Abstract
Lessons Learned.
Due to evolving imaging criteria in brain tumors and variation in magnetic resonance imaging evaluation, it is not ideal to use response rate as a primary objective. Future studies involving antiangiogenic agents should use overall survival.
Disease‐expected toxicities should be considered when defining the clinical significance of an adverse event. For example, vascular thromboembolic events are common in brain tumor patients and should not be attributed to the study drug in the safety analysis.
Background.
Recurrent malignant glioma (rMG) prognosis is poor, with a median patient survival of 3–11 months with bevacizumab (BEV)‐containing regimens. BEV in rMG has 6‐month progression free survival (PFS‐6) of ∼40% and an objective response rate of 21.2%. BEV‐containing regimens improve PFS‐6 to 42.6%–50.3%, indicating that BEV combination therapies may be superior to single agent. Rilotumumab, a hepatocyte growth factor (HGF) antibody, inhibits angiogenesis and expression of angiogenic autocrine factors (e.g., vascular endothelial growth factor [VEGF]) by c‐Met inhibition. Combination of rilotumumab with BEV to block vascular invasion and tumor proliferation may synergistically inhibit tumor growth.
Methods.
Thirty‐six BEV‐naïve rMG subjects received rilotumumab (20 mg/kg and BEV (10 mg/kg) every 2 weeks. Endpoints included objective response rate (using Response Assessment in Neuro‐Oncology [RANO] criteria), PFS‐6, overall survival (OS), and toxicity.
Results.
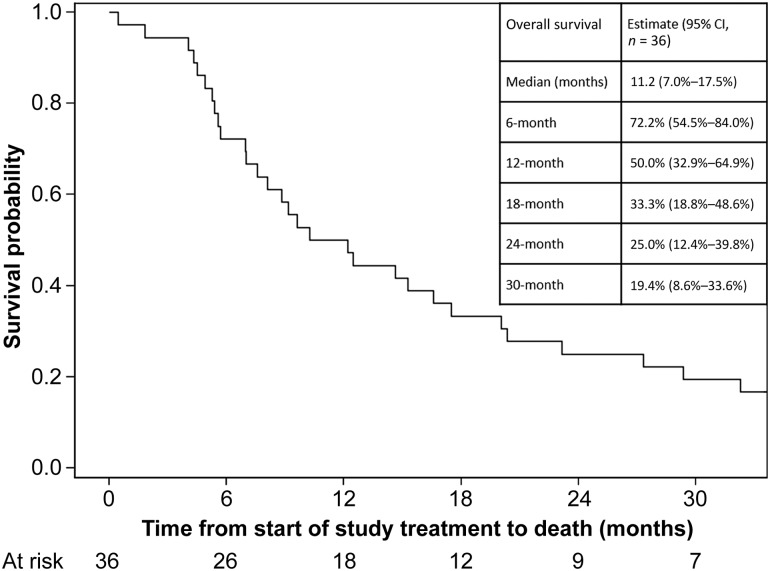
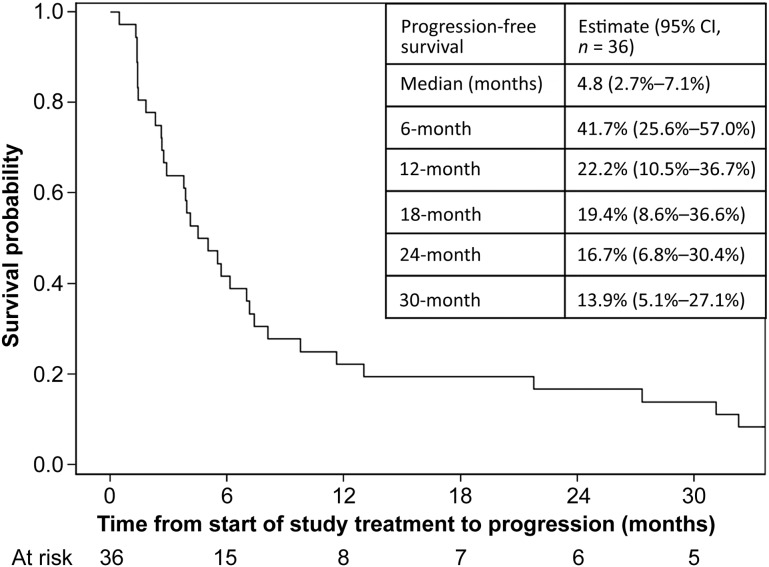
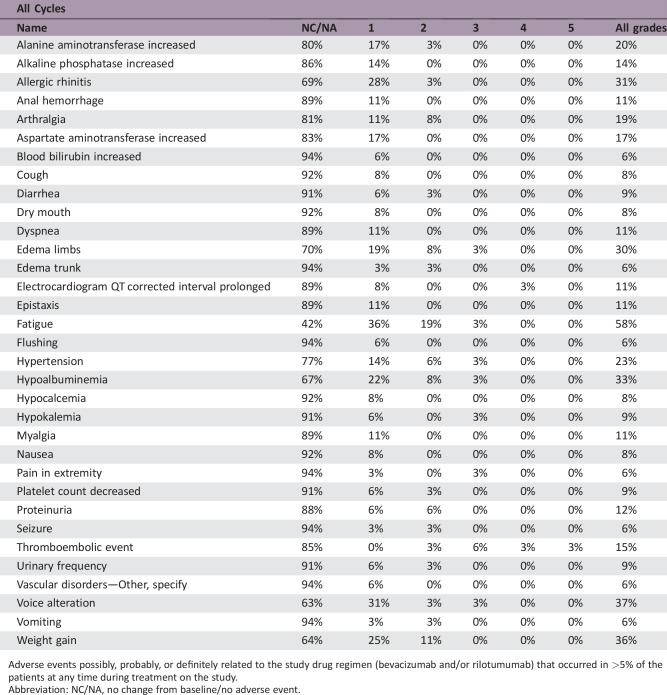
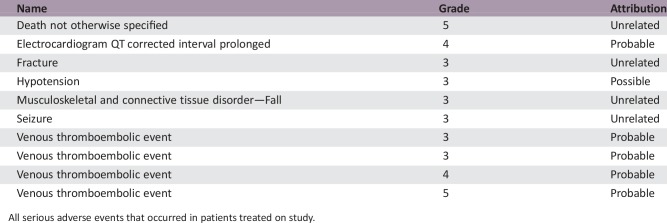
Median patient follow‐up was 65.0 months. Objective response rate was 27.8% (95% confidence interval [CI]: 15.7%–44.1%). Median OS was 11.2 months (95% CI: 7–17.5). PFS‐6 was 41.7% (95% CI: 25.6%–57.0%). Most frequent treatment‐related grade ≤2 events included weight gain, fatigue, allergic rhinitis, and voice alteration; grade ≥3 events included venous thromboembolism (four patients), including one death from pulmonary embolism.
Conclusion.
Rilotumumab with BEV did not significantly improve objective response compared with BEV alone, and toxicity may preclude the use of rilotumumab in combination BEV regimens.
Abstract
经验获取
• 由于脑肿瘤影像标准的不断发展以及磁共振影像评估的变化,以缓解率作为主要指标不够理想。未来有关抗血管生成药物的研究应使用总生存期。
• 在界定不良事件的临床意义时,应考虑疾病的预期毒性。例如,血管血栓栓塞事件在脑肿瘤患者中常见,做安全性分析时不应归因于研究药物。
摘要
背景. 复发性恶性胶质瘤(rMG)预后较差,采用含贝伐单抗(BEV)的治疗方案时,患者生存期中位数为3~11个月。BEV在治疗rMG患者中,6个月无进展生存期(PFS‐6)的比例约40%,客观缓解率为21.2%。含BEV的治疗方案将PFS‐6提高至42.6%~50.3%,提示BEV联合治疗可能优于单药治疗。Rilotumumab是一种肝细胞生长因子(HGF)抗体,通过c‐Met抑制剂抑制血管生成及血管生成自分泌因子[如血管内皮生长因子(VEGF)]的表达。Rilotumumab联合BEV阻断血管浸润和肿瘤增殖,可能协同抑制肿瘤生长。
方法. 36例初治rMG受试者每2周接受一次rilotumumab(20 mg/kg)和BEV(10 mg/kg)治疗。终点包括客观缓解率[利用神经肿瘤临床疗效评估(RANO)标准]、PFS‐6、总生存期(OS)及毒性。
结果. 中位随访65.0个月。客观缓解率为27.8%[95%置信区间(CI):15.7%–44.1%]。中位OS为11.2个月(95%CI:7–17.5)。PFS‐6为41.7% (95% CI:25.6%–57.0%)。最常见的与治疗相关的 ≤2级事件包括体重增加、乏力、变应性鼻炎和声音改变; ≥3级事件包括静脉血栓栓塞(4例),其中1例死于肺栓塞。
结论 与BEV单药治疗相比,rilotumumab联合BEV不能明显改善客观缓解率,而且毒性可能会阻碍rilotumumab与BEV联合治疗方案。
Discussion
This study's hypothesis was that rilotumumab plus BEV, a humanized anti‐VEGF‐A antibody, would work synergistically to block vascular invasion and tumor proliferation causing tumor growth inhibition. Previous studies demonstrate a positive association between HGF and glioma grade. Additionally, abnormal expression of HGF contributes to glioma progression, showing the importance of the HGF mechanism in malignant glioma. Rilotumumab is an antibody that blocks the interaction of HGF with the c‐Met receptor, resulting in reduced tumor cell proliferation and migration. This study was designed to differentiate between a 20% and 40% radiographic response rate using RANO criteria. This study combination resulted in an objective response of 27.8% (complete response: 2.8% plus partial response: 25%), therefore not meeting the threshold for concluding that the regimen merits further investigation. A previous study treating rMG patients with rilotumumab alone demonstrated a median OS of 6.5 months and a median PFS of 4.1 weeks. The statistical comparator study of single‐agent BEV in rMG showed a median OS of ∼7.8 months (95% CI: 5.3–13.5) and a median PFS of 4 months (95% CI: 3–6). In the present study, BEV plus rilotumumab modestly increased the median OS to 11.2 months (95% CI: 7.0–17.5) and the median PFS to 4.8 months (95% CI: 2.7–7.1), see Figures 1 and 2, respectively. Although BEV plus rilotumumab showed ∼3–4‐month improvement in median survival over the individual agents, it did not increase the PFS of BEV alone. The improvement in median OS should be balanced with the toxicity of the combination regimen. Occurrences of grade (GR) ≥2 central nervous system (CNS) hemorrhage or GR4/5 nonhematologic treatment‐related toxicity were determined unacceptable, a priori. “Unacceptable” toxicity rates of ≤5% were desirable, whereas rates ≥20% were undesirable. There were no GR2 CNS hemorrhages, although 6% of patients had GR4/5 nonhematologic treatment‐related toxicity (one GR4 prolonged QTc; one GR4 pulmonary embolism [PE]; one lethal PE). Although venous thromboembolic events are expected in gliomas, and the combination did not exceed unacceptable toxicity, four patients (11%) had grade >3 PE, which is clinically significant.
Figure 1.
Kaplan‐Meier curve of overall survival of patients treated with rilotumumab in combination with bevacizumab. The inset table shows the overall survival specifications for this regimen.
Abbreviation: CI, confidence interval.
Figure 2.
Kaplan‐Meier curve of progression‐free survival in patients treated with rilotumumab in combination with bevacizumab. The inset table shows the progression‐free survival specifications for this regimen.
Abbreviation: CI, confidence interval.
Trial Information
- Disease
World Health Organization (WHO) grade IV malignant glioma
- Stage of Disease/Treatment
Adjuvant
- Prior Therapy
No designated number of regimens
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Overall response rate
- Secondary Endpoint
Overall survival
- Secondary Endpoint
Progression‐free survival
- Secondary Endpoint
Toxicity
- Additional Details of Endpoints or Study Design
- Primary endpoint: radiological response rate as determined by RANO criteria.
- Secondary endpoints: overall survival and 6‐month progression‐free survival. Incidence and severity of CNS hemorrhage and systemic hemorrhage. Incidence of grade >4 hematologic and grade >3 nonhematologic toxicities.
- Sample size justification: A minimax two‐stage design was used to assess the radiographic response rate (i.e., complete response [CR] + partial response [PR]) of rilotumumab and Avastin in the treatment of patients with advanced malignant glioma [1]. The randomized phase II trial, AVF3708g, investigating Avastin versus Avastin plus irinotecan demonstrated a radiographic response rate to Avastin of approximately 20%. Hence, the current study was designed to differentiate between a 20% (null hypothesis) and a 40% (alternative hypothesis) response rate, assuming type I and II error rates of 0.10. If 10 or fewer of the total 36 patients had a radiographic response, the AMG 102 and Avastin treatment combination was to be considered not worthy of further research.
- Analytic methods: The primary endpoint, radiographic response rate, was assessed using RANO criteria. Brain magnetic resonance imaging scans were obtained on patients after each 4‐week treatment cycle, and complete and partial responses were confirmed at least 4 weeks later. The response rate was calculated with Wald confidence intervals. Secondary endpoints of OS and PFS were estimated using Kaplan‐Meier methods; corresponding 95% confidence intervals were calculated using Greenwood's formula. OS was defined as the time between the initiation of study treatment and death or last follow‐up, if alive at the time of analysis; PFS was defined as the time between the initiation of study treatment and the first occurrence of disease progression or death. Patients alive and progression free at the time of analysis were censored as of the date of last follow‐up. Common Terminology Criteria for Adverse Events version 4.0 was used to assess adverse events for safety endpoints. SAS 9.4 (SAS Institute, Inc., Cary, NC) was used for all analyses.
- Investigator's Analysis
Active but results overtaken by other developments
Drug Information
- Drug 1
- Generic/Working Name
Bevacizumab
- Trade Name
Avastin
- Company Name
Genentech
- Drug Type
Antibody
- Drug Class
Angiogenesis—VEGF
- Dose
10 mg/kg
- Route
IV
- Schedule of Administration
Every 2 weeks for up to 12 cycles. Each cycle was 6 weeks, which included three infusions of Avastin every 2 weeks.
- Drug 2
- Generic/Working Name
Rilotumumab
- Trade Name
AMG102
- Company Name
Amgen, Inc.
- Drug Type
Antibody
- Drug Class
MET—cMET
- Dose
20 mg/kg
- Route
IV
- Schedule of Administration
Rilotumumab was administered every 2 weeks following the administration of Avastin for up to 12 cycles. Each cycle was 6 weeks and included three administrations of Avastin at 10 mg/kg followed by rilotumumab at 20 mg/kg.
Patient Characteristics
- Number of Patients, Male
22
- Number of Patients, Female
14
- Age
Median (range): 55.5 (27–74)
- Number of Prior Systemic Therapies
Median (range): 1 (1–3)
- Performance Status: ECOG
-
0 — 0
1 — 33
2 — 3
3 — 0
Unknown — 0
- Cancer Types or Histologic Subtypes
Recurrent malignant glioma WHO grade IV
Primary Assessment Method
- Title
Total patient population
- Number of Patients Screened
42
- Number of Patients Enrolled
36
- Number of Patients Evaluable for Toxicity
36
- Number of Patients Evaluated for Efficacy
36
- Evaluation Method
RANO
- Response Assessment CR
n = 1 (2.8%)
- Response Assessment PR
n = 9 (25.0%)
- Response Assessment SD
n = 17 (47.2%)
- Response Assessment PD
n = 6 (16.7%)
- Response Assessment OTHER
n = 3 (8.3%)
- (Median) Duration Assessments PFS
4.8 months, CI: 95%
- (Median) Duration Assessments OS
11.2 months, CI: 95%
Adverse Events
Adverse events possibly, probably, or definitely related to the study drug regimen (bevacizumab and/or rilotumumab) that occurred in >5% of the patients at any time during treatment on the study.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Serious Adverse Events
All serious adverse events that occurred in patients treated on study.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active but results overtaken by other developments
Patients with recurrent malignant glioma (rMG) have improved progression‐free survival (PFS) with bevacizumab (BEV), a humanized monoclonal antibody that inhibits vascular endothelial growth factor‐A (VEGF‐A). In a 2009 study, single‐agent BEV, followed by BEV plus irinotecan, in rMG resulted in a median overall survival (OS) of 7.8 months (95% confidence interval [CI]: 5.3–13.5) and a median PFS of 4 months (95% CI: 3–6) [2]. The BELOB trial showed single‐agent BEV to have a similar median OS of 8 months (95% CI: 6–9) but showed an increase in median OS to 12 months (95% CI: 8–13) when combined with lomustine [3]. However, overall survival benefits were not seen in a phase III study of BEV combined with lomustine compared with single‐agent lomustine (median OS 9.1 months vs. 8.6 months, respectively), although the combination improved PFS by 2.7 months over lomustine alone [4]. Observations from other phase II studies show improved PFS with BEV‐containing regimens, with no significant impact on OS [2], [5]. Even with such increases in PFS, the prognosis of rMG remains poor with low survival outcomes.
Numerous studies are testing BEV combination therapies in attempts to increase survival [6], [7], [8]. One component that may provide synergy with BEV is rilotumumab, a hepatocyte growth factor (HGF)‐inhibiting antibody that blocks HGF, which prevents activation of the c‐Met receptor, resulting in downstream prevention of tumor cell growth and survival. In addition to the effect on cell growth, HGF is also associated with tumor grade in gliomas [9], [10], [11], and abnormal expression of HGF contributes to glioma progression, showing the importance of the HGF activation mechanism in malignant glioma [12], [13], [14], [15]. By itself, rilotumamb is not as effective as BEV, with phase II study results of single‐agent rilotumumab in rMG patients demonstrating a median OS of 6.5 months and a median PFS of 4.1 weeks [16]. With rilotumumab's ability to inhibit a molecule that is associated with malignant glioma and BEV's impact on PFS in rMG, it was suspected that these two drugs may work synergistically to improve survival outcomes of rMG patients through multi‐pathway tumor growth inhibition.
This clinical trial enrolled 36 rMG subjects to receive rilotumumab (20 mg/kg) and BEV (10 mg/kg) every 2 weeks. Of the 36 subjects, 27 (75%) were at their first recurrence and 33 (91.7%) had a Karnofsky Performance Status ≥80. Based on an earlier BEV‐alone study in rMG, the current study was designed to differentiate between a 20% and 40% response rate. Overall best responses included 1 (2.8%) complete response, 9 (25.0%) partial responses, 17 (47.2%) patients with stable disease, and 6 (16.7%) with progressive disease. The resulting objective response rate (complete or partial response) of 27.8% did not meet the study threshold needed to deem the BEV/rilotumumab combination worthy of further investigation. However, objective response is not a robust measure of efficacy due to the intricacies and evolving criteria for analyzing magnetic resonance images of brain tumor patients who have received BEV. Future studies in this patient population would benefit from using OS as the primary outcome instead of the response rate.
The BEV plus rilotumumab study regimen resulted in a median OS of 11.2 months (95% CI: 7.0–17.5). This is a small, nonsignificant improvement in OS over recently published results by Wick and colleagues, which showed the median OS for BEV with lomustine (9.1 months [95% CI: 8.1–10.1]) was not superior to single‐agent lomustine (8.6 months [95% CI: 7.6–10.4]) [4]. With a median PFS of 4.8 months (95% CI: 2.7–7.1), our study of BEV plus rilotumumab did not show any improvement in PFS over BEV alone. In this study, it is difficult to attribute the extended OS exclusively to the BEV plus rilotumumab regimen given that many subjects progressed quickly and proceeded to receive other therapies that could influence OS. Additionally, the small increase in median OS should be balanced with the toxicity caused by the regimen. Occurrences of grade (GR) ≥2 central nervous system (CNS) hemorrhage or GR4/5 nonhematologic treatment‐related toxicity were determined unacceptable, a priori. “Unacceptable” toxicity rates of ≤5% were desirable, whereas rates ≥20% were undesirable. There were no GR2 CNS hemorrhages, although 6% of patients had a GR4/5 nonhematologic‐related toxicity (one GR4 prolonged electrocardiogram QT corrected interval; one GR4 pulmonary embolism [PE]; one GR5 PE). Although venous thromboembolic events are expected in gliomas, and the combination did not exceed unacceptable toxicity, four patients (11%) had ≥GR3 PE, which is clinically significant. Other common treatment‐related side effects experienced by ≥20% of patients were fatigue (58%), voice alteration (37%), weight gain (36%), hypoalbuminemia (33%), and allergic rhinitis (31%). Overall, BEV plus rilotumumab resulted in an increase of side effects, both expected and new. Ultimately, the BEV plus rilotumumab combination was not further explored in rMG due to a decision made by the Principal Investigator after an ongoing clinical trial in gastric cancer (RILOMET‐1) was terminated early due to an increased number of deaths on the rilotumumab arm compared with the placebo‐controlled arm [17], [18]. Although the BEV plus rilotumumab regimen was not excessively toxic in the rMG patients in this study, there was concern for the safety of the patients due to the number of venous thromboembolisms that occurred in the rMG patients on this study and the increased number of deaths on the rilotumumab arm in the RILOMET‐1 study. No further studies of rilotumumab alone or in combination with BEV have been done in rMG patients.
In conclusion, rilotumumab in combination with BEV did not significantly improve objective response, OS, or PFS compared with BEV alone. Nonetheless, given the improvement of OS (although statistical significance was not evaluated), rilotumumab plus BEV was an active treatment regimen in rMG patients. The toxicity profile of rilotumumab in combination with BEV precludes this regimen from being used in rMG patients.
Acknowledgments
We acknowledge Amgen for their support of this investigator‐initiated trial.
Footnotes
ClinicalTrials.gov Identifier: NCT01113398
Sponsor(s): PI sponsored
Principal Investigators: Mary Lou Affronti, Katherine B. Peters
IRB Approved: Yes
Disclosures
Mary Lou Affronti: Merck (C/A), Eisai, Merck, Tesaro, Amgen (RF); Annick Desjardins: Patents US 15/428510, AU 2013347945, CA 2,892,183, CN 201380070749.7, EP 13856989.2, HK 15112399.5, JP 2015‐544130, Div2017‐084158, PCT/US17/023148, (IP), Genentech/Roche, Celgene (C/A), Genentech/Roche, Celgene, Celldex, Triphase Accelerator Corp, Eli Lilly and Co, Symphogen A/S, Pfizer, Orbus Therapeutics (RF), Istari Oncology (OI); Henry S. Friedman: Genentech/Roche (C/A, H), Istari Oncology (OI, IP, SAB); Gordana Vlahovic: AstraZeneca (E); Katherine B. Peters: Agios, Abbvie, Eisai, Novocure (C/A), Agios, Abbvie, Biomimetix, Eisai, Genentech, Merck, Tessaro (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Simon R. Optimal two‐stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 2. Kreisl TN, Kim L, Moore K et al. Phase II trial of single‐agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009;27:740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taal W, Oosterkamp HM, Walenkamp AM et al. Single‐agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol 2014;15:943–953. [DOI] [PubMed] [Google Scholar]
- 4. Wick W, Gorlia T, Bendszus M et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 2017;377:1954–1963. [DOI] [PubMed] [Google Scholar]
- 5. Friedman HS, Prados MD, Wen PY et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733–4740. [DOI] [PubMed] [Google Scholar]
- 6. Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 2007;13:1253. [DOI] [PubMed] [Google Scholar]
- 7. Reardon DA, Desjardins A, Vredenburgh JJ et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: A phase II study. Br J Cancer 2009;101:1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sathornsumetee S, Desjardins A, Vredenburgh JJ et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol 2010;12:1300–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koochekpour S, Jeffers M, Rulong S et al. Met and hepatocyte growth factor scatter factor expression in human gliomas. Cancer Res 1997;57:5391–5398. [PubMed] [Google Scholar]
- 10. Moriyama T, Kataoka H, Hamasuna R et al. Up‐regulation of vascular endothelial growth factor induced by hepatocyte growth factor/scatter factor stimulation in human glioma cells. Biochem Biophys Res Commun 1998;249:73–77. [DOI] [PubMed] [Google Scholar]
- 11. Rosen EM, Laterra J, Joseph A et al. Scatter factor expression and regulation in human glial tumors. Int J Cancer 1996;67:248–255. [DOI] [PubMed] [Google Scholar]
- 12. Laterra J, Rosen E, Nam M et al. Scatter factor/hepatocyte growth factor expression enhances human glioblastoma tumorigenicity and growth. Biochem Biophys Res Commun 1997;235:743–747. [DOI] [PubMed] [Google Scholar]
- 13. Moriyama T, Kataoka H, Koono M et al. Expression of hepatocyte growth factor/scatter factor and its receptor c‐Met in brain tumors: Evidence for a role in progression of astrocytic tumors (Review). Int J Mol Med 1999;3:531–536. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt NO, Westphal M, Hagel C et al. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer 1999;84:10–18. [DOI] [PubMed] [Google Scholar]
- 15. Lamszus K, Laterra J, Westphal M et al. Scatter factor/hepatocyte growth factor (SF/HGF) content and function in human gliomas. Int J Dev Neurosci 1999;17:517–530. [DOI] [PubMed] [Google Scholar]
- 16. Wen PY, Schiff D, Cloughesy TF et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol 2011;13:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lazar DC, Taban S, Cornianu M et al. New advances in targeted gastric cancer treatment. World J Gastroenterol 2016;22:6776–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cunningham D, Tebbutt NC, Davidenko I et al. Phase III, randomized, double‐blind, multicenter, placebo (P)‐controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first‐line therapy in patients (pts) with advanced MET‐positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET‐1 study J Clin Oncol 2015;33(suppl 15):4000a. [Google Scholar]