Abstract
Lessons Learned.
Everolimus does not have sufficient activity to justify its use as single agent in metastatic melanoma.
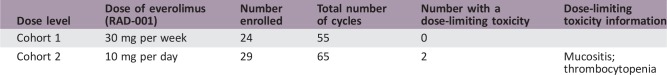
Patients treated with 10 mg per day dose were most likely to require dose reductions.
Everolimus appeared to reduce the numbers of regulatory T cells in approximately half of the treated patients; unfortunately, these effects were not correlated with clinical outcomes.
Background.
Everolimus (RAD‐001) is an orally active rapamycin analogue shown in preclinical data to produce cytostatic cell inhibition, which may be potentially beneficial in treating melanoma. We conducted a phase II study to evaluate the efficacy and safety of everolimus in patients with unresectable metastatic melanoma (MM).
Methods.
This study included two cohorts; cohort 1 received 30 mg of everolimus by mouth (PO) weekly, and cohort 2 was dosed with 10 mg of everolimus PO daily. The endpoints of the study were safety, 16‐week progression‐free survival (PFS), overall survival (OS), and measures of immunomodulatory/antiangiogenic properties with therapy. Tumor samples before therapy and at week 8 of treatment were analyzed. Peripheral blood plasma or mononuclear cell isolates collected prior to therapy and at weeks 8 and 16 and at time of tumor progression were analyzed for vascular endothelial growth factor and regulatory T‐cell (Treg) measurements.
Results.
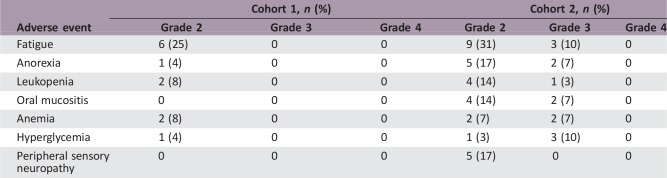
A total of 53 patients were enrolled in cohort 1 (n = 24) and cohort 2 (n = 29). Only 2 patients of the first 20 patients enrolled in cohort 2 had treatment responses (25%; 95% confidence interval, 8.6%–49.1%); this result did not allow full accrual to cohort 2, as the study was terminated for futility. Median OS was 12.2 months for cohort 1 versus 8.1 months in cohort 2; no PFS advantage was seen in either group (2.1 months vs. 1.8 months). Dose‐limiting toxicities included grade 4 myocardial ischemia (3.4%); grade 3 fatigue, mucositis, and hyperglycemia (10.3%); and anorexia and anemia (6.9%). Everolimus significantly reduced the number of Tregs in approximately half of the treated patients; however, these effects were not correlated with clinical outcomes.
Conclusion.
Everolimus does not have sufficient single‐agent activity in MM; however, we have identified evidence of biological activity to provide a potential rationale for future combination studies.
Abstract
经验获取
• 依维莫司的活性不足以证明可用作治疗转移性黑色素瘤的单一药物。
• 每天服用10mg的患者可能最需要减少剂量。
• 在接受依维莫司治疗的患者中,大约一半出现调节性T细胞数量减少;遗憾的是,这些效应与临床结果没有相关性。
摘要
背景. 临床前期数据显示,依维莫司(RAD‐001)是一种口服雷帕霉素活性类似物,具有抑制细胞生长的作用,可能对黑色素瘤的治疗具有潜在的益处。我们进行了一项II期研究,以评估依维莫司治疗不可切除转移性黑色素瘤(MM)患者的疗效和安全性。
方法. 本研究分为两组,第1组每周口服(PO)依维莫司30 mg,第2组每日口服依维莫司10 mg。研究终点包括:安全性、16周无进展生存期(PFS)、总生存期(OS)以及用治疗方法测量免疫调节/抗血管生成特性。分析治疗前及治疗第8周时的肿瘤标本。于治疗前、第8周、第16周及肿瘤进展期间,分别采集外周血浆或单核细胞分离物,对血管内皮生长因子(VEGF)及调节性T细胞(Treg)指标进行分析。
结果. 第1组(n=24)和第2组(n=29)共入组53名患者。第2组前20名入组患者中,仅有2名患者出现治疗反应(25%;95%置信区间:8.6%‐49.1%);这一结果不允许对第2组进行完全招募,因为本研究因无效而终止。第1组的中位OS为12.2个月,第2组为8.1个月;两组均无PFS优势(2.1个月vs 1.8个月)。剂量限制性毒性包括4级心肌缺血(3.4%)、3度疲乏、粘膜炎和高血糖(10.3%)、厌食及贫血(6.9%)。在依维莫司治疗的患者中,约一半患者的Treg数量显著减少,但是这些效应与临床结果无相关性。
结论 在MM治疗中,依维莫司没有足够的单药活性;然而,我们已经确定了生物活性的证据,为今后的联合研究提供了一个潜在的理论基础。
Discussion
Melanoma is the most malignant form of skin cancer, the fifth most common cancer in men and sixth in women in the U.S., with its highest incidence in the white population [1], [2], [3].
In preclinical studies by our group, inhibitors of mammalian target of rapamycin (mTOR) demonstrated a potent inhibitory effect on tumor growth, improved survival, an inhibitory effect of rapamycin on angiogenesis, and significant decrease in the number of capillaries perfusing the tumor [4], [5], [6]. The results of the current study demonstrate that single‐agent therapy with RAD‐001 does not have sufficient activity to justify its use as a single agent in the treatment of metastatic melanoma. Our data also suggest that patients treated with the 10 mg per day dose were most likely to require dose reductions. The treatment did appear to modulate aspects of both immunity and angiogenesis; however, in view of the insufficient clinical efficacy of treatment, these findings can only be viewed as exploratory and illustrative of the potential utility of everolimus in combination with other agents. Everolimus significantly reduced the numbers of Tregs in approximately half of the treated patients; unfortunately, these effects were not correlated with clinical outcomes.
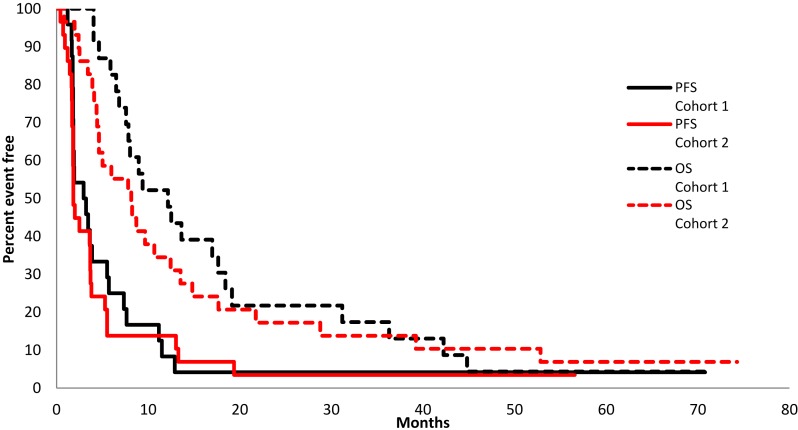
Figure 1.
Kaplan‐Meier plot. Overall survival (OS) and PFS comparing cohort 1, 30 mg by mouth (PO) weekly (n = 24), with cohort 2, 10 mg PO daily (n = 9). Median OS was 12.2 months versus 8.1 months, respectively; no PFS advantage was seen in either group (2.1 months vs. 1.8 months).
Abbreviation: PFS, progression‐free survival.
Investigating the benefits of everolimus as a single agent is the first step toward incorporating this agent into a combination regimen to treat melanoma. Because the 10 mg per day dose appeared to be excessively toxic in this population, future studies will need to use a lower dose. The toxicity profile of everolimus does not overlap with other melanoma therapies.
Trial Information
- Disease
Melanoma
- Stage of Disease/Treatment
Metastatic/Advanced
- Prior Therapy
No designated number of regimens
- Type of Study ‐ 1
Phase I/II
- Type of Study ‐ 2
Phase II study
- Primary Endpoint
Safety
- Secondary Endpoint
Overall response rate
- Secondary Endpoint
Progression‐free survival
- Secondary Endpoint
Correlative endpoint
- Additional Details of Endpoints or Study Design
- The primary endpoint of this trial was the proportion of patients that were failure‐free (FF) at 16 weeks. A patient was defined as FF if the patient was progression‐free and still receiving study treatment at 16 weeks. The study utilized a two‐stage Simon design to test that the true 16‐week FF rate was at most 30% versus the alternative, which was at least 50%. This design had a significance level of 0.10 with a power of 90%. To test this hypothesis, a maximum of 55 evaluable patients were to be accrued. After the first 20 evaluable patients were enrolled, accrual was stopped. If, after the first 20 evaluable patients, there were at most 6 patients who remained FF, no more patients were to be accrued. Otherwise, an additional 25 evaluable patients were to be accrued. In order for the trial to be declared successful, a minimum of 17 patients were to be declared FF at 16 weeks (of the first 45 evaluable patients).
- The results of the interim analysis performed on the first 20 patients enrolled allowed accrual to continue; however, clinical studies in renal cell carcinoma demonstrated early positive results with a slightly higher dose of therapy (10 mg/day). Considering these new observations and in consultation with the National Cancer Institute, a decision was made to restart the trial at the 10 mg/day dose of RAD‐001 and plan to enroll another cohort of 55 patients, using the same two‐stage design and eligibility and decision rules for efficacy.
- Secondary endpoints included overall survival (OS, defined as the time from study registration until death), progression‐free survival (PFS, defined as the time from study entry until disease progression or death when patients died without documentation of disease progression), confirmed response rate (a confirmed response was defined as a CR or PR on consecutive cycles at least 8 weeks apart), LDH (testing LDH's influence on PFS, OS, and treatment response), and correlative laboratory studies (effects of therapy on PET/CT imaging, mTOR inhibition, and immune homeostasis parameters).
- Progression‐free survival at 16 weeks was not achieved. Secondary endpoints, including OS (defined as the time from study registration until death), PFS (defined as the time from study entry until disease progression or death if patients died without documentation of disease progression), and confirmed response rate (a confirmed response was defined as a complete response [CR] or partial response [PR] on consecutive cycles at least 8 weeks apart), were not achieved.
- Investigator's Analysis
- Inactive because results did not meet primary endpoint
Drug Information
- Generic/Working Name
Everolimus (RAD‐001)
- Company Name
Novartis
- Drug Type
Small molecule
- Drug Class
mTOR
- Dose
30 milligrams (mg) per flat dose
- Route
PO
- Schedule of Administration
Doses: Cohort 1 received 30 mg of everolimus PO weekly, and cohort 2 was dosed with 10 mg of everolimus PO daily.
Patient Characteristics
- Number of Patients, Male
37
- Number of Patients, Female
16
- Stage
IV
- Age
Median (range): 61 years (21.0-81.0)
- Performance Status: ECOG
-
0 — 31
1 — 20
2 — 2
3 —
Unknown —
- Cancer Types or Histologic Subtypes
Malignant melanoma
Primary Assessment Method for Phase I Control
- Title
Total Patient Population
- Number of Patients Enrolled
53
- Number of Patients Evaluable for Toxicity
53
- Evaluation method
RECIST, version 1.0
- Response assessment CR
n = 0
- Response assessment PR
n = 1
- Response assessment SD
n = 0
- Response assessment PD
n = 52
Adverse Events
Dose‐Limiting Toxicities
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Inactive because results did not meet primary endpoint
Although the inhibition of PI3K/mammalian target of rapamycin (mTOR)/AKT pathway is a therapeutic strategy for several cancer types, the current study demonstrates that single‐agent therapy with everolimus does not have sufficient activity to justify its use in the treatment of metastatic melanoma. This was our conclusion, despite literature showing that the mTOR pathway is activated in malignant melanoma as opposed to benign nevi [7]. Efforts to evaluate the efficacy of everolimus with other regimens have been performed by different groups; for example, the use of everolimus in combination with temozolamide was evaluated in a single‐arm phase II multi‐institution trial; although the regimen was well tolerated, it failed to meet or exceed the study threshold for promising clinical activity in patients with metastatic melanoma [8]. A subsequent phase II trial combining paclitaxel, carboplatin, and everolimus showed activity in the first‐line treatment of metastatic melanoma; unfortunately, the duration of benefit was brief for most patients [7]. A recent study evaluated the addition of everolimus to carboplatin, paclitaxel, and bevacizumab; this combination was found to be ineffective in metastatic melanoma because of inability to give the full dose of everolimus, predominantly because of cytopenias [9]. Although it was a negative study, the investigators reported that the everolimus combination arm performed exceptionally well, receiving >30 cycles of therapy [9].
Interestingly, the use of everolimus in a preclinical model demonstrated increased programmed death‐ligand 1(PD‐L1) expression in renal cell carcinoma, and the addition of everolimus to anti‐PD‐L1 significantly reduced tumor burden compared with everolimus alone; [10] the use of immunotherapy in combination with everolimus in patients with melanoma warrants further investigation.
Figures and Tables
Figure 2.
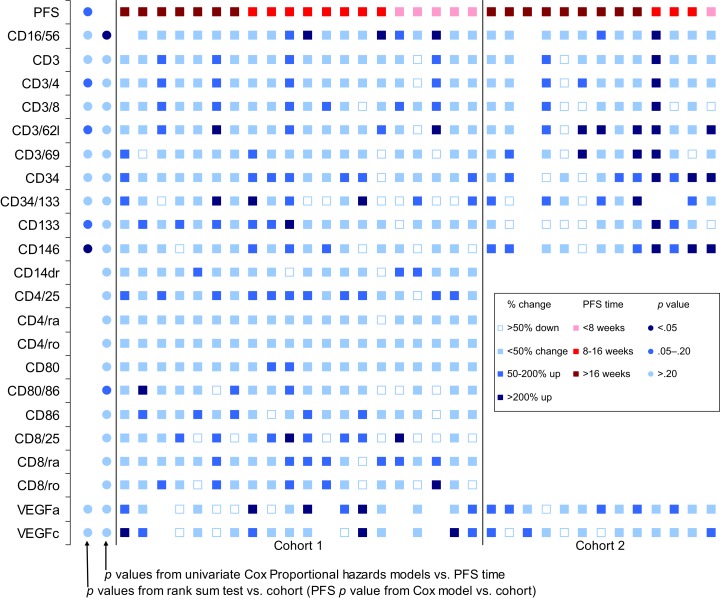
Summary of correlative studies. Effects of RAD‐001 therapy on peripheral blood‐derived parameters of immune homeostasis. Peripheral blood plasma or mononuclear cell isolates collected prior to therapy, at weeks 8 and 16 of therapy as well as at the time of tumor progression, were analyzed. For most measured parameters, RAD‐001 therapy did not appear to significantly influence the measurements; however, therapy did appear to significantly reduce the numbers of regulatory T cells (Treg) in approximately half of the treated patients. These effects were not correlated with clinical outcomes.
Abbreviation: PFS, progression‐free survival.
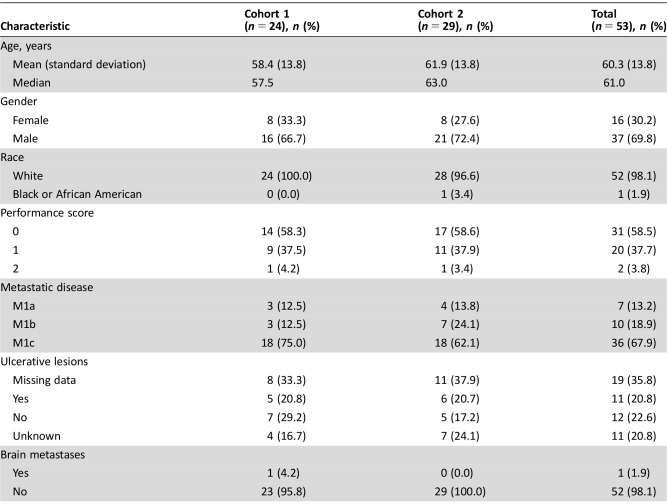
Table 1. Patient characteristics.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821, U10CA180882 (to the Alliance for Clinical Trials in Oncology), and U10CA180790. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Retired
Footnotes
ClinicalTrials.gov Identifier: NCT00098553
Sponsor(s): National Cancer Institute (NIH Award Numbers U10CA180821, U10CA180882 [to the Alliance for Clinical Trials in Oncology], and U10CA180790)
Principal Investigator: Svetomir Markovic
IRB Approved: Yes
Disclosures
Val J. Lowe: GE Healthcare, AVID Radiopharmaceuticals (RF), Celgene, AMAG Pharma, Alexion Inc., Exelixis Inc. (OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Shah DJ, Dronca RS. Latest advances in chemotherapeutic, targeted, and immune approaches in the treatment of metastatic melanoma. Mayo Clin Proc 2014;89:504–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balch CM, Soong SJ, Atkins MB et al. An evidence‐based staging system for cutaneous melanoma. CA Cancer J Clin 2004;54:131–149; quiz 182–184. [DOI] [PubMed] [Google Scholar]
- 3. Miao Y, Hylarides M, Fisher DR et al. Melanoma therapy via peptide‐targeted {alpha}‐radiation. Clin Cancer Res 2005;11:5616–5621. [DOI] [PubMed] [Google Scholar]
- 4. Humar R, Kiefer FN, Berns H et al. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)‐dependent signaling. FASEB J 2002;16:771–780. [DOI] [PubMed] [Google Scholar]
- 5. Luan FL, Ding R, Sharma VK et al. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int 2003;63:917–926. [DOI] [PubMed] [Google Scholar]
- 6. Guba M, von Breitenbuch P, Steinbauer M et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat Med 2002;8:128–135. [DOI] [PubMed] [Google Scholar]
- 7. Hauke RJ, Infante JR, Rubin MS et al. Everolimus in combination with paclitaxel and carboplatin in patients with metastatic melanoma: A phase II trial of the Sarah Cannon Research Institute Oncology Research Consortium. Melanoma Res 2013;23:468–473. [DOI] [PubMed] [Google Scholar]
- 8. Dronca RS, Allred JB, Perez DG et al. Phase II study of temozolomide (TMZ) and everolimus (RAD001) therapy for metastatic melanoma: A North Central Cancer Treatment Group study, N0675. Am J Clin Oncol 2014;37:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McWilliams RR, Allred JB, Slostad JA et al. NCCTG N0879 (Alliance): A randomized phase 2 cooperative group trial of carboplatin, paclitaxel, and bevacizumab ± everolimus for metastatic melanoma. Cancer 2018;124:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirayama Y, Gi M, Yamano S et al. Anti‐PD‐L1 treatment enhances antitumor effect of everolimus in a mouse model of renal cell carcinoma. Cancer Sci 2016;107:1736–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]