The assay is optimized on an automated platform (Dimension Vista System; Siemens Healthineers). In our laboratory, the range encompassing the 99th percentile corresponded to that reported by the manufacturer [23], being 0.000–0.045 ng/mL. The analytical sensitivity corresponds to 0.015 ng/mL and the functional sensitivity to 0.040 ng/mL. The within‐run imprecision, expressed as coefficient of variation, ranged from 2.9% to 6.6%, depending on the sample's TnI concentration.
We considered as troponin positive (TnI+) any value above the decisional cutoff value of 0.045 ng/mL (upper limit of normal [ULN]); troponin detectable (TnIdet) any value between 0.015 (lower limit of detectable) and 0.045 (ULN) ng/mL; and troponin negative (TnI−) any given result <0.015 ng/mL, which is the lowest result given by the assay, indistinguishable from zero. The decision to set troponin elevations during anticancer therapy by the cutoffs specific to the assay platform used in our own lab is in agreement with the recommendations of the Expert Consensus Paper of the American Society of Echocardiography and the European Association of Cardiovascular Imaging [24].
Three cardiologists (P.S., M.S., E.A.), who were blind to troponin determinations, examined medical records of all patients. Patients who were still receiving nivolumab underwent a cardiologic evaluation including physical examination, ECG, and echocardiogram, and other tests were only performed if clinically indicated. The information thus obtained was used to interpret the results of the troponin analysis.
Patients were categorized according to the estimated risk of troponin release, irrespective of nivolumab therapy: (a) very high risk if any cardiac disease, clinical or unequivocal on imaging (chronic heart failure or asymptomatic left‐ventricular dysfunction, coronary artery disease, moderate‐to‐severe cardiac valve disease, hypertensive cardiomyopathy, pulmonary hypertension, chronic atrial fibrillation or flutter) was reported; (b) high risk if extra‐cardiac target organ disease (peripheral artery disease, stage III–IV chronic kidney disease, history of stroke or transient ischemic attack) or diabetes was reported; and (c) low risk if none of the above criteria were observed.
Results
At the time of blood sample analysis, 59 patients had received at least two administrations of nivolumab. Patients’ characteristics are presented in Table 1. The median number of administered cycles was 5 (range 2–27). Forty‐five patients (76%) received at least the first five cycles of nivolumab. Seventeen patients died of a noncardiologic primary cause, and in 11 others, nivolumab had been discontinued by oncologist's judgment. Clinical or subclinical irAEs occurred in 13 patients (3 serious: thyroiditis, hepatitis, pancreatitis). The incidence of irAEs was consistent with previously published data on nivolumab, and these events were managed accordingly [25]. No adverse cardiovascular event occurred.
Table 1. Characteristics of the enrolled patients.
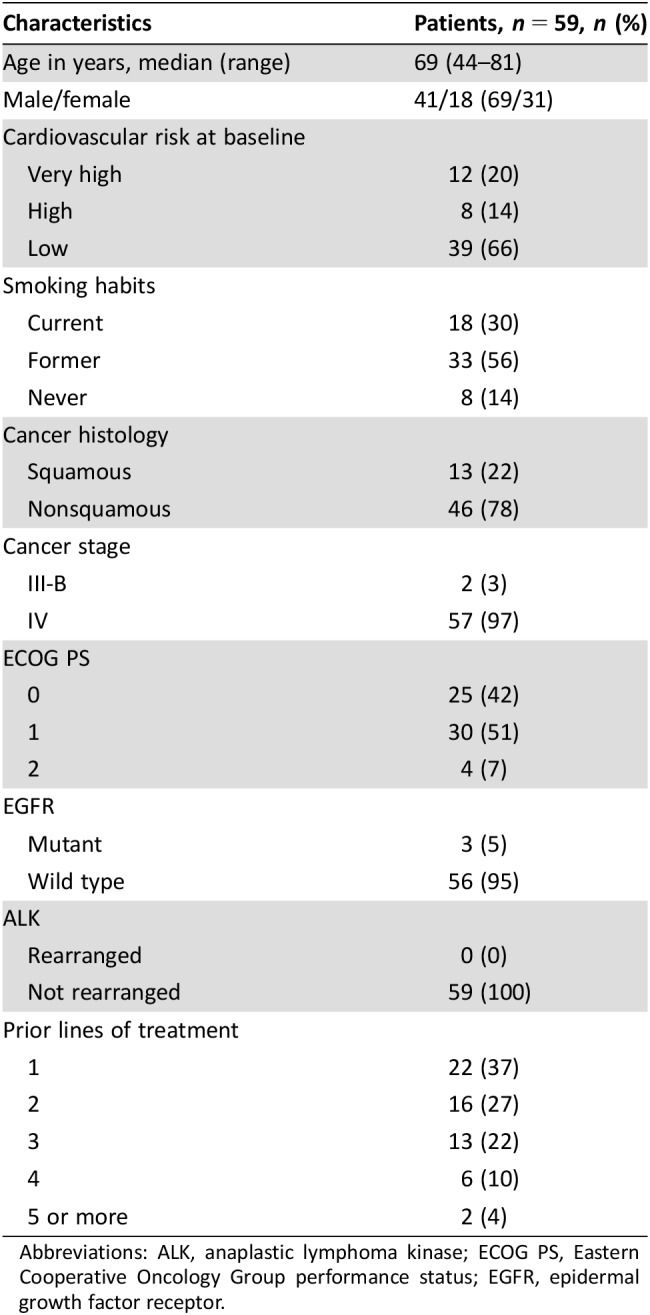
Abbreviations: ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor.
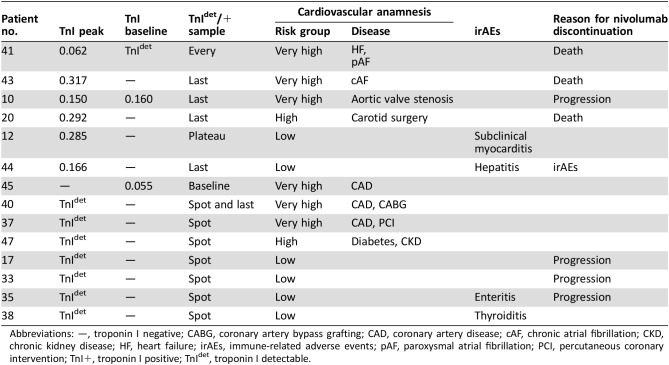
In total, 362 blood samples were available for TnI determination, including the 59 collected at baseline. At least one TnI value was ≥0.015 ng/mL in 14 patients, for a total of 31 samples: 13 TnI+ (mean = 0.153, median = 0.150, range 0.049–0.317 ng/mL) and 18 TnIdet . Distribution of TnI+ and TnIdet in the cohort is represented in Fig. 1, and detailed characteristics of the 14 patients with TnI alterations are shown in Table 2.
Table 2. Patients with troponin I positive or detectable (n = 14), ordered by TnI+/TnIdet and risk group.
Abbreviations: —, troponin I negative; CABG, coronary artery bypass grafting; CAD, coronary artery disease; cAF, chronic atrial fibrillation; CKD, chronic kidney disease; HF, heart failure; irAEs, immune‐related adverse events; pAF, paroxysmal atrial fibrillation; PCI, percutaneous coronary intervention; TnI+, troponin I positive; TnIdet, troponin I detectable.
At baseline, three patients had TnI+ or TnIdet value. All these patients were at very high risk due to clinical history or instrumental evidence of cardiovascular disease, and two showed TnI+ also on nivolumab treatment. During nivolumab exposure, six patients (10%) had at least one TnI+ value. A TnI+ patient had TnI+ or TnIdet in all samples, including the one collected before nivolumab exposure. This patient was at very high risk due to chronic heart failure but did not show cardiac worsening during nivolumab therapy.
In four TnI+ patients, troponin elevation occurred only in the last samples, just before nivolumab discontinuation. The decision to withdraw nivolumab was due to disease progression and worsening clinical status in three patients who were adjudicated as high or very high cardiovascular risk and to an immune‐related acute hepatitis requiring hospitalization and treatment with hydrocortisone and mycophenolate in a fourth patient who was adjudicated as low risk.
In the sixth TnI+ patient (patient 12), TnI+ was observed from the 8th to the 22nd week, preceded and followed by TnIdet in multiple samples, including the very first sample after nivolumab initiation. The cardiovascular risk had been rated low, and no cardiologic signs or symptoms were reported during nivolumab therapy. At the time of troponin determinations, 3 months after the last TnIdet, the patient was still alive, nivolumab was ongoing, troponin had become consistently negative, and no echocardiographic alteration was found.
Among the seven patients with TnIdet, mainly occurring in isolated “spot” samples, three were at high or very high risk; in two low‐risk patients, TnI detection was coincident with noncardiologic immune‐related adverse events, both responsive to steroidal treatment. The last two TnIdet patients were at low risk, and no clinical event occurred coincidentally with TnI detected sample.
Among the 45 patients who did not show troponin abnormalities during nivolumab therapy, 6 were adjudicated as very high risk, 6 as high risk, and 33 as low risk.
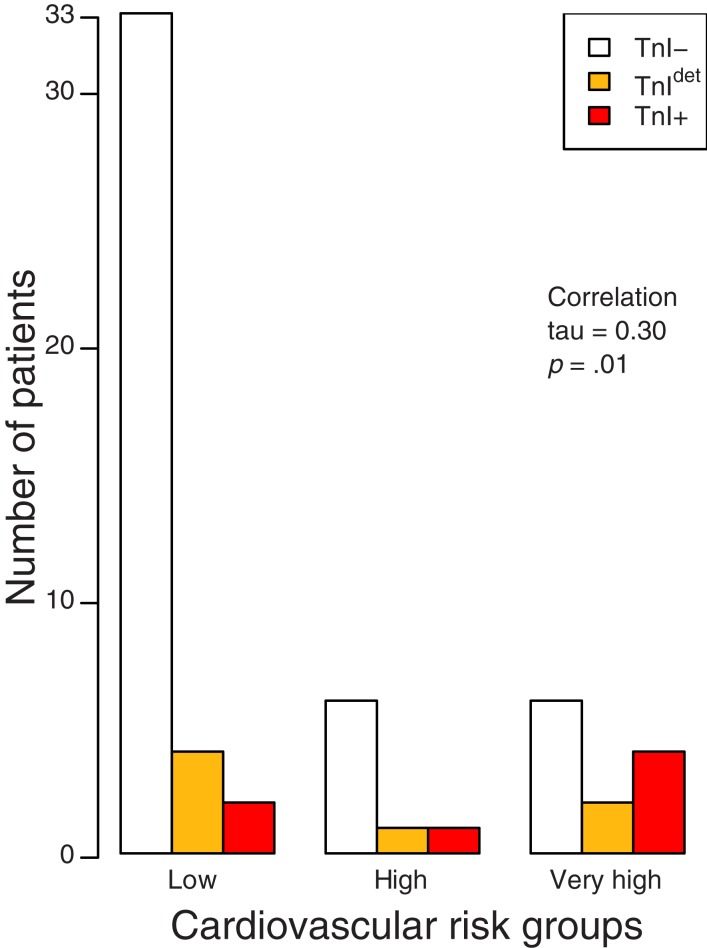
When the patients were divided according to the highest level of troponin (Fig. 2), categorized as TnI−, TnIdet, and TnI+, a correlation was found between the predefined group of cardiac risk (low, high, and very high) and the troponin alteration (coefficient of correlation tau: 0.30, p = .01).
Figure 2.
Relation between cardiac risk group and TnI+/TnIdet. Patients are divided by cardiac risk in three groups (low, high, and very high) and by troponin I in three groups (negative, detectable, positive). Kendall method is used for rank correlation.
Abbreviations: TnI−, troponin I negative; TnI+, troponin I positive; TnIdet, troponin I detectable.
Discussion
None of the 59 patients treated with nivolumab had cardiovascular events during the observation period. However, serial assessments of troponin plasma levels documented minor cases of myocardial cytolysis. In an attempt to figure out the possible causes of troponin elevation in these patients, we have looked at several factors: troponin peak values and time curve, pre‐existing cardiac disease, ECG, echocardiography, signs and symptoms coincident to troponin elevation, and cancer progression and clinical conditions of the patient [18], [19], [20], as well as the occurrence of noncardiac nivolumab‐related adverse events.
We have noticed that troponin elevation can be due to three reasons. The first reason is the presence of heart disease. The case of the asymptomatic patient with stable, chronic heart failure and trivial troponin abnormalities detected even before the start of nivolumab is a perfect example of this. The lack of symptoms is not surprising because it is known that troponin elevation may occur not only in the acute decompensated phase of heart failure, but also during the chronic phase [26]. The second reason is the deterioration of the clinical status and/or cancer progression, sometimes in association with pre‐existing, subclinical cardiac problems. In both conditions, troponin elevation may be the consequence of myocardial oxygen demand‐supply mismatch, which may result from many cardiac and noncardiac factors including myocardial wall stress, anemia, tachycardia, hypoxemia, inflammation, and exposure to catecholamine or cytokines [20]. Accordingly, troponin increases might be viewed as a bad prognostic index, but not as a specific biomarker of nivolumab toxicity.
The third possible reason of troponin elevation is nivolumab cardiotoxicity, as illustrated by the case of the patient who had for several weeks a minimal increase of troponin, at plateau, followed by late normalization of this biomarker. This patient had neither history of cardiac problems nor cardiac signs or symptoms for the duration of the treatment, and echocardiogram performed 3 months apart was normal. This troponin release was interpreted as the result of subclinical, self‐limiting, benign myocarditis. We acknowledge that our judgment relies only on a single diagnostic criterion of myocarditis [21]. On the other hand, we have carefully excluded other causes of troponin increase: the very long time curve was inconsistent with ischemic disease, and the patient had a very low cardiovascular risk profile, no symptoms, nor echocardiographic wall‐motion abnormalities. It should be said that troponin elevation alone has already been considered as a surrogate biomarker of possible subclinical myocarditis, such as in a previous study on healthy active subjects exposed to a pro‐immune trigger such as smallpox or influenza immunization [27]. We also noticed that three patients had troponin elevation (either TnI+ or TnIdet) that coincided with the presence of nivolumab‐related immune adverse events involving thyroid, liver, and gastrointestinal tract. Such troponin changes might be viewed as a marker of a subclinical immune‐mediated inflammatory process in the myocardium that accompanies a more severe immune adverse reaction in other districts. Alternatively, troponin might indicate the myocardial distress secondary to hemodynamic instability, neuro‐humoral activation, and generalized inflammatory condition secondary to thyroiditis, enteritis, or hepatitis.
It is worth noting that our interpretation of troponin elevation as the result of nivolumab‐related myocarditis is in agreement with a large body of experimental evidence showing that PD‐1 deletion or treatment with antibodies against PD‐1 or programmed death‐ligand 1 cause myocarditis [28], [29], [30], [31], [32], [33], [34], [35]. Moreover, a careful reading of experimental studies shows that interventions targeting checkpoint blockade are usually a cause mild myocardial inflammatory process, which may exacerbate in the presence of a second stressful condition. One might speculate that anti‐PD‐1 therapy just gives myocardium an enhanced susceptibility to inflammation that results in a full‐blown myocarditis only in the presence of particular concomitant conditions, such as infectious agents [34].
From a clinical point of view, even if severe myocarditis in the course of immune checkpoint blockade is a rare event, clinicians must be vigilant because of its nonspecific symptomatology and fulminant progression. It is not known what strategies could be adopted to monitor patients, but it would be reasonable to apply tight controls during the first few weeks of treatment, in which the risk would seem to be particularly high. The review of a large safety database of 20,594 patients treated with nivolumab alone or in combination with ipilimumab showed that the 18 documented cases of myocarditis were diagnosed very soon after the beginning of therapy (median 17 days, range 13–64) [15]. In an attempt to detect cardiovascular complications early, the authors of this article report their practice of performing weekly testing of troponin levels during weeks 1–3 of treatment, but they do not go into details in how to deal with troponin positivity. Most recently, Norwood et al. provided the first description of subclinical or smoldering myocarditis with mild symptoms following immune checkpoint blockade with a single dose of ipilimumab and nivolumab [36]. They performed a troponin testing as additional workup in a 49‐year‐old woman with metastatic melanoma and no cardiac risk factors who developed nausea unresponsive to treatment 14 days after the first dose of ipilimumab and nivolumab. Troponin was initially 0.19 (3× ULN). High‐dose glucocorticoid therapy led to rapid resolution of nausea and decreased TnI that, however, climbed again to 0.78 (13× ULN) following a steroid taper. Interestingly, ECG, echocardiogram, serum brain natriuretic peptide, exercise single‐photon emission computed tomography (SPECT) myocardial perfusion study and cardiac nuclear magnetic resonance (NMR) were normal, although endomyocardial biopsy showed areas of early collagen deposition admixed with inflammatory cells. Serum cardiac troponin I levels gradually improved with chronic prednisone. It is unclear whether this case represented a poorly symptomatic subacute myocarditis or an early presentation of a fulminant process that was forestalled with prompt immunosuppression.
Moving on to practical matters, we must ask ourselves whether troponin testing is helpful in the management of asymptomatic patients treated with nivolumab or whether it may create confusion. The results of the present study may help to clarify this issue. We found that 1 patient in 59 could have experienced a subclinical myocarditis, with the first troponin alteration 14 days after initiation of therapy with nivolumab. It is likely, therefore, that myocarditis is not a rare event, especially within the first weeks of therapy, and the alerts of severe myocarditis reported in medical literature are just the tip of the iceberg. Thus, in light of our and previous studies, it would be reasonable to apply tight controls of troponin during the first few weeks, in which the risk would seem to be particularly high. On the other hand, it is to be recalled that troponin is not a specific marker of immune checkpoint blockade‐related myocarditis, but of myocardial damage of any kinds. In our study, a careful cardiologic evaluation showed that most of troponin positivity (5 of 6) was not suggestive of nivolumab‐related myocarditis.
Therefore, if it is decided to plan for a troponin monitoring program, the conditions to interpret results must also be created. This means that, firstly, a careful cardiac assessment should be performed before starting therapy; secondly, the attention to symptoms or signs that may sound alarm bells for heart problems should be maintained at a high level throughout the treatment; and thirdly, it must be kept in mind that anemia, fever, worsening of clinical status, and hemodynamic instability from any cause may facilitate troponin release.
In case of troponin abnormalities, patients must be questioned regarding chest pain, dizziness, palpitations, and dyspnea. In addition, blood pressure recording, physical examination, ECG, and echocardiography should be carried out quickly. Particular attention has to be paid to atrioventricular and intraventricular conduction times (PR and QRS intervals on the surface ECG) because severe conduction‐system abnormalities have been described. Should any problem be documented, patients must be quickly managed according to current practical guidelines.
Patients with cardiac diseases are more likely to show troponin abnormalities that might not directly depend on immunotherapy. On the other hand, patients with no history of cardiac disease, but with troponin abnormalities shortly after beginning of therapy, deserve special attention. If they remain asymptomatic with normal ECG and echocardiogram, close monitoring by repeating exams a few days apart is appropriate, but if they start to describe symptoms or show abnormalities at ECG or ultrasound examination, albeit modest, it could be a myocarditis at an early stage. In such cases, the risk of worsening and developing ventricular dysfunction, shock, or cardiac arrest due to ventricular fibrillation or atrioventricular block is unpredictable, and the interruption of checkpoint blockade therapy is mandatory and immunosuppressive therapy should be promptly initiated. Furthermore, the need for hospitalization in a cardiologic department with an intensive care unit should be seriously considered.
Available data appear to indicate a low sensitivity of cardiac MRI for detecting immune checkpoint blockade‐induced myocarditis [16], [36], but further studies should clarify the role of this technique. Endomyocardial biopsy, the underused diagnostic gold standard for myocarditis [37], should be performed when is suspected that it would influence therapy. This is particularly the case for unclear situations in which the oncologist does not know whether to stop treatment or not.
Conclusion
The results of the present study indicate that troponin testing performed during the first weeks of therapy with immune checkpoint‐blocking agents may provide useful information, as long as it is interpreted within the specific clinical scenario, and for this reason, we support a careful cardiovascular evaluation at baseline and an increasingly close cooperation between oncologists and cardiologists. In addition, it would be desirable for future clinical trials to include troponin testing and, possibly, have a safety committee to examine cases.
Contributed equally
Author Contributions
Conception/design: Matteo Sarocchi, Francesco Grossi, Paolo Spallarossa
Provision of study material or patients: Matteo Sarocchi, Francesco Grossi, Eleonora Arboscello, Andrea Bellodi, Carlo Genova, Maria Giovanna Dal Bello, Erika Rijavec, Giulia Barletta, Giovanni Rossi, Federica Biello, Michele Mussap, Claudio Brunelli, Paolo Spallarossa
Collection and/or assembly of data: Maria Giovanna Dal Bello, Michele Mussap
Data analysis and interpretation: Matteo Sarocchi, Francesco Grossi, Carlo Genova, Giorgio Ghigliotti, Marco Canepa, Michele Mussap, Paolo Spallarossa
Manuscript writing: Matteo Sarocchi, Francesco Grossi, Paolo Spallarossa
Final approval of manuscript: Matteo Sarocchi, Francesco Grossi, Eleonora Arboscello, Andrea Bellodi, Carlo Genova, Maria Giovanna Dal Bello, Erika Rijavec, Giulia Barletta, Giovanni Rossi, Federica Biello, Giorgio Ghigliotti, Marco Canepa, Michele Mussap, Claudio Brunelli, Paolo Spallarossa
Disclosures
Matteo Sarocchi: Incyte (H); Francesco Grossi: Bristol‐Myers Squibb, Merck Sharp & Dohme, Pierre Fabre (C/A, H), AstraZeneca, Roche (C/A); Eleonora Arboscello: ARIAD, Incyte, Novartis (C/A, H); Carlo Genova: AstraZeneca, Boehringer‐Ingelheim, Bristol‐Myers‐Squibb (H); Erika Rijavec: Bristol‐Myers Squibb (H); Giulia Barletta: Boehringer‐Ingelheim, AstraZeneca, Pierre Fabre (H); Paolo Spallarossa: Incyte, Teva, Bristol‐Myers Squibb (C/A), Incyte, Teva, Servier (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Guo L, Zhang H, Chen B. Nivolumab as programmed death‐1 (PD‐1) inhibitor for targeted immunotherapy in tumor. J Cancer 2017;8:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharma P, Retz M, Siefker‐Radtke A et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single‐arm, phase 2 trial. Lancet Oncol 2017;18:312–322. [DOI] [PubMed] [Google Scholar]
- 3. Ferris RL, Blumenschein G, Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non–small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 7. Herbaux C, Gauthier J, Brice P et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood 2017;129:2471–2478. [DOI] [PubMed] [Google Scholar]
- 8. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: The beginning of the end of cancer? BMC Med 2016;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang J, Yuan R, Song W et al. PD‐1, PD‐L1 (B7‐H1) and tumor‐site immune modulation therapy: The historical perspective. J Hematol Oncol 2017;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Velasco G, Je Y, Bossé D et al. Comprehensive meta‐analysis of key immune‐related adverse events from CTLA‐4 and PD‐1/PD‐L1 inhibitors in cancer patients. Cancer Immunol Res 2017;5:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Behling J, Kaes J, Münzel T et al. New‐onset third‐degree atrioventricular block because of autoimmune‐induced myositis under treatment with anti‐programmed cell death‐1 (nivolumab) for metastatic melanoma. Melanoma Res 2017;27:155–158. [DOI] [PubMed] [Google Scholar]
- 14. Heinzerling L, Ott PA, Hodi FS et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 2016;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson DB, Balko JM, Compton ML et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Läubli H, Balmelli C, Bossard M et al. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer 2015;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng F, Loscalzo J. Autoimmune cardiotoxicity of cancer immunotherapy. Trends Immunol 2017;38:77–78. [DOI] [PubMed] [Google Scholar]
- 18. Jaffe AS. The 10 commandments of troponin, with special reference to high sensitivity assays. Heart 2011;97:940–946. [DOI] [PubMed] [Google Scholar]
- 19. Mahajan VS, Jarolim P. How to interpret elevated cardiac troponin levels. Circulation 2011;124:2350–2354. [DOI] [PubMed] [Google Scholar]
- 20. Eggers KM, Lindahl B. Application of cardiac troponin in cardiovascular diseases other than acute coronary syndrome. Clin Chem 2017;63:223–235. [DOI] [PubMed] [Google Scholar]
- 21. Caforio AL, Pankuweit S, Arbustini E et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- 22. Kelley WE, Lockwood CM, Cervelli DR et al. Cardiovascular disease testing on the Dimension Vista system: Biomarkers of acute coronary syndromes. Clin Biochem 2009;42:1444–1451. [DOI] [PubMed] [Google Scholar]
- 23. Mion MM, Bragato G, Casarotti A et al. Clinical performance of cardiac Troponin I: A comparison between the POCT AQT90 FLEX and the Dimension Vista analyzer in an emergency setting. Clin Biochem 2017;50:763–767. [DOI] [PubMed] [Google Scholar]
- 24. Plana JC, Galderisi M, Barac A et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27:911–939. [DOI] [PubMed] [Google Scholar]
- 25. Genova C, Rossi G, Rijavec E et al. Releasing the brake: Safety profile of immune check‐point inhibitors in non‐small cell lung cancer. Expert Opin Drug Saf 2017;16:573–585. [DOI] [PubMed] [Google Scholar]
- 26. Torre M, Jarolim P. Cardiac troponin assays in the management of heart failure. Clin Chim Acta 2015;441:92–98. [DOI] [PubMed] [Google Scholar]
- 27. Engler RJ, Nelson MR, Collins LC Jr et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PloS One 2015;10:e0118283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grabie N, Gotsman I, DaCosta R et al. Endothelial programmed death‐1 ligand 1 (PD‐L1) regulates CD8+ T‐cell mediated injury in the heart. Circulation 2007;116:2062–2071. [DOI] [PubMed] [Google Scholar]
- 29. Tarrio ML, Grabie N, Bu DX et al. PD‐1 protects against inflammation and myocyte damage in T cell‐mediated myocarditis. J Immunol 2012;188:4876–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lucas JA, Menke J, Rabacal WA et al. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol 2008;181:2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang J, Okazaki I, Yoshida T et al. PD‐1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol 2010;22:443–452. [DOI] [PubMed] [Google Scholar]
- 32. Nishimura H, Okazaki T, Tanaka Y et al. Autoimmune dilated cardiomyopathy in PD‐1 receptor‐deficient mice. Science 2001;291:319–322. [DOI] [PubMed] [Google Scholar]
- 33. Okazaki T, Tanaka Y, Nishio R et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD‐1‐deficient mice. Nat Med 2003;9:1477–1483. [DOI] [PubMed] [Google Scholar]
- 34. Okazaki T, Okazaki I, Wang J et al. PD‐1 and LAG‐3 inhibitory co‐receptors act synergistically to prevent autoimmunity in mice. J Exp Med 2011;208:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seko Y, Yagita H, Okumura K et al. Roles of programmed death‐1 (PD‐1)/PD‐1 ligands pathway in the development of murine acute myocarditis caused by coxsackievirus B3. Cardiovasc Res 2007;75:158–167. [DOI] [PubMed] [Google Scholar]
- 36. Norwood TG, Westbrook BC, Johnson DB et al. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer 2017;5:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kindermann I, Barth C, Mahfoud F et al. Update on myocarditis. J Am Coll Cardiol 2012;59:779–792. [DOI] [PubMed] [Google Scholar]