Recently, several cases of immune checkpoint inhibitor‐related cardiotoxicity have been reported, with complications ranging from cardiogenic shock to sudden death. Clear guidelines for surveillance, diagnosis, and management of one such adverse event, myocarditis, are not currently available. This article describes a clinical vignette and discusses the salient features and management strategies for immune checkpoint inhibitor‐associated myocarditis.
Abstract
Immune checkpoint inhibitors (ICIs) are approved for a wide range of malignancies. They work by priming the immune system response to cancer and have changed the landscape of available cancer treatments. As anticipated, modulation of the regulatory controls in the immune system with ICIs results in diverse immune‐related adverse events, targeting any organ or gland. These toxicities are rarely fatal and generally regress after treatment discontinuation and/or prescription of corticosteroids. Recently, several cases of ICI‐related cardiotoxicity have been reported with complications ranging from cardiogenic shock to sudden death. The true incidence of ICI‐associated myocarditis is likely underestimated, due to a combination of factors including the lack of specificity in the clinical presentation, the potential of overlap with other cardiovascular and general medical illnesses, the challenges in the diagnosis, and a general lack of awareness of this condition. Currently, there are no clear guidelines for surveillance, diagnosis, or management of this entity. There are multiple unresolved issues including, but not limited to, identifying those at risk of this uncommon toxicity, elucidating the pathophysiology, determining if and what type of surveillance is appropriate, optimal work‐up of suspected patients, and methods for resolution of myocarditis. Here we describe a clinical vignette and discuss the salient features and management strategies of ICI‐associated myocarditis.
Key Points.
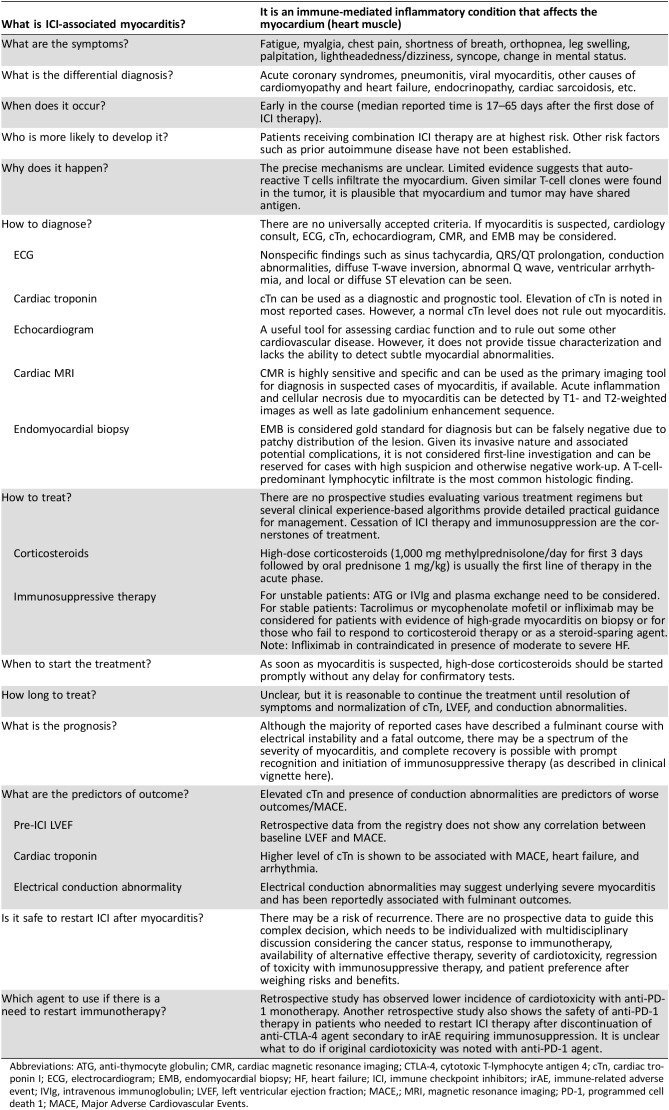
The incidence of immune checkpoint inhibitor (ICI)‐associated myocarditis is unclear and has been reported to range from 0.06% to 1% of patients prescribed an ICI.
Myocarditis may be difficult to diagnose.
The risk factors for ICI‐associated myocarditis are not well understood but may include underlying autoimmune disease and diabetes mellitus.
The prevalence of myocarditis has been reported to be higher with combination immune therapies.
Myocarditis with ICI's typically occurs early, with an elevated troponin, may present with an normal left ventricular ejection fraction and may have a fulminant course.
The optimal management of myocarditis associated with ICI's is unclear but most cases are treated with high-dose steroids.
Patient Story
A 41‐year‐old woman with no cardiac risk factors but a prior history of Hashimoto's thyroiditis was diagnosed with metastatic melanoma. She presented with mild dyspnea 6 days after completing four cycles of combined immune checkpoint inhibitor (ICI) therapy with ipilimumab and nivolumab. On exam, she was tachycardic and mildly volume overloaded but was otherwise stable. Sinus tachycardia was noted on electrocardiogram (ECG); there were no conduction abnormalities (Fig. 1A). Cardiac troponin I (cTn) was mildly elevated with normal level of N‐terminal‐pro brain natriuretic peptide (NT‐proBNP). A chest computed tomography (CT) scan did not show evidence of pneumonitis but did show cardiomegaly and pulmonary congestion. An echocardiogram revealed global left ventricular (LV) systolic dysfunction with an ejection fraction (EF) of 15%. She had a coronary angiography, which did not show evidence of obstructive coronary artery disease. A cardiac magnetic resonance imaging (CMR) showed T2 hyper‐intensity and patchy mid‐myocardial delayed enhancement involving the interventricular septum (Fig. 1B) with an LVEF of 12%, features consistent with myocarditis. A right heart catheterization revealed an elevated pulmonary capillary wedge pressure (25 mmHg) with a reduced cardiac index (1.8 L/minute/m2). On endomyocardial biopsy, there was an intense lymphocytic infiltrate and mild interstitial fibrosis (Fig. 1C), and an immunostain was positive for CD‐3 and CD‐8 T cells (Fig. 1D, 1E). The biopsy findings were also consistent with myocarditis. She was treated with high‐dose corticosteroids (1,000 mg methylprednisolone/day daily for 3 days followed by a slow tapering regimen of oral prednisone) and neurohormonal antagonists. She underwent a repeat CMR 4 months later, which showed resolution of previously noted delayed myocardial enhancement and that her LVEF had improved to 54%.
Figure 1.
Electrocardiogram (ECG), cardiac magnetic resonance imaging (MRI), and endomyocardial biopsy findings in a patient with immune checkpoint inhibitor‐associated myocarditis. (A): 12‐lead ECG showing sinus tachycardia. (B): Cardiac MRI: Arrow showing mid‐myocardial delayed enhancement of interventricular septum. (C): High‐power view of endomyocardial biopsy (EMB) sample shows an intense lymphocytic infiltrate and mild fibrosis. (D): CD‐3 immunostain of EMB sample shows that the majority of the inflammatory infiltrate consists of CD‐3‐positive T lymphocytes. (E): CD‐8 immunostain of EMB sample shows presence of cytotoxic (CD‐8 positive) T cells.
Immune Checkpoint Inhibitors
Antitumor immunity is enhanced by blocking intrinsic down‐regulators of immunity, such as cytotoxic T‐lymphocyte antigen 4 (CTLA‐4) and programmed cell death 1 (PD‐1) or its ligand, programmed cell death ligand 1 (PD‐L1) [1], [2]. Various ICIs have shown efficacy and increased overall survival for patients with several cancers and, so far, six agents (one CTLA‐4 blocking antibody—ipilimumab; two PD‐1 blocking antibodies—nivolumab and pembrolizumab; and three PD‐L1 blocking antibodies—atezolizumab, avelumab, and durvalumab) have been approved for 10 different cancers by the U.S. Food and Drug Administration [3].
Cardiotoxicity
Due to increased activity of the immune system, ICIs can be associated with immune‐related adverse events (irAEs) [4]. Although gastrointestinal tract, endocrine glands, skin, and liver are most commonly involved, any organ system can be affected by irAEs [3], [4]. Cardiotoxicity in the form of myocarditis has recently been reported [5], [6], [7], [8], [9]. The incidence of ICI‐associated myocarditis is unclear. In a pharmacovigilance study, myocarditis was noted in 0.27% of patients receiving combination therapy and 0.09% of patients on a single ICI [5]. In contrast, in a recent retrospective case‐control study, 1% of patients prescribed an ICI developed myocarditis [6]. The risk factors for ICI‐associated myocarditis are not well understood [3], [8]. It is possible, such as in our case, that patients with underlying autoimmune disease may be at increased risk [3], [10], [11]. Additional risk factors may include pre‐existing cardiac disease and diabetes mellitus [3], [6]. Combination ICI therapy is associated with an increased risk of other irAEs and is also a risk factor for ICI‐associated myocarditis [5], [6]. Specifically, in a small registry of ICI‐related myocarditis, the prevalence was 0.5% with anti‐PD‐1 alone as compared with 2.4% with combined anti‐PD‐1 and anti‐CTLA‐4 therapy [6]. Similarly, the risk of myocarditis development may differ between various classes of ICIs. The prevalence of myocarditis was lowest with anti‐PD‐1 agent (0.5%), whereas it was noted to be higher with anti‐PD‐L1 (2.4%) and anti‐CTLA‐4 monotherapy (3.3%) [6]; however, the noted difference in the prevalence of irAEs between various classes of ICIs may be an overestimation, particularly the difference noted between anti‐PD‐1 and anti‐PD‐L1 agents, given that the previous studies report similar toxicity profile of both classes [12].
A feature favoring a pre‐existing subclinical immunology risk factor is the general recognition that ICI‐associated myocarditis occurs early with a median time of 1–2 months and with most of the cases occurring within 3 months of starting ICI therapy [5], [6]. However, although cardiovascular adverse events occurred more frequently in the early phase of the treatment, they can occur at any time [7]. The presenting symptoms can also vary widely and may range from mild, nonspecific symptoms such as fatigue and myalgia, chest pain, and shortness of breath to syncope and sudden cardiac death [5], [6], [7], [9]. It may also present as a tachyarrhythmia (atrial or ventricular) or heart block [5], [6]. Although fulminant myocarditis with heart failure and arrhythmias has been more commonly reported, subclinical or smoldering myocarditis with minimal signs and symptoms may also occur [13].
Pathophysiology
The exact mechanism of irAEs is not understood. Evolving data suggest that common high frequency T‐cell receptor sequences are found exist in cardiac muscle and tumor, raising the possibility of a shared antigen theory [5], [14]. Additionally, the relatively early onset of myocarditis after initiating ICI therapy and involvement of selective patients without a clear explanation supports hypotheses regarding the role of pre‐existing conditions that predispose to the development of myocarditis. In animal models, both CTLA‐4 and PD‐1 protect the heart from immune‐mediated damage after stress [15], [16], [17], and genetic manipulation of this axis has provided some insight. Specifically, CTLA‐4 knockout mice develop rapidly fatal autoimmune myocarditis mediated by CD8+ T cells [15], whereas deletion of PD‐1 in mice leads to spontaneous myocarditis and dilated cardiomyopathy that is caused by anti‐cTn autoantibodies [16], [17]. In mouse models of T‐cell‐mediated myocarditis, myocardial PD‐L1 up‐regulation is noted, likely a cytokine‐induced cardio‐protective mechanism, and the upregulation is critical for limiting immune‐mediated cardiac injury and may be abrogated by anti‐PD‐L1 antibody [18].
Management
Diagnosis
There are no clear guidelines for diagnosis and treatment of this relatively newly emerging entity, and, with increasing knowledge, practice will evolve. In Table 1, we describe the current, albeit early, knowledge of the salient clinical features, diagnosis, and treatment strategies. A high level of vigilance is required given that immune‐mediated myocarditis may present with nonspecific symptoms and may potentially have a fulminant progression.
Table 1. Salient features of ICI‐associated myocarditis.
Abbreviations: ATG, anti‐thymocyte globulin; CMR, cardiac magnetic resonance imaging; CTLA‐4, cytotoxic T‐lymphocyte antigen 4; cTn, cardiac troponin I; ECG, electrocardiogram; EMB, endomyocardial biopsy; HF, heart failure; ICI, immune checkpoint inhibitors; irAE, immune‐related adverse event; IVIg, intravenous immunoglobulin; LVEF, left ventricular ejection fraction; MACE,; MRI, magnetic resonance imaging; PD‐1, programmed cell death 1; MACE, Major Adverse Cardiovascular Events.
If a patient has symptoms suggestive of myocarditis, an ECG and troponin should be checked immediately. It is important to acknowledge that although these tests are frequently used for diagnosis of myocarditis, they lack the sensitivity and specificity for diagnosis and can be abnormal due to other cardiovascular conditions. For example, an ECG in a patient with myocarditis may show nonspecific findings such as sinus tachycardia, QRS/QT prolongation, conduction abnormalities, diffuse T‐wave inversion, Q waves, ventricular arrhythmias, and local or diffuse ST elevation [19], [20]. Although ECG abnormalities can be found in the majority of patients with myocarditis at initial presentation, a normal ECG does not rule out myocarditis [21]. Similarly, although most reported fulminant cases are associated with elevated serum troponin, an elevated troponin is not specific for myocarditis, and a normal troponin, especially in cases that appear late after initiation of ICIs, does not exclude ICI‐associated myocarditis [6]. However, the utility of troponin levels is not limited just to diagnosis of myocarditis as the extent of the elevated troponin has also been shown to be prognostic with a higher troponin associated with worse cardiovascular outcomes [6]. Other cardiac biomarkers, including BNP or NT‐proBNP, are markers of myocardial stretch and should be checked in symptomatic patients, but they may also be normal in specific phenotypes [22], [23].
An echocardiogram is a standard first‐line test for the assessment of patients with suspected ICI‐associated myocarditis given its widespread availability and ease of performance. However, the LVEF may be normal even in fulminant myocarditis, and a normal LVEF does not exclude the occurrence of a major adverse cardiac event [5], [6]. All patients presenting with new cardiovascular symptoms, an abnormal ECG, and an elevated cTn should have coronary ischemia excluded. Depending on the clinical presentation, this can be performed with traditional invasive coronary angiography, a cardiac CT, or, less favorably, a stress test. Additional testing to be considered includes a viral‐serology panel, including influenza, to exclude other causes of myocarditis. A CMR is the gold standard noninvasive test for the diagnosis of myocarditis. The strengths of CMR include its excellent spatial resolution and its additive ability to provide tissue characterization [24]. Specifically, myocarditis is associated with increased capillary permeability, leading to increased myocardial water content and cellular necrosis, which can be detected by CMR on T1‐ and T2‐weighted images [25]. This was noted in our case with the observation of late gadolinium enhancement. A combination of these CMR criteria has a sensitivity of 76% and specificity of 96% for myocarditis [26], [27]. CMR has also shown to be an effective tool for risk stratification and prognostication in general cases of myocarditis [28], [29]. Despite the potential benefits of CMR, its limited availability and the difficulty in obtaining this relatively lengthy test in severely ill patients are major obstacles that restrict its widespread use in every patient with suspected myocarditis. Importantly, an absence of abnormal findings on either echocardiogram or CMR does not rule out myocarditis [27].
An endomyocardial biopsy is considered the gold standard for the diagnosis of myocarditis [30]. However, due to its invasive nature, the risk of cardiac perforation, and the localized nature of the biopsy sample, the test is not performed as a first‐line test despite being considered the “gold standard.” If biopsy is obtained from the affected area, histological examination may show inflammatory infiltrates (usually T‐cell‐predominant lymphocytic infiltrate) in the myocardium not typical of ischemic damage from coronary artery disease. Immunostains for cell‐specific markers such as T lymphocytes (CD3) or macrophages (CD68) or human leukocyte antigens may improve the sensitivity of the test [31].
Given that patients with ICI‐associated myocarditis may develop tachy‐ and bradyarrhythmias, suspected or confirmed patients should be closely monitored with cardiac telemetry and ECGs.
It is important to consider broad differential diagnosis in a patient with suspected myocarditis. Pneumonitis, another irAE, may also present with similar symptoms, and appropriate work‐up should be considered especially if work‐up for myocarditis is unrevealing. Many of these patients may have received other potentially cardiotoxic therapies in the past, which may also cause cardiac dysfunction. Particularly, patients with BRAFV600 mutation metastatic melanoma may have received combination therapy with BRAF/MEK inhibitors, which are associated with LV systolic dysfunction [32]. Additionally, other diagnoses should be considered. For example, cases of sarcoidosis with immunotherapy have been reported [33], which may affect the heart and present in a manner very similar to ICI‐associated myocarditis with heart block and heart failure.
Treatment
Although there are no prospective studies evaluating various potential treatment regimens, some early clinical experience‐based algorithms provide some initial guidance for management [5], [8].
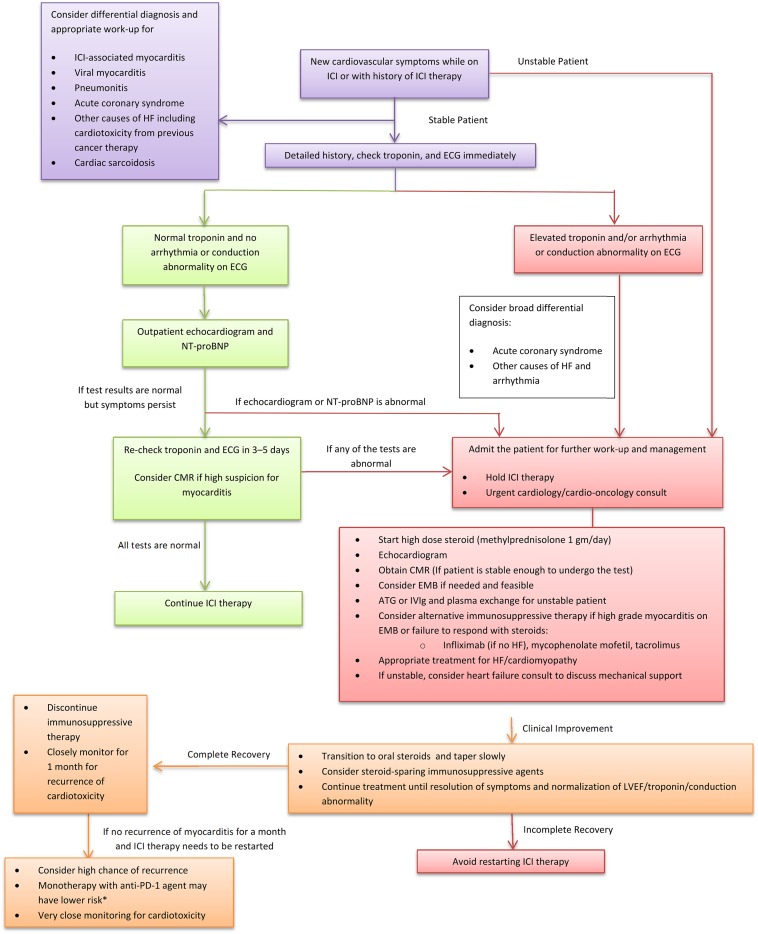
Cumulatively, cessation of ICI therapy and immunosuppression are the cornerstones of ICI‐associated myocarditis treatment (Fig. 2). Timing of treatment is likely important given the potential for rapid progression to fulminant disease with cardiovascular compromise; therefore, prompt initiation of the immunosuppression is recommended without any further delay for confirmatory testing.
Figure 2.
Proposed algorithm for management of ICI‐associated myocarditis. The proposed management algorithm suggests a stepwise approach for a patient with suspected myocarditis.*, the data are very limited and are based on retrospective analysis.
Abbreviations: ATG, antithymocyte globulin; CMR, cardiac magnetic resonance imaging; ECG, electrocardiogram; EMB, endomyocardial biopsy; HF, heart failure; ICI, immune checkpoint inhibitors; IVIg, intravenous immunoglobulin; PD‐1, programmed cell death 1; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal‐pro‐brain natriuretic peptide.
A high dose of corticosteroids (i.e., methylprednisolone 1,000 mg per day for 3 days followed by prednisone 1 mg/kg) should be considered the first line of therapy in the acute phase. Data from two registries have suggested that prompt initiation of high‐dose corticosteroids is beneficial for recovery of left ventricular systolic function as well as for reducing the burden of major adverse cardiac events [6], [7]. Beyond treatment with high‐dose corticosteroids, there are few data to suggest the optimal subsequent therapy should steroids fail. Potential alternatives to consider, should high‐dose steroids not result in the resolution of myocarditis, include infliximab. However, it is important to note that efficacy data with infliximab are mixed, and the use of infliximab has been associated with the development of heart failure among patients with rheumatoid arthritis [34]. If patient is unstable, anti‐thymocyte globulin, intravenous immunoglobulin, and plasma exchange should be considered [35], [36]. In stable patients either with evidence of high‐grade myocarditis on biopsy or who fail to respond to corticosteroid therapy, additional therapy with tacrolimus or mycophenolate mofetil should be considered based on their proven efficacy as immunosuppressive agents in cardiac allograft rejection [37]. Concomitant standard heart failure and anti‐arrhythmic management should also be initiated, especially if the LVEF is reduced. It is unclear how long a patient should be treated with immunosuppressive therapy, but it is reasonable to continue until resolution of symptoms and normalization of LVEF, biomarker, and conduction abnormality.
The development of cardiovascular adverse events is particularly challenging because it has potential implications in overall cancer management and outcomes of patients. Although the interruption of cancer therapies could increase the risk of disease progression, cardiac events could lead to early complications and death. Retrospective data suggest that in patients with advanced melanoma and non‐small cell lung cancer who have had an initial favorable response with ICI and needed to discontinue due to irAEs, the response was maintained even after discontinuation of treatment [38], [39], and restarting ICI may not be required. Although the guidelines recommend a definite discontinuation of immunotherapy in cases of life‐threatening (grade 4) and severe (grade 3) adverse events, the decision to rechallenge with ICI therapy after development of ICI‐associated myocarditis is complex and needs to be individualized with multidisciplinary discussion considering the cancer status, response to immunotherapy, availability of alternative effective therapy, severity of cardiotoxicity, regression of toxicity with immunosuppressive therapy and patient preference. If the patient needs to be rechallenged with immunotherapy, monotherapy with a different agent along with very close cardiovascular monitoring should be considered. Specifically, retrospective analysis of the registry data, albeit limited, suggests that monotherapy with anti‐PD‐1 agent was associated with lowest risk of cardiotoxicity [6]. A similar finding was noted in a retrospective study of patients with melanoma, which showed that anti‐PD‐1 therapy was safely given after serious ipilimumab (anti‐CTLA‐4) or combination therapy with CTLA‐4/PD‐1 related adverse events [40], [41].
Screening and Surveillance
Both screening and surveillance are considered when a significant cardiotoxicity can occur from cancer therapies. Specifically, prior to anthracyclines, measurement of an LVEF is suggested [42]. However, data suggest that measurement of LVEF prior to ICI therapy may not provide utility. For example, in one case series, 70% of patients who developed myocarditis on ICI therapy had a normal pre‐ICI LVEF [6]. In most series, an abnormal ECG and cTn is noted at presentation. Therefore, a surveillance approach of serial ECG and cTn could be considered. As the median time to myocarditis is early, checking cTn levels at baseline and at each cycle may therefore be of value. An elevated cTn should warrant consideration of myocarditis and immediate referral to cardiology/cardio‐oncology for further evaluation. Additional questions include who to monitor and for how long. Surveillance may be appropriate for trials in the adjuvant or neoadjuvant setting, combination ICI regimens, and co‐administration of ICIs with other agents with established cardiovascular toxicities.
Future Directions
Our knowledge of ICI‐related myocarditis is rapidly evolving and will continue to evolve as the testing and indications for ICIs expand. In 2017, 940 immuno‐oncology agents were being tested in 3,042 clinical trials with a target enrollment of 577,076 patients [43]. There are many key information gaps, including, importantly, a lack of a standardized definition for ICI‐associated myocarditis, which would enable a consistent assessment by broad groups of clinicians. However, because ICI‐associated myocarditis is a new syndrome, our understanding of this condition is rapidly evolving, and any definition is subject to change. Other important considerations include identifying clinical, genetic, and immunological risk factors for ICI‐associated myocarditis, validation of surveillance pathways with robust test characteristics, and establishing treatment algorithms with research focused on identification of targeted interventions that may reduce the current reliance on high‐dose steroids. A key component will be multidisciplinary collaborations, which should include oncologists, general physicians, cardiologists, cardio‐oncologists, and immunologists. These collaborations involving academics and clinicians should also include industry and regulatory authorities.
Author Contributions
Conception/design: Sarju Ganatra, Tomas G. Neilan
Provision of study material or patients: Sarju Ganatra, Tomas G. Neilan
Collection and/or assembly of data: Sarju Ganatra, Tomas G. Neilan
Data analysis and interpretation: Sarju Ganatra, Tomas G. Neilan
Manuscript writing: Sarju Ganatra, Tomas G. Neilan
Final approval of manuscript: Sarju Ganatra, Tomas G. Neilan
Disclosures
Tomas G. Neilan: Takeda (C/A). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robert C, Thomas L, Bondarenko I et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 3. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 4. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016;44:51–60. [DOI] [PubMed] [Google Scholar]
- 5. Johnson DB, Balko JM, Compton ML et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahmood SS, Fradely MG, Cohen JV et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am College Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Escudier M, Cautela J, Malissen N et al. Clinical features, management, and outcomes of immune checkpoint inhibitor‐related cardiotoxicity. Circulation 2017;136:2085–2087. [DOI] [PubMed] [Google Scholar]
- 8. Wang DY, Okoye GD, Neilan TG et al. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep 2017;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berg DD, Vaduganathan M, Nohria A et al. Immune‐related fulminant myocarditis in a patient receiving ipilimumab therapy for relapsed chronic myelomonocytic leukaemia. Eur J Heart Fail 2017;19:682–685. [DOI] [PubMed] [Google Scholar]
- 10. Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer 2017;123:1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson DB, Sullivan RJ, Ott PA et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2016;2:234–240. [DOI] [PubMed] [Google Scholar]
- 12. Naidoo J, Page DB, Li BT et al. Toxicities of the anti‐PD‐1 and anti‐PD‐L1 immune checkpoint antibodies. Ann Oncol 2016;27:1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Norwood TG, Westbrook BC, Johnson DB et al. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer 2017;5:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reuben A, Petaccia de Macedo M, McQuade J et al. Comparative immunologic characterization of autoimmune giant cell myocarditis with ipilimumab. Oncoimmunology 2017;6:e1361097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Love VA, Grabie N, Duramad P et al. CTLA‐4 ablation and interleukin‐12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ Res 2007;101:248–257. [DOI] [PubMed] [Google Scholar]
- 16. Nishimura H, Okazaki T, Tanaka Y et al. Autoimmune dilated cardiomyopathy in PD‐1 receptor‐deficient mice. Science 2001;291:319–322. [DOI] [PubMed] [Google Scholar]
- 17. Okazaki T, Tanaka Y, Nishio R et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD‐1‐deficient mice. Nat Med 2003;9:1477–1483. [DOI] [PubMed] [Google Scholar]
- 18. Grabie N, Gotsman I, DaCosta R et al. Endothelial programmed death‐1 ligand 1 (PD‐L1) regulates CD8+ T‐cell mediated injury in the heart. Circulation 2007;116:2062–2071. [DOI] [PubMed] [Google Scholar]
- 19. Nakashima H, Honda Y, Katayama T. Serial electrocardiographic findings in acute myocarditis. Intern Med 1994;33:659–666. [DOI] [PubMed] [Google Scholar]
- 20. Testani JM, Kolansky DM, Litt H et al. Focal myocarditis mimicking acute ST‐elevation myocardial infarction: Diagnosis using cardiac magnetic resonance imaging. Tex Heart Inst J 2006;33:256–259. [PMC free article] [PubMed] [Google Scholar]
- 21. Deluigi CC, Ong P, Hill S et al. ECG findings in comparison to cardiovascular MR imaging in viral myocarditis. Int J Cardiol 2013;165:100–106. [DOI] [PubMed] [Google Scholar]
- 22. Abrar S, Ansari MJ, Mittal M et al. Predictors of mortality in paediatric myocarditis. J Clin Diagn Res 2016;10:SC12–SC16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ogawa T, Veinot JP, Kuroski de Bold ML et al. Angiotensin II receptor antagonism reverts the selective cardiac BNP upregulation and secretion observed in myocarditis. Am J Physiol Heart Circ Physiol 2008;294:H2596–H2603. [DOI] [PubMed] [Google Scholar]
- 24. Friedrich MG, Sechtem U, Schulz‐Menger J et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol 2009;53:1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farhad H, Staziaki PV, Addison D et al. Characterization of the changes in cardiac structure and function in mice treated with anthracyclines using serial cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahrholdt H, Goedecke C, Wagner A et al. Cardiovascular magnetic resonance assessment of human myocarditis: A comparison to histology and molecular pathology. Circulation 2004;109:1250–1258. [DOI] [PubMed] [Google Scholar]
- 27. Abdel‐Aty H, Boye P, Zagrosek A et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: Comparison of different approaches. J Am Coll Cardiol 2005;45:1815–1822. [DOI] [PubMed] [Google Scholar]
- 28. Grani C, Eichhorn C, Biere L et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol 2017;70:1964–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aquaro GD, Perfetti M, Camastra G et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY Study. J Am Coll Cardiol 2017;70:1977–1987. [DOI] [PubMed] [Google Scholar]
- 30. Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: Implications for role of sampling error. Mayo Clin Proc 1989;64:1235–1245. [DOI] [PubMed] [Google Scholar]
- 31. Maisch B, Portig I, Ristic A et al. Definition of inflammatory cardiomyopathy (myocarditis): on the way to consensus. A status report. Herz 2000;25:200–209. [DOI] [PubMed] [Google Scholar]
- 32. Flaherty KT, Infante JR, Daud A et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suozzi KC, Stahl M, Ko CJ et al. Immune‐related sarcoidosis observed in combination ipilimumab and nivolumab therapy. JAAD Case Rep 2016;2:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kwon HJ, Cote TR, Cuffe MS et al. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med 2003;138:807–811. [DOI] [PubMed] [Google Scholar]
- 35. Rodriguez ER, Skojec DV, Tan CD et al. Antibody‐mediated rejection in human cardiac allografts: Evaluation of immunoglobulins and complement activation products C4d and C3d as markers. Am J Transplant 2005;5:2778–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kobashigawa J, Crespo‐Leiro MG, Ensminger SM et al. Report from a consensus conference on antibody‐mediated rejection in heart transplantation. J Heart Lung Transplant 2011;30:252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kobashigawa JA, Miller LW, Russell SD et al. Tacrolimus with mycophenolate mofetil (MMF) or sirolimus vs. cyclosporine with MMF in cardiac transplant patients: 1‐year report. Am J Transplant 2006;6:1377–1386. [DOI] [PubMed] [Google Scholar]
- 38. Santini FC, Rizvi H, Wilkins O et al. Safety of retreatment with immunotherapy after immune‐related toxicity in patients with lung cancers treated with anti‐PD(L)‐1 therapy. J Clin Oncol 2017;35(suppl 15):2012a. [Google Scholar]
- 39. Schadendorf D, Wolchok JD, Hodi FS et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. J Clin Oncol 2017;35:3807–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Menzies AM, Johnson DB, Ramanujam S et al. Anti‐PD‐1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–376. [DOI] [PubMed] [Google Scholar]
- 41. Pollack MH, Betof A, Dearden H et al. Safety of resuming anti‐PD‐1 in patients with immune‐related adverse events (irAEs) during combined anti‐CTLA‐4 and anti‐PD1 in metastatic melanoma. Ann Oncol 2018;29:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Armenian SH, Lacchetti C, Barac A et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2017;35:893–911. [DOI] [PubMed] [Google Scholar]
- 43. Tang J, Shalabi A, Hubbard‐Lucey VM. Comprehensive analysis of the clinical immuno‐oncology landscape. Ann Oncol 2018;29:84–91. [DOI] [PubMed] [Google Scholar]