This review provides clinicians with an overview of the available data on endocrine treatment in premenopausal women with hormone receptor positive metastatic breast cancer and summarizes the treatment options available in routine clinical practice.
Keywords: Metastatic breast cancer, Hormone receptor positive, Premenopausal, Endocrine therapy
Abstract
There is growing interest in the endocrine treatment (ET) of premenopausal women with hormone receptor positive (HR+) metastatic breast cancer (MBC). This review summarizes available data on endocrine therapy for this patient subset and aims to define the most appropriate treatment approach. The combination of luteinizing hormone‐releasing hormone (LHRH) agonists plus tamoxifen seems effective and safe and is considered as being superior to either approach alone; still, single‐agent therapy remains an acceptable treatment option. Due to their mechanism of action, aromatase inhibitors alone are not suitable for the treatment of premenopausal patients, but the combination with LHRH agonists may result in excellent disease control. Fulvestrant, in conjunction with LHRH agonists, also yields interesting results regarding clinical benefit rate and time to progression; currently, other orally available selective estrogen receptor downregulators are under clinical evaluation. Recently, targeted drugs have been added to ET in order to reverse endocrine resistance, but only limited information regarding their activity in premenopausal patients is available. The cyclin dependent kinase 4 and 6 inhibitor palbociclib when combined with fulvestrant and LHRH agonists was shown to prolong progression‐free survival over endocrine therapy alone in pretreated patients; similar results were obtained with the addition of abemacicilib or ribociclib to endocrine therapy. Currently, activity of the mammalian target of rapamycin inhibitor everolimus in combination with letrozole and goserelin is under assessment in premenopausal patients after progression on tamoxifen (MIRACLE trial).
Implications for Practice.
This review provides clinicians with an overview on the available data regarding endocrine treatment of hormone receptor positive (HR+) metastatic breast cancer (MBC) in premenopausal women and summarizes the treatment options available in routine clinical practice. Knowledge of an up‐to‐date therapeutic approach in women with premenopausal HR+ MBC will lead to better disease management, thereby improving disease control and quality of life while minimizing side effects.
摘要
绝经前女性激素受体阳性(HR+)转移性乳腺癌(MBC)内分泌治疗(ET)越来越受到关注。本文总结了有关该患者人群内分泌治疗的现有数据,旨在确定最合适的治疗方法。促黄体生成激素释放激素(LHRH)激动剂与他莫昔芬联合使用似乎安全有效,并且被认为优于任何一种单药治疗,然而,单药疗法仍然是一种可接受的治疗选项。由于芳香化酶抑制剂作用机制的原因,单药治疗不适合绝经前期的患者,但与 LHRH 激动剂联合应用可更好地控制疾病。氟维司群与 LHRH 激动剂联合使用,在临床受益率和至进展时间方面也出现了值得关注的结果。目前,其他现有的口服选择性雌激素受体下调调节剂正在接受临床评估。最近,为了逆转内分泌抵抗,已将靶向药物添加到 ET 中,但有关其在绝经前期患者的作用的信息有限。与单纯内分泌治疗相比,将细胞周期蛋白依赖性激酶4和6抑制剂palbociclib与氟维司群和 LHRH 激动剂联合使用,可以延长经治患者的无进展生存期。在内分泌治疗中加入 abemacicilib 或ribociclib也取得了类似的结果。目前正在他莫昔芬经治后进展的绝经前期患者中,评估雷帕霉素抑制剂依维莫司与来曲唑、戈舍瑞林联合应用对哺乳动物靶点的作用(MIRACLE试验)。
实践意义: 本文为临床医生提供了关于绝经前女性激素受体阳性(HR+)转移性乳腺癌(MBC)内分泌治疗(ET)的现有数据概述,并总结了常规临床实践中的治疗方案。了解绝经前HR+的MBC女性最新治疗方法将有助于更好的管理疾病,从而提高疾病控制率和生活质量,同时最大限度地减少副作用。
Introduction
During the past 30 years, the incidence of metastatic breast cancer (MBC) in women aged 25–39 years has slightly increased from 1.53 (95% confidence interval [CI] 1.01–22.1) per 100,000 in 1976 to 1.9 (95% CI 2.31–3.59) per 100,000 in 2009 [1], increasing the interest in appropriate treatment strategies for this specific patient subset. In general, BC arising in young patients is characterized by a more aggressive phenotype [2], and several studies underline that young age is an independent predictor of adverse outcome [3], [4]; indeed, women diagnosed below the age of 40 are more likely to develop metastatic disease and die from BC [3], [5], [6]. As endogenous estrogens are clearly involved in BC development and progression [6], endocrine therapy (ET) remains the main pillar of systemic treatment [7]. Despite these facts, young MBC patients are underrepresented in endocrine therapy trials, and up to now, no comprehensive update review exists. Therefore, this overview aims to analyze the available data on ET in premenopausal women with hormone receptor positive (HR+) MBC and indicates potential future directions of research.
Endocrine Therapy for Metastatic Breast Cancer
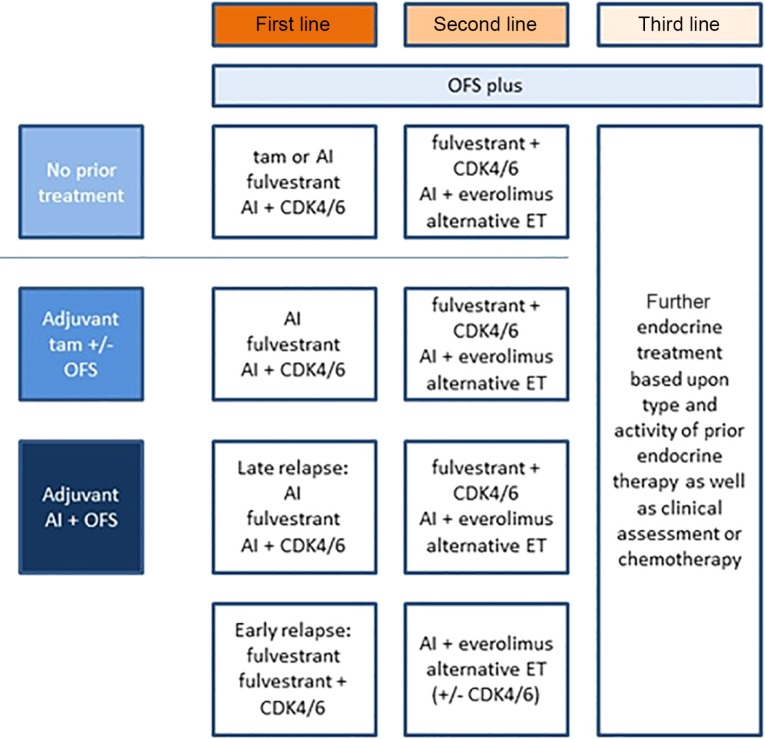
In postmenopausal HR+/human epidermal growth receptor 2 (HER2) negative MBC, endocrine therapy is considered the treatment of choice, and this consideration applies for premenopausal patients as well. Clinical practice guidelines outline appropriate methods of treatment and care and address specific clinical situations [8], [9], [10]. Here, we summarize available evidence with regard to ET specifically in premenopausal patients. A potential treatment algorithm is provided in Figure 1.
Figure 1.
Endocrine therapy for premenopausal women with hormone receptor positive metastatic breast cancer. Abbreviations: AI, aromatase inhibitor; ET, endocrine treatment; OFS, ovarian function suppression; tam, tamoxifen.
Ovarian Ablation: Surgical Versus Medical Therapy
In premenopausal women, the ovaries are the predominant source of estrogen; oophorectomy has been suggested as the first systemic therapy for BC and has been used for over a century [11]. It promptly reduces circulating estrogens but causes permanent fertility loss and requires hospitalization. Oophorectomy and ovarian irradiation have been considered equally effective, with overall response rate (ORR) ranging from 30% [12], [13] to 79% [14], [15]. Reversible medical ovarian function suppression (OFS) can be accomplished via the administration of luteinizing hormone‐releasing hormone (LHRH) agonists. The characterization of gonadotropin‐releasing hormone in 1971 allowed for the development of synthetic LHRH analogues [16]. Chronic administration of these substances causes permanent internalization of pituitary LHRH receptors, rendering gonadotropic cells refractory to endogenous LHRH. Depot formulations of LHRH agonists showed similar effects with no difference in adverse events while allowing for a less frequent administration [17], [18]. In 1993, the monthly formulation of goserelin received U.S. Food and Drug Administration approval, although the 3‐monthly formulation was approved for use in prostate cancer patients only, as insufficient data are available to support its use in BC. In line, current guidelines suggest caution as the suppression of estrogen production may be incomplete. A recent open‐label, randomized phase III study, however, comparing a 3‐monthly with monthly administration of goserelin in premenopausal women with HR+ MBC, observed similar pharmacodynamics and safety profiles with comparable suppression of estrogen levels [19]. Regarding the different available LHRH agonists, similar ORRs were seen: goserelin (31%) [20], buserelin (14%–42%) [21], [22], [23], leuprolide (34%–44%) [24], [25], and triptorelin (45%–70%) [26], [27].
Two trials compared goserelin with oophorectomy (or irradiation), both reporting no differences in terms of overall survival (OS; Table 1) [28], [32]. Nowadays, oophorectomy can be performed via laparoscopy with a relatively low complication rate (0%–6%) [33], is cost‐effective [34], and can guarantee a good quality of life with a side‐effect rate that seems to be not higher than with the use of LHRH analogues [35], [36]. Therefore, surgical castration should be considered as an alternative method of ovarian suppression, and the choice between the available options should be carefully taken considering patients' preferences.
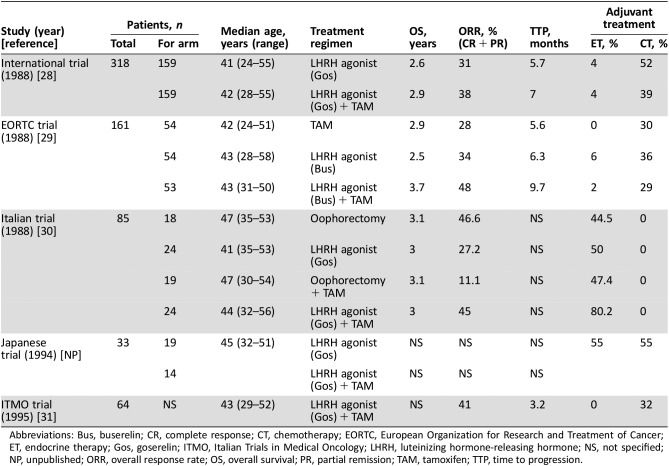
Table 1. Clinical trials including premenopausal breast cancer patients treated with LHRH and tamoxifen.
Abbreviations: Bus, buserelin; CR, complete response; CT, chemotherapy; EORTC, European Organization for Research and Treatment of Cancer; ET, endocrine therapy; Gos, goserelin; ITMO, Italian Trials in Medical Oncology; LHRH, luteinizing hormone‐releasing hormone; NS, not specified; NP, unpublished; ORR, overall response rate; OS, overall survival; PR, partial remission; TAM, tamoxifen; TTP, time to progression.
Tamoxifen
Tamoxifen is a selective estrogen receptor (ER) modulator with an agonistic effect in certain tissue such as bone, liver, and the cardiovascular system and an antagonistic effect on other sites such as uterus and breast. Initially developed in the 1960s, it has been used as first‐line therapy in MBC since the 1970s and was shown to harbor significant activity in premenopausal women [37], [38], [39], [40], [41]. Only two small trials have compared the efficacy of surgical castration with tamoxifen [38], [39]. In the trial by Ingle et al., treatment responses were seen in 37% of patients treated with oophorectomy and in 27% of patients receiving tamoxifen (10 mg twice daily); this difference was not statistically significant. In addition, progression‐free survival (PFS) and OS did not differ between the two treatment arms [40]. In the trial by Buchanan et al., a higher dose of tamoxifen was used (20 mg twice daily); again, no significant differences in terms of ORR (21% vs. 24%) or OS were observed [38].
In order to test the hypothesis of providing complete estrogen blockade by combining tamoxifen with LHRH agonists, one study randomized 318 pre‐ and perimenopausal patients to goserelin with or without tamoxifen [28]. Similar ORRs (38% vs. 31%) were obtained, whereas a modest benefit in terms of median time to progression (TTP) in favor of the combination arm was observed (28 vs. 23 weeks; p = .03); median OS, however, was comparable between the groups. In another study by Klijn et al., the combination of buserelin and tamoxifen in premenopausal patients with MBC was compared with the sequence of upfront LHRH agonist therapy followed by tamoxifen or tamoxifen followed by buserelin [29]. Here, the combination demonstrated superiority in terms of ORR (48% vs. 34% vs. 28%, p = .031), median PFS (PFS 9.7 vs. 6.3 vs. 5.6 years, p = .03), and OS (3.7 vs. 2.5 vs. 2.9 years, p = .01). Moreover, in the combination arm, the tamoxifen‐stimulated pituitary‐ovarian axis was completely suppressed. In a meta‐analysis [42] of four randomized trials (n = 506), the combination of tamoxifen and an LHRH agonist improved OS (hazard ratio = 0.70, 95% CI 0.58–0.85, test for heterogeneity p = .5), PFS (hazard ratio = 0.78, 95% CI 0.63–0.96, test for heterogeneity p = .08), and ORR compared with OFS alone. Still, some concerns have to be mentioned: The number of patients included was small; HR status was confirmed in 62% of patients only; patients had received different types of prior adjuvant chemo‐ and endocrine therapy; localization of metastatic disease was heterogeneous; no formal cross over to tamoxifen as second‐line therapy existed in patients treated with LHRH agonists alone; and no toxicity and quality‐of‐life data were reported. Despite these limitations, the combination of tamoxifen and LHRH agonists may be considered the standard approach, with single‐agent therapy remaining an acceptable treatment option.
Aromatase Inhibitors
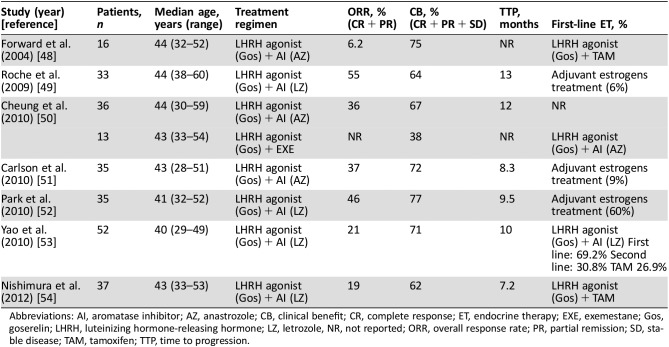
Although the current treatment algorithm in early‐stage BC in premenopausal women is changing, many patients still receive tamoxifen with or without an LHRH agonist in the adjuvant setting, and a different endocrine therapy would be preferred for metastatic disease. Aromatase, a cytochrome P‐450‐dependent enzyme responsible for the conversion of adrenal androgen substrates to estrogens, is the unique source of estrogen after cessation of ovarian estrogen production; in postmenopausal women, the superiority of aromatase inhibitors (AIs) over tamoxifen as endocrine therapy for MBC has been established [43], [44]. In premenopausal patients, AIs must be used in combination with OFS, as otherwise, ovarian estrogen production remains unaffected. Limited data on first‐generation AIs in premenopausal women with HR+ MBC are available with single‐agent aminoglutethimide yielding a complete response (CR) or partial remission (PR) in 27.8% of patients. Of note, a CR was also observed in an HR‐negative patient; therefore, these data need to be interpreted with due caution [45]. Further development of AIs in premenopausal patients occurred in combination with LHRH agonists due to the observation that LH and follicle‐stimulating hormone levels may rise in patients treated with AIs alone [46]. Supporting this combination approach, two studies [46], [47] of formestane (a second‐generation AI) plus an LHRH agonist reported a significant reduction of median estradiol levels compared with an LHRH agonist alone. Several phase II trials investigated the combination of third‐generation AIs with LHRH agonists (Table 2). Based upon available data, such combinations are a viable treatment option even after tamoxifen failure. Still, the level of evidence supporting the use of AIs in premenopausal MBC patients remains lower as compared with early‐stage disease [55], [57].
Table 2. Clinical trials with the combination of LHRH and aromatase inhibitor.
Abbreviations: AI, aromatase inhibitor; AZ, anastrozole; CB, clinical benefit; CR, complete response; ET, endocrine therapy; EXE, exemestane; Gos, goserelin; LHRH, luteinizing hormone‐releasing hormone; LZ, letrozole, NR, not reported; ORR, overall response rate; PR, partial remission; SD, stable disease; TAM, tamoxifen; TTP, time to progression.
Selective Estrogen Receptor Downregulators
Fulvestrant is a first‐generation selective estrogen receptor downregulator (SERD) that competitively binds to ER with greater affinity than tamoxifen and acts by downregulating ER and progesterone receptor (PgR). Therefore, in theory, it could be used as single agent in premenopausal patients. Despite this, several preclinical data suggested that fulvestrant worked better in the presence of a low‐estrogen environment [58]. In postmenopausal patients, fulvestrant 250 mg and AIs have shown comparable efficacy as second‐line treatment [59], [60], [61], [62]. The first‐line CONFIRM trial randomized postmenopausal MBC patients to fulvestrant 500 mg versus 250 mg, with longer PFS and OS observed with the high‐dose, loading‐dose regimen [63]. In premenopausal women, a single preoperative dose of fulvestrant 250 mg did not significantly alter the levels of ER, PgR, and Ki67; in contrast, fulvestrant 750 mg produced a significant change in the same markers. These observations led to the hypothesis that a higher dose of fulvestrant or a combination with LHRH agonists is required in premenopausal women in order to achieve an adequate estrogen blockade. The study by Bartsch et al. [64] therefore evaluated the combination of fulvestrant 250 mg plus goserelin in 26 premenopausal as first‐ to fourth‐line ET in premenopausal MBC patients. This regimen yielded a CR in 1 patient, PR in 3 patients, and stable disease (>6 months) in 11 patients, resulting in a promising clinical benefit rate (CBR) (57.7%) and ORR (15.4%); median TTP was 6 months. Although limited by its nonrandomized design, long accrual period, low number of patients, and a suboptimal dose of fulvestrant (250 mg) as well as by the heterogeneous study population, these results are encouraging. Recently, the control arm of the PALOMA 3 trial obtained a comparable median PFS of 5.6 months with fulvestrant 500 mg plus goserelin in premenopausal patients who had progressed on prior ET.
Obviously, the dose of fulvestrant is one of the key points: The phase II FIRST trial indicated superior activity of fulvestrant 500 mg over anastrozole in terms of TTP in postmenopausal patients [65]. In the phase III FALCON trial, PFS was significantly longer in the fulvestrant 500 mg arm compared with the anastrozole arm. The only available data regarding high‐dose fulvestrant in premenopausal patients with MBC were derived from the aforementioned PALOMA 3 study. In summary, these results suggested that the combination of fulvestrant 500 mg plus goserelin is a reasonable treatment approach in premenopausal women. Recently, the KCSG BR10‐04 study showed that premenopausal patients with advanced BC treated with fulvestrant plus goserelin had an increased PFS (hazard ratio = 0.61, 95% CI 0.370–0.998, p = .049) but not OS compared with goserelin alone, especially in patients younger than 40 years (hazard ratio = 0.41, 95% CI 0.181–0.936, p = .034). No difference was observed in terms of PFS and OS when anastrozole was added to goserelin compared with goserelin alone [66].
Different combinations of ET were also assessed. The FACT trial showed no benefit for the combination of fulvestrant and anastrozole as first‐line treatment in post‐ and premenopausal women, the latter receiving a combination with LHRH agonists [67]. In contrast, in the SWOG 0226 study, an improvement in terms of TTP (13.5 vs. 15 months) and OS (41.3 vs. 47.7 months) was obtained when fulvestrant 250 mg was added to anastrozole [68]; of note, this effect was mainly driven by endocrine‐naïve patients. Finally, in the SoFEA study, the combination of fulvestrant 250 mg with anastrozole compared with fulvestrant plus placebo or exemestane alone yielded comparable results in terms of PFS and OS [69]. Therefore, the combination of fulvestrant with AIs is currently not considered as treatment standard.
Despite the considerable activity of fulvestrant, there is evidence to suggest that even at the 500 mg dose, suboptimal occupancy of the ER may occur in some patients, which may correlate with rapid disease progression [70]. These data, combined with the intramuscular route of administration, underscore the need for novel SERDs. Recently, data on Elacestrant were published; this orally available SERD exhibited significant antitumor activity both as a single agent and in combination with palbociclib or everolimus in patient‐derived BC xenograft models [71]; therefore, further investigation of this compound is warranted.
Despite the considerable activity of fulvestrant, there is evidence to suggest that even at the 500 mg dose, suboptimal occupancy of the ER may occur in some patients, which may correlate with rapid disease progression. These data, combined with the intramuscular route of administration, underscore the need for novel SERDs.
Combinations and New Directions: mTOR, CDK4/6, and PI3KCA Inhibitors
Signal transduction inhibitors have been added to ET in order to overcome endocrine resistance. The mammalian target of rapamycin (mTOR) is a signaling kinase in the PI3K/mTOR/akt‐pathway that mediates cell growth and metabolism; it is commonly dysregulated in BC. In the BOLERO‐2 trial, postmenopausal women with HR+ MBC progressing after or on therapy with AIs were randomized to exemestane plus everolimus or placebo; a clinically relevant PFS prolongation (7.8 vs. 3.2 months) and a higher ORR was observed in the everolimus group [72], [73]. Currently, the ongoing MIRACLE trial (NCT02313051) randomizes premenopausal HR+ MBC patients after progression on tamoxifen to receive goserelin plus letrozole with or without everolimus.
Cell proliferation requires the progression from the G1 phase to the S phase, which is regulated by the cyclin‐dependent‐kinases 4 and 6 (CDK4/CDK6). Palbociclib, an oral small molecule inhibitor of CDK4/CDK6, has shown relevant activity when combined with ET [74]. The phase II PALOMA‐1/Trio‐18 study randomized postmenopausal patients to letrozole plus palbociclib or letrozole alone as first‐line treatment; the combination obtained significantly longer PFS and higher CBR in all subgroups, including patients who had previously received ET [75], [76]. The phase III PALOMA‐2 study confirmed these results; both studies, however, were conducted in postmenopausal patients only. In contrast, the PALOMA‐3 trial assessed the combination of fulvestrant with palbociclib or placebo in patients who had failed on previous endocrine treatment, including 108 premenopausal patients, who received additional goserelin. Of note, results in the premenopausal cohort were comparable to the overall population, with a clinically relevant improvement in median PFS (9.5 vs. 5.6 months) and CBR (69% vs. 44%) in favor of the palbociclib group [77], [78].
Similar to the results of PALOMA‐2, the MonaLEEsa‐2 trial established that the addition of the CDK4/6‐inhibitor ribociclib to letrozole resulted in a significant prolongation of PFS in postmenopausal woman who had received no prior therapy for advanced HR+ BC [79]. The MonaLEEsa‐7 trial is the first phase III trial investigating CDK4/6 inhibitor‐based regimens as front‐line treatment specifically for pre/perimenopausal women with advanced BC. The addition of ribociclib to tamoxifen/nonsteroidal AI (NSAI) and goserelin led to an increased PFS (median PFS 23.8 vs. 13.0, hazard ratio = 0.55, 95% CI 0.44–0.69, p < .001) and CBR (79.8% vs. 67.3%, p < .001) compared with placebo tamoxifen/NSAI and goserelin, with a manageable safety profile [80].
Finally, the MONARCH 2 study showed an improvement in PFS and ORR with a tolerable safety profile in women with HR+/HER2‐negative MBC when the CDK4/6‐inhibitor abemaciclib was added to fulvestrant. Importantly, results were independent from the menopausal status, and peri‐/premenopausal patients received an LHRH agonist in addition [81].
Phosphatidylinositol 3‐kinase (PI3K) pathway activation is a hallmark of endocrine‐resistant HR+ MBC. The BELLE‐2 trial demonstrated that the addition of the pan‐class I PI3K inhibitor buparlisib to fulvestrant in postmenopausal patients with MBC whose disease had progressed on or after AI treatment improved PFS over ET alone; on the downside, a relevant increase of toxicity was observed as well. In a post hoc analysis, a greater effect of buparlisib was reported in patients harboring PIK3CA mutations [82]. The results of the BELLE‐3 trial are consistent, but again, the clinical use of buparlisib appeared limited by its unfavorable toxicity profile [83]. Tolerability of α‐isoform‐specific PI3K inhibitors is apparently superior, and such drugs are currently under clinical investigation in the SOLAR 1 (alpelisib) [84] and SANDPIPER (taselisib) trials [85]. Data regarding the activity of PI3K inhibitors in premenopausal women are still lacking.
Discussion
The optimal endocrine treatment approach in premenopausal patients with MBC is still poorly defined. Current clinical guidelines recommend that patients with luminal disease should be treated preferentially with ET, whereas chemotherapy should be reserved for rapidly progressing, symptomatic or endocrine‐resistant disease. Still, in many countries, chemotherapy is the preferred first‐line option in younger patients [86]. Information regarding the efficacy of endocrine therapy in premenopausal patients is limited by the small number of patients enrolled into clinical trials, long accrual time, and lack of stratification for previous adjuvant therapy or for relevant prognostic factors; in addition, no information concerning postprogression treatment is available. The vast majority of trials evaluating novel endocrine treatment options included postmenopausal patients only. Therefore, no corresponding results are available for women who remain premenopausal.
Information regarding the efficacy of endocrine therapy in premenopausal patients is limited by the small number of patients enrolled into clinical trials, long accrual time, and lack of stratification for previous adjuvant therapy or for relevant prognostic factors; in addition, no information concerning postprogression treatment is available.
Given available data, the combination of LHRH agonists with tamoxifen is preferred compared with the use of either agent alone; oophorectomy is a valid alternative approach to LHRH agonists, especially in the metastatic setting, where fertility preservation might be less important for the patients. Moreover, it is the option of choice for those patients who would like to avoid monthly injections. Despite these considerations, it is of major importance to carefully discuss with each patient which approach to choose, considering the pros and cons of both methods and the patient's preference. The combination of AIs or fulvestrant with LHRH agonists harbors promising activity even after prior tamoxifen exposure. Furthermore, in premenopausal patients who have failed on previous endocrine treatment, palbociclib plus fulvestrant and goserelin was superior to endocrine treatment alone, and this effect was similar to the outcome in postmenopausal patients. Finally, ribociclib added to tamoxifen or NSAI and goserelin is a potential new treatment option for premenopausal patients not previously treated with ET for advanced disease. Therefore, current guidelines recommend starting OFS in order to induce menopause; thereafter, recommended treatment mirrors that of postmenopausal patients.
In summary, endocrine therapy should be considered a standard first‐line treatment option for the majority of premenopausal patients with MBC because of its favorable efficacy/safety balance as compared with chemotherapy. Several endocrine therapy options as well as combinations of endocrine therapy with targeted agents are available today, and treatment should be chosen considering risk factors, response to previous therapy, and patient preference.
Conclusion
Similar to options for postmenopausal patients, endocrine therapy is an active and safe treatment option with limited side effects in premenopausal women with HR+ MBC. Further data regarding the combination of endocrine treatment with novel targeted agents will help to define the best treatment strategy for this population. Currently, OFS with LHRH agonists or surgical castration is preferred, and patients should be treated according to recommendations for postmenopausal women.
Author Contributions
Conception/design: Richard Tancredi, Jenny Furlanetto, Sibylle Loibl
Data analysis and interpretation: Richard Tancredi, Jenny Furlanetto, Sibylle Loibl
Manuscript writing: Richard Tancredi, Jenny Furlanetto, Sibylle Loibl
Final approval of manuscript: Richard Tancredi, Jenny Furlanetto, Sibylle Loibl
Disclosures
The authors indicated no financial relationships.
References
- 1. Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA 2013;309:800–805. [DOI] [PubMed] [Google Scholar]
- 2. Loibl S, Jackisch C, Lederer B et al. Outcome after neoadjuvant chemotherapy in young breast cancer patients: A pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Res Treat 2015;152:377–387. [DOI] [PubMed] [Google Scholar]
- 3. Anders C, Hsu D, Broadwater G et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008;26:3324–3330. [DOI] [PubMed] [Google Scholar]
- 4. Albain KS, Allred DC, Clark GM. Breast cancer outcomes and predictors of outcome: Are there age differentials? J Natl Cancer Inst Monogr 1994;16:35–42. [PubMed] [Google Scholar]
- 5. Gnerlich J, Deshpande A, Jeffe D et al. Elevated breast cancer mortality in young women (<40 yrs) compared with older women is attributed to poorer survival in early stage disease. J Am Coll Surg 2009;208:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol 2012;1:533–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parl FF. Estrogens, estrogen receptor and breast cancer. In: Estrogen Receptor Expression in Breast Cancer. Amsterdam, The Netherlands: IOS Press, 2000:135–204. [Google Scholar]
- 8. Paluch‐Shimon S, Pagani O, Partridge AH et al. Second international consensus guidelines for breast cancer in young women (BCY2). Breast 2016;26:87–99. [DOI] [PubMed] [Google Scholar]
- 9. Rugo HS, Rumble RB, Macrae E et al. Endocrine therapy for hormone receptor‐positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 2016;34:3069–3103. [DOI] [PubMed] [Google Scholar]
- 10. Cardoso F, Costa A, Senkus E et al. 3rd ESO‐ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol 2017;28:16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beatson CT. On treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment with illustrative cases. Lancet 1896;2:104–107. [PMC free article] [PubMed] [Google Scholar]
- 12. Veronesi U, Pizzocaro G, Rossi A. Oophorectomy for advanced carcinoma of the breast. Surg Gynecol Obstet 1975;141:569–570. [PubMed] [Google Scholar]
- 13. Ingle JN, Krook JE, Green SJ et al. Randomized trial of bilateral oophorectomy versus tamoxifen in premenopausal women with metastatic breast cancer. J Clin Oncol 1986;4:178–185. [DOI] [PubMed] [Google Scholar]
- 14. Oriana S, Böhm S, Baeli A et al. Clinical response and survival according to estrogen receptor levels after bilateral ovariectomy in advanced breast cancer. Eur J Surg Oncol 1989;15:39–42. [PubMed] [Google Scholar]
- 15. Conte CC, Nemoto T, Rosner D et al. Therapeutic oophorectomy in metastatic breast cancer. Cancer 1989;64:150–153. [DOI] [PubMed] [Google Scholar]
- 16. Karten MJ, Rivier JE. Gonadotropin‐releasing hormone analogue design. Structure function studies toward the development of agonists and antagonists: Rationale and prospective. Endocrine Rev 1986;7:44–66. [DOI] [PubMed] [Google Scholar]
- 17. Boccardo F, Rubagotti A, Amoroso D et al. Endocrinological and clinical evaluation of two depot formulations of leuprolide acetate in pre‐ and perimenopausal breast cancer patients. Cancer Chemother Pharmacol 1999;43:461–466. [DOI] [PubMed] [Google Scholar]
- 18. Dowsett M, Jacobs S, Aherne J et al. Clinical and endocrine effects of leuprorelin acetate in pre‐ and postmenopausal patients with advanced breast cancer. Clin Ther 1992;14(suppl A):97–103. [PubMed] [Google Scholar]
- 19. Noguchi S, Kim HJ, Jesena A et al. Phase 3, open‐label, randomized study comparing 3‐monthly with monthly goserelin in pre‐menopausal women with estrogen receptor‐positive advanced breast cancer. Breast Cancer 2016;23:771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blamey RW, Jonat W, Kaufmann M et al. Goserelin depot in the treatment of premenopausal advanced breast cancer. Eur J Cancer 1992;28A:810–814. [DOI] [PubMed] [Google Scholar]
- 21. Lissoni P, Barni S, Crispino S et al. Endocrine and clinical effects of LHRH analogue in pretreated advanced breast cancer. Tumori 1998;74:303–308. [DOI] [PubMed] [Google Scholar]
- 22. Klijn JG. Long‐term LHRH‐agonist treatment in metastatic breast cancer as a single treatment and in combination with other additive endocrine treatments. Med Oncol Tumor Pharmacother 1984;1:123–128. [DOI] [PubMed] [Google Scholar]
- 23. Klijn JG, De Jong FH, Lamberts SW et al. LHRH‐agonist treatment in clinical and experimental human breast cancer. J Steroid Biochem 1985;23:867–873. [DOI] [PubMed] [Google Scholar]
- 24. Dowsett M, Metha A, Mansi J et al. A dose comparative clinical study of leuprorelin in premenopausal breast cancer patients. Br J Cancer 1990;62:834–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harvey HA, Lipton A, Max DT et al. Medical castration produced by the GnRH analogue leuprolide to treat metastatic breast cancer. J Clin Oncol 1985;3:1068–1072. [DOI] [PubMed] [Google Scholar]
- 26. Neskovic‐ Konstantinovic ZB, Vuletic LB, Nikolic‐ Stanojievic LI et al. Therapeutic and endocrine effects of Decapetyl, synthetic LH‐RH agonistic analogue in premenopausal women with metastatic breast cancer. A pilot phase II study. Oncology 1994;51:95–101. [DOI] [PubMed] [Google Scholar]
- 27. Garcia‐Giralt E, Beuzeboc P, Dieras V et al. Phase II trial of decapeptyl (D‐TRP‐6), a potent luteinizing hormone‐releasing hormone analogue in untreated advanced breast cancer Am J Clin Oncol 1996;19:455–458. [DOI] [PubMed] [Google Scholar]
- 28. Jonat W, Kaufmann M, Blamey RW et al. A randomised study to compare the effect of the luteinising hormone releasing hormone (LHRH) analogue goserelin with or without tamoxifen in pre‐ and perimenopausal patients with advanced breast cancer. Eur J Cancer 1995;31A:137–142. [DOI] [PubMed] [Google Scholar]
- 29. Klijn JG, Beex LV, Mauriac L et al. Combined treatment with buserelin and tamoxifen in premenopausal metastatic breast cancer: A randomized study. J Natl Cancer Inst 2000;92:903–911. [DOI] [PubMed] [Google Scholar]
- 30. Boccardo F, Rubagotti A, Perrotta A et al. Ovarian ablation versus goserelin with or without tamoxifen in pre‐perimenopausal patients with advanced breast cancer: Results of a multicentric Italian study. Ann Oncol 1994;5:337–342. [DOI] [PubMed] [Google Scholar]
- 31. Buzzoni R, Biganzoli L, Bajetta E et al. Combination goserelin and tamoxifen therapy in premenopausal advanced breast cancer: A multicentre study by the ITMO group. Italian Trials in Medical Oncology. Br J Cancer 1995;71:1111–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor CW, Green S, Dalton WS et al. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor positive metastatic breast cancer: An intergroup study. J Clin Oncol 1998;16:994–999. [DOI] [PubMed] [Google Scholar]
- 33. Angioni S, Pontis A, Sedda F et al. Single‐port versus conventional multiport access prophylactic laparoscopic bilateral salpingo‐oophorectomy in high‐risk patients for ovarian cancer: A comparison of surgical outcomes. Onco Targets Ther 2015;8:1575–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hagemann AR, Zighelboim I, Odibo AO et al. Cost‐benefit of laparoscopic versus medical ovarian suppression in premenopausal breast cancer. Breast J 2011;17:103–105. [DOI] [PubMed] [Google Scholar]
- 35. Park IH, Ro J, Lee KS et al. Phase II parallel group study showing comparable efficacy between premenopausal metastatic breast cancer patients treated with letrozole plus goserelin and postmenopausal patients treated with letrozole alone as first‐line hormone therapy. J Clin Oncol 2010;28:2705–2711. [DOI] [PubMed] [Google Scholar]
- 36. Boccardo F, Rubagotti A, Perrotta A et al. Ovarian ablation versus goserelin with or without tamoxifen in pre‐perimenopausal patients with advanced breast cancer: Results of a multicentric Italian study. Ann Oncol 1994;5:337–342. [DOI] [PubMed] [Google Scholar]
- 37. Santen RJ, Manni A, Harvey H et al. Endocrine treatment of breast cancer in women. Endocr Rev 1990;11:221–265. [DOI] [PubMed] [Google Scholar]
- 38. Buchanan RB, Blamey RW, Durrant KR et al. A randomized comparison of tamoxifen with surgical oophorectomy in premenopausal patients with advanced breast cancer J Clin Oncol 1986;4:1326–1330. [DOI] [PubMed] [Google Scholar]
- 39. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med 1998;339:1609–1618. [DOI] [PubMed] [Google Scholar]
- 40. Ingle JN, Krook JE, Green SJ et al. Randomized trial of bilateral oophorectomy versus tamoxifen in premenopausal women with metastatic breast cancer. J Clin Oncol 1986;4:178–185. [DOI] [PubMed] [Google Scholar]
- 41. Fossati R, Confalonieri C, Torri V et al. Cytotoxic and hormonal treatment for metastatic breast cancer: A systematic review of published randomized trials involving 31,510 women. J Clin Oncol 1998;16:3439–3460. [DOI] [PubMed] [Google Scholar]
- 42. Klijn JG, Blamey RW, Boccardo F, et al. Combined Hormone Agents Trialists' Group and the European Organization for Research and Treatment of Cancer. Combined tamoxifen and luteinizing hormone‐releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta‐analysis of four randomized trials. J Clin Oncol 2001;19:343–353. [DOI] [PubMed] [Google Scholar]
- 43. Bonneterre J, Thürlimann B, Robertson JF et al. Anastrozole versus tamoxifen as first‐line therapy for advanced breast cancer in 668 postmenopausal women: Results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 2000;18:3748–3757. [DOI] [PubMed] [Google Scholar]
- 44. Mouridsen H, Gershanovich M, Sun Y et al. Phase III study of letrozole versus tamoxifen as first‐line therapy of advanced breast cancer in postmenopausal women: Analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003;21:2101–2109. [DOI] [PubMed] [Google Scholar]
- 45. Wander HE, Blossey HC, Nagel GA. Aminoglutethimide in the treatment of premenopausal patients with metastatic breast cancer. Eur J Cancer Clin Oncol 1986;22:1371–1374. [DOI] [PubMed] [Google Scholar]
- 46. Stein RC, Dowsett M, Hedley A et al. The clinical and endocrine effects of 4‐hydroxyandrostenedione alone and in combination with goserelin in premenopausal women with advanced breast cancer. Br J Cancer 1990;62:679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Celio L, Martinetti A, Ferrari L et al. Premenopausal breast cancer patients treated with a gonadotropin‐releasing hormone analog or in combination with an aromatase inhibitor: A comparative endocrine study. Anticancer Res 1999;19:2261–2268. [PubMed] [Google Scholar]
- 48. Forward DP, Cheung KL, Jackson L et al. Clinical and endocrine data for goserelin plus anastrozole as second‐line endocrine therapy for premenopausal advanced breast cancer. Br J Cancer 2004;90:590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roche H, Thierry D, Chieze S et al. Anastrozole and goserelin combination as first treatment for premenopausal receptor positive advanced or metastatic breast cancer: A phase II trial. J Clin Oncol 2009;27:1079a. [Google Scholar]
- 50. Cheung, KL, Agrawal A, Folkerd E et al. Suppression of ovarian function in combination with an aromatase inhibitor as treatment for advanced breast cancer in pre‐menopausal women. Eur J Cancer 2010;46:2936–2942. [DOI] [PubMed] [Google Scholar]
- 51. Carlson RW, Theriault R, Schurman CM et al. Phase II trial of anastrozole plus goserelin in the treatment of hormone receptor‐positive, metastatic carcinoma of the breast in premenopausal women. J Clin Oncol 2010;28:3917–3921. [DOI] [PubMed] [Google Scholar]
- 52. Park IH, Ro J, Lee KS et al. Phase II parallel group study showing comparable efficacy between premenopausal metastatic breast cancer patients treated with letrozole plus goserelin and postmenopausal patients treated with letrozole alone as first‐line hormone therapy. J Clin Oncol 2010;28:2705–2711. [DOI] [PubMed] [Google Scholar]
- 53. Yao S, Xu B, Li Q et al. Goserelin plus letrozole as first‐ or second‐line hormonal treatment in premenopausal patients with advanced breast cancer. Endocr J 2011;58:509–516. [DOI] [PubMed] [Google Scholar]
- 54. Nishimura R, Anan K, Yamamoto Y et al. Efficacy of goserelin plus anastrozole in premenopausal women with advanced or recurrent breast cancer refractory to an LH‐RH analogue with tamoxifen: Results of the JMTO BC08‐01 phase II trial. Oncol Rep 2013;29:1707–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pagani O, Regan MM, Walley BA et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014;371:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Regan MM, Francis PA, Pagani O et al. Absolute benefit of adjuvant endocrine therapies for premenopausal women with hormone receptor‐positive, human epidermal growth factor receptor 2‐negative early breast cancer: TEXT and SOFT trials. J Clin Oncol 2016;34:2221–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Francis PA, Regan MM, Fleming GF et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 2015;372:436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jelovac D, Macedo L, Goloubeva OG et al. Additive anti‐tumor effect of aromatase inhibitor letrozole and antiestrogen fulvestrant in a postmenopausal breast cancer model. Cancer Res 2005;65:5439. [DOI] [PubMed] [Google Scholar]
- 59. Robertson JF, Osborne CK, Howell A et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women. A prospective combined analysis of two multicenter trials. Cancer 2003;98:229–238. [DOI] [PubMed] [Google Scholar]
- 60. Osborne CK, Pippen J, Jones SE et al. Double‐blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: Results of a North American trial. J Clin Oncol 2002;20:3386–3395. [DOI] [PubMed] [Google Scholar]
- 61. Howell A, Robertson JF, Quaresma Albano J et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol 2002;20:3396–3403. [DOI] [PubMed] [Google Scholar]
- 62. Howell A, Pippen J, Elledge RM et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma: A prospectively planned combined survival analysis of two multicenter trials. Cancer 2005;104:236–239. [DOI] [PubMed] [Google Scholar]
- 63. Di Leo A, Jerusalem G, Petruzelka L et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor‐positive advanced breast cancer. J Clin Oncol 2010;28:4594–4600. [DOI] [PubMed] [Google Scholar]
- 64. Bartsch R, Bago‐Horvath Z, Berghoff A et al. Ovarian function suppression and fulvestrant as endocrine therapy in premenopausal women with metastatic breast cancer. Eur J Cancer 2012;48:1932–1938. [DOI] [PubMed] [Google Scholar]
- 65. Robertson JF, Llombart‐Cussac A, Rolski J et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first‐line treatment for advanced breast cancer: Results from the FIRST study. J Clin Oncol 2009;27:4530–4535. [DOI] [PubMed] [Google Scholar]
- 66. Kim JY, Im SA, Jung KH et al. A phase II, randomized, open‐label 3‐arm clinical trial of fulvestrant (F) plus goserelin (G) versus anastrozole (A) plus goserelin (G) versus goserelin (G) alone for hormone receptor (HR) positive, tamoxifen (T) pretreated premenopausal women with recurrent or metastatic breast cance r(MBC) (KCSG BR10‐04). J Clin Oncol 2017;35(suppl 15):1041a. 28113032 [Google Scholar]
- 67. Bergh J, Jonsonn PE, Lidbrink EK et al. FACT: An open‐label randomized phase II study of fulvestrant and anastrozole in combination compared with anastrozole alone as a first‐line therapy for patients with receptor positive postmenopausal breast cancer. J Clin Oncol 2012;30:1919–1925. [DOI] [PubMed] [Google Scholar]
- 68. Mehta RS, Barlow WE, Albain KS et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 2012;367:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Johnston SR, Kilburn LS, Ellis P et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non‐steroidal aromatase inhibitors in postmenopausal patients with hormone‐receptor‐positive locally advanced or metastatic breast cancer (SoFEA): A composite, multicentre, phase 3 randomised trial. Lancet Oncol 2013;14:989–998. [DOI] [PubMed] [Google Scholar]
- 70. van Kruchten M, de Vries EG, Glaudemans AW et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov 2015;5:72–81. [DOI] [PubMed] [Google Scholar]
- 71. Bihani T, Patel HK, Arlt H et al. Elacestrant (RAD1901), a selective estrogen receptor degrader (SERD), has antitumor activity in multiple ER(+) breast cancer patient‐derived xenograft models. Clin Cancer Res 2017;23:4793–4804. [DOI] [PubMed] [Google Scholar]
- 72. Baselga J, Campone M, Piccart M et al. Everolimus in postmenopausal hormone‐receptor‐positive advanced breast cancer. N Engl J Med 2012;366:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Piccart M, Hortobagyi GN, Campone M et al. Everolimus plus exemestane for hormone‐receptor‐positive, human epidermal growth factor receptor‐2‐negative advanced breast cancer: Overall survival results from BOLERO‐2. Ann Oncol 2014;25:2357–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Finn RS, Dering J, Conklin D et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor‐positive human breast cancer cell lines in vitro. Breast Cancer Res 2009;11:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Finn RS, Crown JP, Lang I, et al. The cyclin‐dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first‐line treatment of oestrogen receptor‐positive, HER2 negative, advanced breast cancer (PALOMA‐1/TRIO‐18): A randomized phase 2 study. Lancet Oncol 2015;16:25–35. [DOI] [PubMed] [Google Scholar]
- 76. Finn RS, Crown JP, Ettl J et al. Efficacy and safety of palbociclib in combination with letrozole as first‐line treatment of ER‐positive, HER2‐negative, advanced breast cancer: Expanded analyses of subgroups from the randomized pivotal trial PALOMA‐1/TRIO‐18. Breast Cancer Res 2016;18:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cristofanilli M, Turner NC, Bondarenko I et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone‐receptor‐positive, HER2‐negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA‐3): Final analysis of the multicentre, double‐blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–439. [DOI] [PubMed] [Google Scholar]
- 78. Turner NC, Ro J, André F et al. Palbociclib in hormone‐receptor‐positive advanced breast cancer. N Engl J Med 2015;373:209–219. [DOI] [PubMed] [Google Scholar]
- 79. Barroso‐Sousa R, Shapiro GI, Tolaney SM. Clinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancer. Breast Care (Basel) 2016;11:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tripathy D, Sohn J, Im S‐A et al. First‐line ribociclib vs placebo with goserelin and tamoxifen or a non‐steroidal aromatase inhibitor in premenopausal women with hormone receptor‐positive, HER2‐negative advanced breast cancer: Results from the randomized phase III MONALEESA‐7 trial. Presented at SABCS 2017; Abstract GS2‐05. [Google Scholar]
- 81. Sledge GW Jr, Toi M, Neven P et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2– advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–2884. [DOI] [PubMed] [Google Scholar]
- 82. Baselga J, Im SA, Iwata H et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor‐positive, HER2‐negative, advanced breast cancer (BELLE‐2): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2017;18:904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Di Leo A, Lee KS, Ciruelos E et al. BELLE‐3: A phase III study of buparlisib and fulvestrant in postmenopausal women with HR+, HER2‐, AI‐treated, locally advanced or metastatic breast cancer, who progressed on or after mTOR inhibitor‐based treatment. J Clin Oncol 2015;33(suppl 15):TPS626a. [Google Scholar]
- 84. Andre F, Campone M, Ciruelos EM et al. SOLAR‐1: A phase III study of alpelisib + fulvestrant in men and postmenopausal women with HR+/HER2– advanced breast cancer (BC) progressing on or after prior aromatase inhibitor therapy. J Clin Oncol 2016;34(suppl 15)TPS618a. [Google Scholar]
- 85. Baselga J, Cortés J, DeLaurentiis M et al. SANDPIPER: Phase III study of the PI3‐kinase (PI3K) inhibitor taselisib (GDC‐0032) plus fulvestrant in patients (pts) with estrogen receptor (ER)‐positive, HER2‐negative locally advanced or metastatic breast cancer (BC) enriched for pts with PIK3CA‐mutant tumors. J Clin Oncol 2017;35(suppl 15):TPS1119a. [Google Scholar]
- 86. Marchetti P, Maass N, Gligorov J et al. Patient database analysis of fulvestrant 500 mg in the treatment of metastatic breast cancer: A European perspective. Breast 2017;32:247–255. [DOI] [PubMed] [Google Scholar]