Although seldom severe or life‐threatening, dermatologic toxicities can result in physical and emotional discomfort for patients; therefore, appropriate management of such toxicities is important. This review provides oncologists with an understanding of dermatologic adverse events associated with the third‐generation EGFR‐TKI osimertinib.
Keywords: Dermatologic adverse events, Non‐small cell lung cancer, Epidermal growth factor receptor, Tyrosine kinase inhibitor, T790M
Abstract
Dermatologic adverse events (dAEs) are common with the use of epidermal growth factor receptor‐tyrosine kinase inhibitor (EGFR‐TKI) therapy. First‐ and second‐generation agents (erlotinib, gefitinib, and afatinib) are frequently associated with acneiform rash, pruritus, xerosis, and paronychia; the incidence and characterization of these dAEs have been well described. However, there is evidence that the dAE profile is different with third‐generation EGFR‐TKIs. Herein, we describe the dAEs associated with third‐generation EGFR‐TKIs and our clinical experience with osimertinib, a third‐generation EGFR‐TKI approved by the U.S. Food and Drug Administration for the treatment of metastatic, EGFR T790M mutation‐positive non‐small cell lung cancer in patients whose disease has progressed on or after EGFR‐TKI therapy. Case summaries of patients from two of our institutions who received osimertinib and were referred to a dermatologist for dAEs are also presented. Overall, the evidence suggests that osimertinib is associated with less severe and less frequent dAEs than first‐ and second‐generation EGFR‐TKIs and that therefore a different approach is warranted. Finally, we outline dAE management approaches for osimertinib in the context of those typically employed with first‐ and second‐generation EGFR‐TKIs.
Implications for Practice.
Appropriate prevention and management of dermatologic adverse events (dAEs) associated with the use of epidermal growth factor receptor‐tyrosine kinase inhibitors (EGFR‐TKIs) may help patients to continue therapy and lessen any negative impact on their quality of life. EGFR‐TKIs are frequently associated with acneiform rash, pruritus, xerosis, and paronychia; however, dAEs associated with third‐generation EGFR‐TKIs are lower in frequency and severity. Before therapy, health care providers should discuss the potential osimertinib‐associated dAEs and encourage patients to report their dAEs. Patients should also be educated on prophylactic measures to minimize the severity of dAEs and the importance of adherence to the treatment regimen.
Introduction
The treatment paradigm for patients with epidermal growth factor receptor (EGFR) mutation‐positive, metastatic non‐small cell lung cancer (NSCLC) that progresses on an initial tyrosine kinase inhibitor (TKI) is evolving with investigational third‐generation EGFR‐TKIs and the approval of osimertinib, an oral, central nervous system‐active, third‐generation EGFR‐TKI; this agent is an effective treatment for patients with EGFR T790M mutation‐positive advanced NSCLC [1], [2], [3], [4], [5].
First‐ and second‐generation EGFR‐TKIs have safety and tolerability profiles that are well characterized and associated with mechanism‐based dermatologic toxicities such as acneiform rash, pruritus, xerosis, and paronychia. The dermatologic adverse event (dAE) profile of third‐generation EGFR‐TKIs appears to be milder than that found for first‐ and second‐generation agents [4]. Osimertinib was specifically developed to have greater potency against EGFR‐TKI‐sensitizing and T790M resistance mutations than wild‐type EGFR, potentially reducing wild‐type EGFR‐related toxicities that adversely affect normal tissues such as the skin, hair, nails, and gut [1], [6].
Although they are rarely severe or life‐threatening, dermatologic toxicities may result in physical and emotional morbidity for patients [7]. It is therefore imperative that these toxicities be appropriately managed [8], [9]. This review aims to provide oncologists with an understanding of dAEs associated with the third‐generation EGFR‐TKI osimertinib. We summarize dAEs seen with first‐ and second‐generation EGFR‐TKIs, and our clinical experience in treating dAEs observed with osimertinib (including case summaries from two of our institutions representing the U.S. and Asia), and describe management strategies for dAEs associated with EGFR‐TKI treatment.
Dermatologic Toxicities Among First‐ and Second‐Generation EGFR‐TKIs
The first‐ and second‐ generation EGFR‐TKIs (gefitinib, erlotinib, and afatinib) are approved for first‐line therapy for patients with metastatic NSCLC with EGFR‐sensitizing mutations (deletions in exon 19 and point mutations in exon 21) [10], [11], [12]. Both first‐ and second‐generation agents inhibit EGFR harboring sensitizing mutations and are associated with a clinical response rate of ∼70% (range, 62%–83%) [13], [14], [15], [16], [17], [18], [19].
Among the first‐line EGFR therapies, there are differences in safety and tolerability profiles, which may reflect their different affinities for EGFR [20], [21], [22], [23]. They are also associated with particular dAEs as a result of their mechanism of action and molecular targets in other tissues [24]. Specifically, wild‐type EGFR is expressed in the basal cell layer of the epidermis, the outer root sheath of hair follicles, the epithelium of sweat and sebaceous glands, and in periungual tissue [6], [24], [25]. Inhibition of EGFR may result in abnormal proliferation, migration, and differentiation, leading to disruption of skin integrity with the recruitment of inflammatory cells [6], [26].
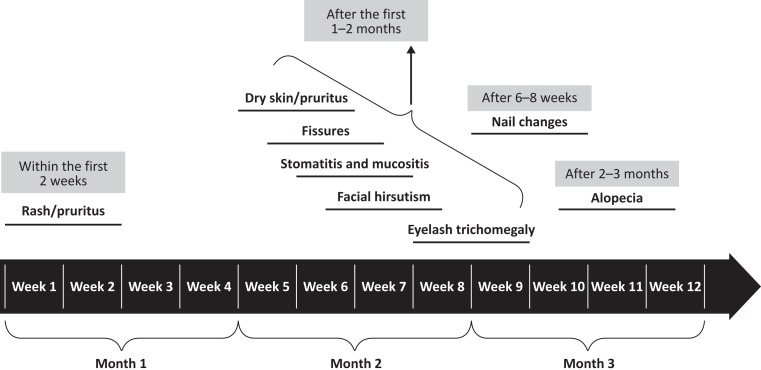
dAEs have been reported in clinical trials of EGFR‐TKIs and are summarized in Table 1. Several reviews have focused on the management of dAEs associated with first‐ and second‐generation EGFR‐TKI treatment, and the time of onset of dAEs has been well described (Fig. 1) [8], [9], [27]. Therefore, we will only briefly review the dAE data presented in the pivotal or registrational trials of first‐ and second‐generation agents.
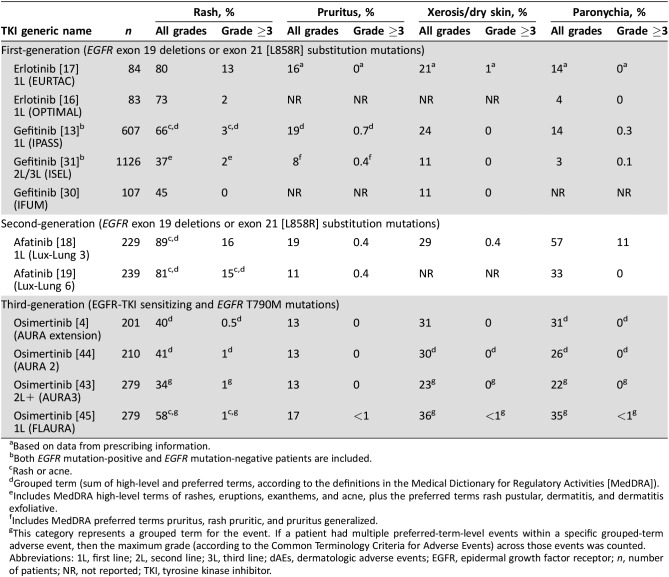
Table 1. Incidence of dermatologic adverse events associated with EGFR‐TKIs approved by the U.S. Food and Drug Administration for the treatment of metastatic EGFR mutation‐positive non‐small cell lung cancer (based on data from pivotal clinical trials).
Based on data from prescribing information.
Both EGFR mutation‐positive and EGFR mutation‐negative patients are included.
Rash or acne.
Grouped term (sum of high‐level and preferred terms, according to the definitions in the Medical Dictionary for Regulatory Activities [MedDRA]).
Includes MedDRA high‐level terms of rashes, eruptions, exanthems, and acne, plus the preferred terms rash pustular, dermatitis, and dermatitis exfoliative.
Includes MedDRA preferred terms pruritus, rash pruritic, and pruritus generalized.
This category represents a grouped term for the event. If a patient had multiple preferred‐term‐level events within a specific grouped‐term adverse event, then the maximum grade (according to the Common Terminology Criteria for Adverse Events) across those events was counted.
Abbreviations: 1L, first line; 2L, second line; 3L, third line; dAEs, dermatologic adverse events; EGFR, epidermal growth factor receptor; n, number of patients; NR, not reported; TKI, tyrosine kinase inhibitor.
Figure 1.
Timeline of the onset of dermatologic adverse events (dAEs) associated with endothelial growth factor receptor‐tyrosine kinase inhibitors (EGFR‐TKIs). The figure provides a general overview of the occurrence of dAEs associated with first‐, second‐, and third‐generation EGFR‐TKIs.
Rash is the most common dAE associated with EGFR‐TKIs [10], [11], [12]. Rash from first‐ and second‐generation EGFR‐TKIs typically presents as an acneiform eruption with a combination of inflamed papules and pustules, which are often associated with a serous and/or hemorrhagic crust [6], [8], [26], [28]. Rash typically presents within 2 weeks of starting EGFR‐TKI therapy [24], [26], [29]. Of patients who received erlotinib across two studies, 73%–80% reported some form of rash, including acneiform dermatitis, erythematous rash, follicular rash, papular rash, or other types of rash, with a relatively lower incidence of grade ≥3 rash (2%–13%) [16], [17] according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI‐CTCAE). In three studies, patients who received gefitinib showed a lower incidence of rash compared with erlotinib (37%–66%) and a lower incidence of grade ≥3 rash (0%–3%) [13], [30], [31]. Most patients (81%–89%) receiving the second‐generation EGFR‐TKI, afatinib, experienced rash, and 15%–16% of these were grade ≥3 events [18], [19].
As noted, most dAEs associated with EGFR‐TKIs were mild (grade 1 or 2), but when severe or intolerable, these toxicities can lead to dose modification or discontinuation [9], [32]; for instance, in patients receiving afatinib, the incidence of discontinuation related to paronychia was 0.9%, and with erlotinib, 13% of patients in clinical trials had a dose modification because of rash [12], [18].
In addition to the data from clinical trials of approved agents in Table 1, dAEs associated with first‐ and second‐generation agents have also been investigated by meta‐analyses. A meta‐analysis of patients receiving erlotinib (n = 2,717) and gefitinib (n = 3,002) found an overall incidence of pruritus of 21% for each agent and the incidence for high‐grade pruritus to be 2.3% and 1%, respectively [33]. A meta‐analysis of risk and incidence of nail toxicities associated with erlotinib and other EGFR inhibitors found that, among patients (n = 488) receiving erlotinib for the treatment of NSCLC, endometrial cancer, or hepatocellular carcinoma, 16.3% (95% confidence interval [CI], 12.4–21.1) had nail toxicity of any grade, and 1.8% (95% CI, 0.8–3.8) had high‐grade nail toxicity [34].
Dacomitinib is a second‐generation EGFR‐TKI in development for which incidence data on dAEs have been reported [35]. In the phase III ARCHER 1050 trial (NCT01774721), in which patients received either first‐line dacomitinib or gefitinib, although dacomitinib was associated with superior clinical efficacy, the agent was associated with a greater incidence of dAEs such as paronychia, dermatitis acneiform, dry skin, and pruritus [35]. The phase II ARCHER 1042 study showed that prophylactic doxycycline in dacomitinib‐treated patients could significantly reduce certain dAEs [36].
Dermatologic Toxicities Among Third‐Generation EGFR‐TKIs
The National Comprehensive Cancer Network recommends that patients whose disease has progressed during a first‐ or second‐generation EGFR‐TKI treatment undergo molecular testing to determine the mechanism of drug resistance [37]. In approximately 60% of cases, a second mutation in exon 20 of EGFR (T790M) leads to tumors that are resistant to first‐ and second‐generation EGFR‐TKIs [4], [38], [39], [40], [41]. Although there are a number of third‐generation agents in development (olmutinib [BI1482694/HM61713], EGF816, PF‐06747775), the only third‐generation EGFR‐TKI currently approved by the U.S. Food and Drug Administration for the treatment of patients with metastatic EGFR T790M mutation‐positive NSCLC whose disease has progressed on or after EGFR‐TKI therapy is osimertinib [42].
Osimertinib is effective in patients with a T790M mutation in EGFR. Of 198 patients with T790M mutation‐positive NSCLC who received osimertinib in the AURA phase II extension component study, the objective response rate was 62% (95% CI, 54%–68%), the median duration of response (DoR) was 15.2 months (95% CI, 11.3 months to not calculable), and the median progression‐free survival (PFS) was 12.3 months (95% CI, 9.5–13.8 months) [4]. In the randomized phase III AURA3 trial comparing osimertinib with standard chemotherapy, 279 patients with T790M mutation‐positive NSCLC received osimertinib, and the median DoR was 9.7 months (95% CI, 8.3–11.6 months). The median PFS via blinded independent central review was 11.0 months versus 4.2 months for those receiving osimertinib and chemotherapy, respectively (hazard ratio [HR] 0.28; 95% CI, 0.20–0.38; p < .001) [43].
The most common dAEs associated with osimertinib therapy reported in AURA3 included rash (34%), dry skin (23%), and paronychia (22%); grade ≥3 rash was rare (1%), and no grade ≥3 dry skin was reported. Pruritus occurred in 13% of patients with no grade ≥3 events [43]. Phase II clinical trials document similar rates; the incidence of rash across three studies of osimertinib ranged from 34% to 41%, and instances of grade ≥3 rash were reported in no more than 1% of patients [4], [43], [44]. The incidence of pruritus was 13%, and nail toxicity (including paronychia) was 22%–31% in those treated with osimertinib [4], [43], [44]. Taken as a whole, these numbers are lower than those reported with first‐ and second‐generation EGFR‐TKIs. The only head‐to‐head study to date is FLAURA, which directly compared osimertinib with standard of care (first‐generation TKIs gefitinib or erlotinib) in 556 treatment‐naïve patients with EGFR mutation‐positive locally advanced or metastatic NSCLC [45]. The median DoR in FLAURA was 17.2 months (95% CI, 13.8–22.0 months) with osimertinib versus 8.5 months (95% CI, 7.3–9.8 months) with standard of care. The median investigator‐assessed PFS (the primary endpoint) was 18.9 months versus 10.2 months for those receiving osimertinib and standard of care, respectively (HR for disease progression or death, 0.46; 95% CI, 0.37–0.57; p < .001). Rates of dAEs in FLAURA were generally similar in both treatment arms, with the exception of rashes and acne, which occurred more frequently in the standard‐of‐care arm (78%; grade ≥3, 7%) than in the osimertinib arm (58%; grade ≥3, 1%) [45].
Osimertinib has two active metabolites, AZ7550 and AZ5104, which circulate in plasma at about 10% of the parent concentration and which have similar inhibitory profiles to osimertinib [42]. The potency of AZ7550 is similar to that of osimertinib, whereas AZ5104 has greater potency than osimertinib against exon 19 deletion, T790M mutants, and wild‐type EGFR [42], which may partially explain its dAEs.
Osimertinib Case‐Series Summaries
The cases of patients receiving osimertinib at two institutions were summarized to better evaluate trends in dAEs. Medical records at the National Taiwan University Hospital (NTUH) and the Memorial Sloan Kettering Cancer Center (MSKCC) were reviewed for patients with NSCLC who were referred to a dermatologist to manage their dAEs associated with osimertinib use. For each case, the following data were extracted: age, sex, disease diagnosis, osimertinib dose and line of therapy, characterization of dAE, intervention for dAE, and dAE outcome. Skin toxicities were graded according to NCI‐CTCAE version 4.03. All data presented are descriptive. The retrospective chart reviews were approved by the Research and Ethics Committee of NTUH (201309042RINB and 201511047RINC) and the MSKCC institutional review board (IRB #16‐458).
Patient characteristics from both centers are summarized in Table 2 (see supplemental online Tables 1 and 2 for individual patient data). The NTUH patients (n = 34) had a median age of 60.5 years, and 76% were women. Of these, 47% had one prior line of therapy, 44% had two prior lines of therapy, and 9% received osimertinib in the first line. Among the 34 patients, the following skin toxicities were noted: transient acneiform eruptions in 10 patients (29%), with 1 patient having grade ≥3 acneiform eruption; pruritus in 10 patients (29%), with 1 patient having grade ≥3 pruritus; xerosis or xerotic eczema in 13 patients (38%); and paronychia in 11 patients (32%), of whom 5 patients had grade ≥3 paronychia (Table 3). The MSKCC patients (n = 18) had a median age of 63 years, and 89% were women; all patients had received at least one prior line of therapy. Skin toxicities in this group were all grade ≤2 and included six patients (33%) with acneiform rash, pruritus in five patients (28%), xerosis in 15 patients (83%), and paronychia in two patients (11%). In summary, common skin toxicities in the 52 patients included acneiform rash (31%), pruritus (29%), xerosis (54%), and paronychia (25%). The incidence of grade ≥3 events was low.
Table 2. Patient characteristics for the retrospective cases of patients treated with osimertinib.
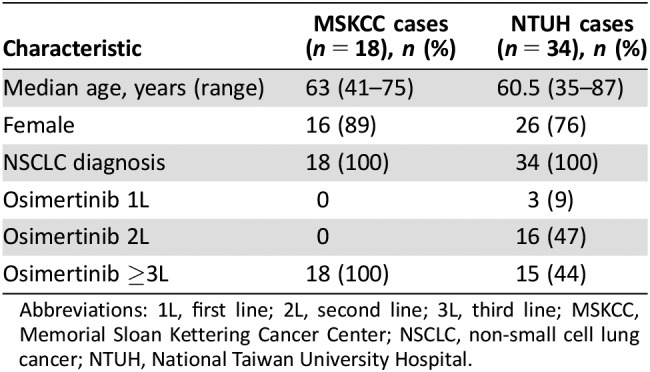
Abbreviations: 1L, first line; 2L, second line; 3L, third line; MSKCC, Memorial Sloan Kettering Cancer Center; NSCLC, non‐small cell lung cancer; NTUH, National Taiwan University Hospital.
Table 3. Osimertinib‐related dAEs in patients evaluated by a dermatologist.
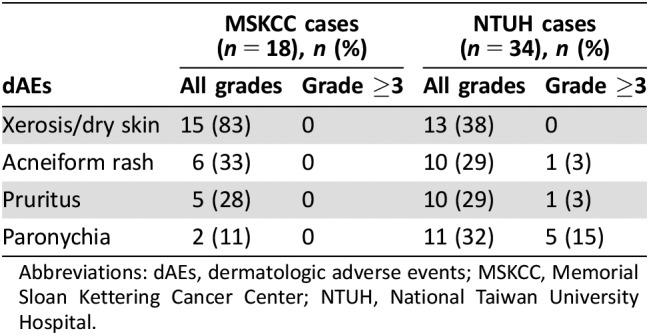
Abbreviations: dAEs, dermatologic adverse events; MSKCC, Memorial Sloan Kettering Cancer Center; NTUH, National Taiwan University Hospital.
Characterization and Management Recommendations
Management of dAEs associated with osimertinib therapy is similar to that of other generations of EGFR‐TKIs but varies with the grade of the event (Table 4). There is typically only a brief period of time between ending the initial EGFR‐TKI therapy and beginning osimertinib, so patients will often need continued management of dAEs based on the residual effects of the original first‐ and second‐generation EFGR‐TKIs. In addition, it is conceivable that the continued presence of rash during second‐line treatment with osimertinib may be falsely attributed to the second‐line agent.
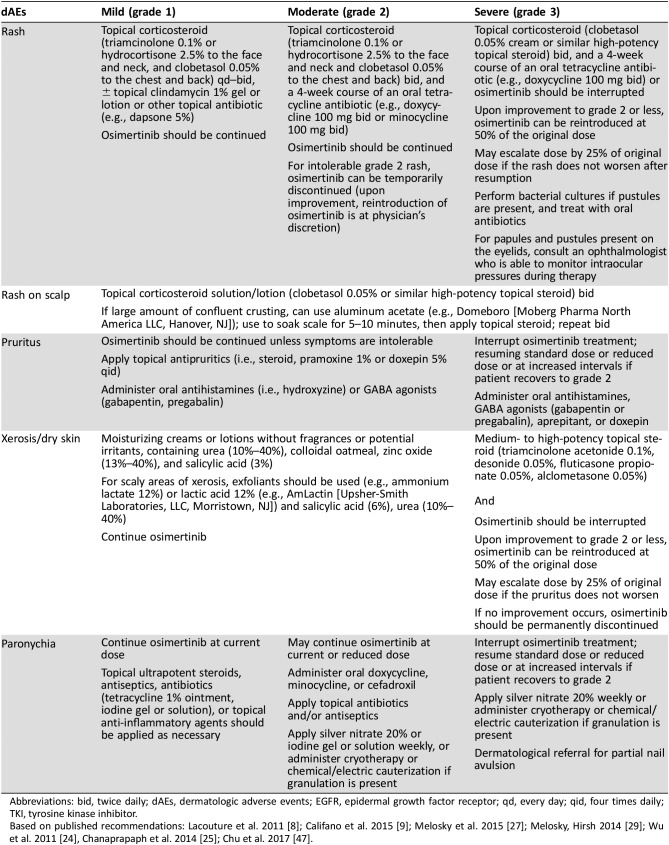
Table 4. Management‐strategy recommendations for osimertinib‐induced dAEs.
Abbreviations: bid, twice daily; dAEs, dermatologic adverse events; EGFR, epidermal growth factor receptor; qd, every day; qid, four times daily; TKI, tyrosine kinase inhibitor.
Acneiform Rash
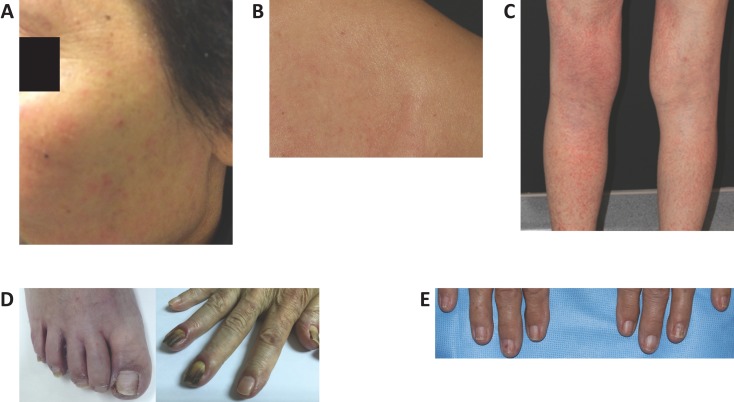
The acneiform rash associated with osimertinib differs in both frequency and intensity from that seen with first‐ and second‐generation EGFR‐TKIs (Fig. 2A). The first‐ and second‐generation EGFR‐TKIs are associated with an acneiform rash comprising inflamed papules and pustules, and often serous and/or hemorrhagic crust [6], [8], [26], [28] in 37%–89% of patients (grade ≥3 in 2%–16%) [27]. It typically occurs in areas with a high density of sebaceous glands (i.e., face, scalp, shoulders, upper back, and upper chest) [8], [27]. The rash associated with osimertinib is less severe and less commonly associated with pruritus (see Pruritus). Grade 3–4 rash has been reported in no more than 1% of osimertinib‐treated patients [42]. One study reported rates of acneiform rash in osimertinib‐treated patients of 33% (7/21), but grade ≥3 events were not observed [46].
Figure 2.
Dermatologic adverse events in patients treated with osimertinib. (A): Face acneiform rash. (B): Pruritus on back. (C): Xerotic dermatitis on legs. (D): Paronychia of the great toe and dry skin (left) and paronychia of the fingers (right). (E): Brittle nails of the fingers.
Although there are a number of prophylactic recommendations to reduce the risk of acneiform rash when using first‐ and second‐generation EGFR‐TKIs, including topical corticosteroids and topical and oral antibiotics [27], the low frequency of acneiform rash with osimertinib does not warrant the same approach; however, advising patients to contact the oncology team if toxicities appear is recommended. Management strategies for acneiform rash after onset are similar to those for rash associated with first‐ and second‐generation EGFR‐TKIs. For grade 1 acneiform rash, topical corticosteroids (e.g., hydrocortisone 2.5% to the face and neck, clobetasol 0.05% to the chest and back) and antibiotics (e.g., clindamycin 1% solution or gel, dapsone 5% gel, fusidic acid 2% cream, tetracycline 1% ointment) twice daily are recommended. For grade 2 acneiform rash, topical corticosteroids (e.g., triamcinolone 0.1% or hydrocortisone 2.5% to the face and neck, clobetasol 0.05% to the chest and back) and an oral antibiotic (i.e., doxycycline 100 mg twice daily or minocycline 100 mg twice daily) are effective. For grade 3 rash, drug interruption along with topical corticosteroids (e.g., clobetasol 0.05% twice daily or similar high‐potency topical steroid twice daily) and an oral antibiotic (i.e., doxycycline 100 mg twice daily or minocycline 100 mg twice daily) are recommended [8], [47]. Discontinuation of osimertinib related to rash was rare in clinical trials; only 1 patient of 210 (0.5%) who received osimertinib in AURA 2 discontinued (because of maculopapular rash) [44]. A dermatologist should be consulted for any grade 3 or intolerable grade 2 rash or any rash not responding to basic management strategies, including dose reduction or interruption identified above; dermatologic assessment is also warranted if a secondary infection or intolerable reaction is suspected.
Pruritus
Pruritus associated with first‐ and second‐generation EGFR‐TKIs is often reported in conjunction with papulopustular (acneiform) rash and dry skin. It may present within 2 weeks of starting EGFR‐TKI therapy or after 1–2 months of EGFR‐TKI therapy [24]. Pruritus associated with osimertinib is distinct, as it often presents in the absence of rash (Fig. 2B) and is generally diffuse and of moderate or severe intensity.
In our case summaries of 52 patients receiving osimertinib, pruritus was found in 15 (29%); among them, 8 (53%) were grade 1, 6 (40%) were grade 2, and 1 (7%) was grade 3. The case of grade 3 pruritus persisted for 9 months and was coincident with grade 1 xerosis, but the patient remained on therapy. In four patients treated at MSKCC who completed a health‐related quality‐of‐life questionnaire on the severity of pruritus (ItchyQoL) [48], the mean (± standard deviation) score was 39.6 ± 4.5; the theoretical score range of the questionnaire is 22–110, representing a range from low impact to high impact on quality of life [49]. This suggests that the pruritus associated with osimertinib therapy had a mild to moderate impact on the patients’ functioning or emotional well‐being.
Preventive measures for pruritus include the optimization of dry skin care recommendations, including frequent use of moisturizers, and fragrance‐free detergents and soaps. For grades 1 and 2 pruritus, the EGFR‐TKI can be continued without dose modification [47]. Treatments include topical antipruritics (e.g., corticosteroids, pramoxine, salicylic acid) and oral antihistamines or GABA agonists. EGFR‐TKI therapy can be interrupted for grade 3 pruritus. For patients with insomnia, sedative antihistamines may be used. A consultation with a dermatologist is recommended for grade 3 pruritus, intolerable grade 2 pruritus, or pruritus unresponsive to any of the above management strategies.
Xerosis
Xerosis associated with osimertinib is generally mild to moderate (grades 1 or 2; Fig. 2C). For first‐ and second‐generation EGFR‐TKIs, dry skin manifests after 1–2 months of therapy and often accompanies or succeeds the papulopustular rash [8]. Xerosis may manifest as pruritus, fine scaling, and fissures [24]. It may also progress into xerotic dermatitis [8] and lead to bacterial or viral superinfection with Staphylococcus aureus, herpes simplex, or other bacterial and viral infections [24].
In our case summaries of 52 patients receiving osimertinib, xerosis was the most frequent dAE, with 54% of patients reporting some grade of this condition. This dAE was also mostly mild, with 19/28 (68%) cases grade 1 in severity; the remaining cases were grade 2; there were no grade 3 cases. Xerosis was notably less common in NTUH patients, which may be due to the high humidity in the subtropical region. Our incidence of xerosis was higher than that described in AURA 3 (54% vs. 23%), which may be due to more diligent adverse event reporting; however, our cases were all low grade with no reports of grade ≥3 xerosis.
Prevention measures for xerosis are the same for all TKIs; patients should avoid extreme temperatures such as severe cold, dry weather, or significant heat, as well as direct sun exposure, which can cause sunburn [8], [47]. For mild to moderate (grades 1 or 2) xerosis, fragrance‐free moisturizing creams can be used, as well as exfoliants for scaly areas. For more severe (grade 3) xerosis, topical steroids are recommended [8]. If xerosis does not respond to these management strategies or is associated with erosions and ulcerations, consultation with a dermatologist may be warranted, but we recommend that such patients continue EGFR‐TKI therapy unless symptoms are very severe.
Paronychia and Nail Changes
There were 11 patients with paronychia within the case summaries at NTUH (Fig. 2D), and fewer (n = 2) reported at MSKCC. In many cases, paronychia persisted from the first‐ and second‐generation EGFR‐TKI therapy through the start of osimertinib treatment; however, in some cases, paronychia persisted for as long as 12 months, which would suggest that these cases could have been attributed to or exacerbated by osimertinib. This dAE also had the greatest number of grade ≥3 events, with five cases cited, whereas with each instance of pruritus and acneiform rash, only one grade ≥3 event was reported. In our case series, the majority of dAEs were low grade; however, the incidence of grade ≥3 paronychia was higher than that reported in the AURA 3 study. All cases of grade ≥3 paronychia occurred in NTUH patients; a humid subtropical climate, frequent walking or jogging outdoors, and decreased attention to nail care might have contributed to the higher severity of this dAE in NTUH patients. Paronychia that persisted over the course of treatment with osimertinib required ongoing management.
To assist in the prevention of paronychia, patients should wear comfortable shoes and avoid aggressive manicuring and pedicuring [8]. Wearing gloves while cleaning the household or dishes is recommended to minimize periungual trauma. Oral antiobiotics and topical antimicrobials (such as povidone iodine) have been shown to reduce the risk of paronychia by approximately half, as demonstrated in a study of patients with metastatic colorectal cancer being treated with panitumumab, an EGFR‐targeting monoclonal antibody associated with skin toxicity [50].
Clinicians are advised to assess patients every 2–4 weeks, starting from the second month of EGFR‐TKI treatment, and intervene when the first symptoms of paronychia appear. After the first 4 months, patients can be assessed every 4–6 weeks, and physicians should remain vigilant for signs of secondary infection. For both grade 1 and 2 paronychia, EGFR‐TKI therapy can be continued at the same dose, but it should be noted that grade 1 symptoms can escalate to grade 2 quickly [47]. Topical ultrapotent steroids and/or antiseptics, antibiotics, or anti‐inflammatory agents are recommended for grade 1 paronychia. Patients may also find warm water soaks beneficial. For grade 2 symptoms, silver nitrate may be additionally helpful. EGFR‐TKI therapy should be interrupted for grade 3 toxicity and can be resumed once symptoms return to grade ≤2. In addition to silver nitrate, cryotherapy or chemical or electric cauterization should be employed if granulation has developed. Nail avulsion should also be considered.
Other nail‐related adverse events have also been shown to occur with EGFR‐TKI therapy. Two patients in our case series had brittle nails (Fig. 2E). Biotin has been shown to be effective for this condition in the general population, and a biotin supplement can be considered [8]. Regular moisturization of the nails with a topical emollient, avoidance of water damage, and treatment with topical poly‐ureaurethane 16% nail solution (Nuvail, Innocutis, Charleston, SC) or topical hydrosoluble nail lacquer (Genadur, Medimetriks Pharmaceuticals, Fairfield, NJ) can also be helpful for brittle nails.
Other Third‐Generation EGFR‐TKIs in Development
Additional third‐generation EGFR‐TKIs are the subject of ongoing clinical trials. Although the dAE profiles of third‐generation agents are less well characterized, with reporting limited to incidence data, there are some studies that more fully describe dAEs [51], [52], [53]. Olmutinib is approved in South Korea, based on an open‐label, multicenter, phase I–II trial (NCT01588145) [54]. Preliminary data from this study showed that olmutinib‐treated patients experienced rash (41%; grade ≥3, 5%), pruritus (42%; grade ≥3, 1%), dry skin (28%; grade ≥3, 1%), and skin exfoliation (26%; grade ≥3, 1%), with one patient discontinuing therapy because of skin exfoliation [51]. However, in September 2016, Korean regulatory authorities issued a safety warning as a result of two cases of toxic epidermal necrolysis, of which one proved fatal, and a case of Stevens–Johnson syndrome. Development of olmutinib outside of Asia has been halted [55]. The pivotal global ELUXA 1 phase II trial (NCT02485652) for olmutinib for second‐line or later therapy is still ongoing, and another study is planned to evaluate the efficacy and safety of olmutinib in the first‐line setting (NCT02444819).
Interim results from a multicenter dose‐escalation study (NCT02108964) of 152 patients treated with EGF816 showed that dAEs suspected to be drug‐related included rash in 54% of patients (16% grade ≥3), pruritus in 34% (<1% grade ≥3), dry skin in 25%, and paronychia in 11%; one patient experienced grade 3–4 purpura [52]. The incidence of treatment‐related rash increased with increased dose. The rash was described as distinct from that associated with EGFR‐TKIs that target wild‐type EGFR, in that the EGF816‐associated rash was mostly found on the arms and legs and was maculopapular in nature, compared with the typical acneiform or pustular rash localized to the face, chest, and back in patients receiving first‐ and second‐generation EGFR‐TKIs [52].
An ongoing phase I trial to test PF‐06747775 in patients with T790M mutant‐positive NSCLC has completed the dose‐escalation portion of the study (NCT02349633). The analysis found no dose‐limiting toxicities; however, grade 3 skin toxicities occurred in 31% of patients (all of whom received >150 mg of the study drug) [53]. Development of two other third‐generation agents, rociletinib and ASP8273, has been terminated, and clinical trials have been closed [56], [57].
Conclusion
Patient education, early recognition, and proactive management of EGFR‐TKI‐related dAEs are critically important for improving patients’ quality of life and treatment adherence, and for avoiding inappropriate dose reductions or discontinuations [9], [27], [58]. With the exception of the prophylactic antibiotics and steroids that may be employed for first‐ and second‐generation EGFR‐TKIs, management of dAEs is similar for first‐, second‐, and third‐generation EGFR‐TKIs and is based on dAE grade. dAEs associated with osimertinib, including rash, xerosis, paronychia, and pruritus, are generally mild (grade 1), and grade ≥3 dAEs are rare. Based on our clinical experience and clinical trial data, the frequency and severity of dAEs associated with osimertinib are lower than those seen with first‐ and second‐generation EGFR‐TKIs.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The authors thank Alanna Kennedy, Ph.D., and Marissa Nolan, Ph.D., of the Lockwood Group (Stamford, CT) for providing medical writing support, which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca (Wilmington, DE). Additional editorial support was provided by Oxford PharmaGenesis (Oxford, UK) and was funded by AstraZeneca (Wilmington, DE). Support for the retrospective case reviews was provided by the National Taiwan University Hospital (grant 106‐S3535 to C.Y.C.) and AstraZeneca (to M.E.L.). M.E.L. is supported in part by the NIH/NCI Cancer Center Support Grant P30‐CA008748.
Author Contributions
Conception/design: Chia‐Yu Chu, Mario E. Lacouture
Provision of study material or patients: Chia‐Yu Chu, Mario E. Lacouture
Collection and/or assembly of data: Chia‐Yu Chu, Mario E. Lacouture
Data analysis and interpretation: Chia‐Yu Chu, Mario E. Lacouture
Manuscript writing: Chia‐Yu Chu, Jennifer Choi, Beth Eaby‐Sandy, Corey J. Langer, Mario E. Lacouture
Final approval of manuscript: Chia‐Yu Chu, Jennifer Choi, Beth Eaby‐Sandy, Corey J. Langer, Mario E. Lacouture
Disclosures
Chia‐Yu Chu: Boehringer Ingelheim International GmbH, Novartis (C/A); Boehringer Ingelheim International GmbH, Novartis, AstraZeneca, Roche (H); Beth Eaby‐Sandy: AstraZeneca, Merck, Helsinn, Takeda (H); Abbvie (C/A); Corey J. Langer: AstraZeneca, Genentech/Roche (C/A, SAB); Mario E. Lacouture: Adgero, AstraZeneca, Boehringer Ingelheim International GmbH, Debiopharm, Foamix, Genentech/Roche, Janssen, Veloce Pharmaceuticals (C/A, RF). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Cross DA, Ashton SE, Ghiorghiu S et al. AZD9291, an irreversible EGFR TKI, overcomes T790M‐mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ward RA, Anderton MJ, Ashton S et al. Structure‐ and reactivity‐based development of covalent inhibitors of the activating and gatekeeper mutant forms of the epidermal growth factor receptor (EGFR). J Med Chem 2013;56:7025–7048. [DOI] [PubMed] [Google Scholar]
- 3. Ballard P, Yates JW, Yang Z et al. Preclinical comparison of osimertinib with other EGFR‐TKIs in EGFR‐mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016;22:5130–5140. [DOI] [PubMed] [Google Scholar]
- 4. Yang JC, Ahn MJ, Kim DW et al. Osimertinib in pretreated T790M‐positive advanced non‐small‐cell lung cancer: AURA study phase II extension component. J Clin Oncol 2017;35:1288–1296. [DOI] [PubMed] [Google Scholar]
- 5. Goss G, Tsai CM, Shepherd FA et al. MA16.11 CNS response to osimertinib in patients with T790M‐positive advanced NSCLC: Pooled data from two phase II trials. J Thorac Oncol 2017;12(suppl 1):S440–S441. [DOI] [PubMed] [Google Scholar]
- 6. Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer 2006;6:803–812. [DOI] [PubMed] [Google Scholar]
- 7. Joshi SS, Ortiz S, Witherspoon JN et al. Effects of epidermal growth factor receptor inhibitor‐induced dermatologic toxicities on quality of life. Cancer 2010;116:3916–3923. [DOI] [PubMed] [Google Scholar]
- 8. Lacouture ME, Anadkat MJ, Bensadoun RJ et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor‐associated dermatologic toxicities. Support Care Cancer 2011;19:1079–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Califano R, Tariq N, Compton S et al. Expert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UK. Drugs 2015;75:1335–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AstraZeneca. IRESSA (gefitinib). Highlights of prescribing information. July 2015. Available at www.iressa-usa.com. Accessed September 25, 2017.
- 11.Boehringer Ingelheim Pharmaceuticals. GILOTRIF (afatinib). Highlights of prescribing information. October 2016. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/201292s010lbl.pdf. Accessed September 25, 2017.
- 12.Genentech. TARCEVA (erlotinib). Highlights of prescribing information. October 2016. Available at https://www.gene.com/download/pdf/tarceva_prescribing.pdf. Accessed September 25, 2017.
- 13. Mok TS, Wu YL, Thongprasert S et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–957. [DOI] [PubMed] [Google Scholar]
- 14. Mitsudomi T, Morita S, Yatabe Y et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–128. [DOI] [PubMed] [Google Scholar]
- 15. Maemondo M, Inoue A, Kobayashi K et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–2388. [DOI] [PubMed] [Google Scholar]
- 16. Zhou C, Wu YL, Chen G et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011;12:735–742. [DOI] [PubMed] [Google Scholar]
- 17. Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012;13:239–246. [DOI] [PubMed] [Google Scholar]
- 18. Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–3334. [DOI] [PubMed] [Google Scholar]
- 19. Wu YL, Zhou C, Hu CP et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014;15:213–222. [DOI] [PubMed] [Google Scholar]
- 20. Bronte G, Rolfo C, Giovannetti E et al. Are erlotinib and gefitinib interchangeable, opposite or complementary for non‐small cell lung cancer treatment? Biological, pharmacological and clinical aspects. Crit Rev Oncol Hematol 2014;89:300–313. [DOI] [PubMed] [Google Scholar]
- 21. Modjtahedi H, Cho BC, Michel MC et al. A comprehensive review of the preclinical efficacy profile of the ErbB family blocker afatinib in cancer. Naunyn Schmiedebergs Arch Pharmacol 2014;387:505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burotto M, Manasanch EE, Wilkerson J et al. Gefitinib and erlotinib in metastatic non‐small cell lung cancer: A meta‐analysis of toxicity and efficacy of randomized clinical trials. The Oncologist 2015;20:400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haspinger ER, Agustoni F, Torri V et al. Is there evidence for different effects among EGFR‐TKIs? Systematic review and meta‐analysis of EGFR tyrosine kinase inhibitors (TKIs) versus chemotherapy as first‐line treatment for patients harboring EGFR mutations. Crit Rev Oncol Hematol 2015;94:213–227. [DOI] [PubMed] [Google Scholar]
- 24. Wu PA, Balagula Y, Lacouture ME et al. Prophylaxis and treatment of dermatologic adverse events from epidermal growth factor receptor inhibitors. Curr Opin Oncol 2011;23:343–351. [DOI] [PubMed] [Google Scholar]
- 25. Chanprapaph K, Vachiramon V, Rattanakaemakorn P. Epidermal growth factor receptor inhibitors: A review of cutaneous adverse events and management. Dermatol Res Pract 2014;2014:734249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lynch TJ Jr, Kim ES, Eaby B et al. Epidermal growth factor receptor inhibitor‐associated cutaneous toxicities: An evolving paradigm in clinical management. The Oncologist 2007;12:610–621. [DOI] [PubMed] [Google Scholar]
- 27. Melosky B, Leighl NB, Rothenstein J et al. Management of EGFR TKI‐induced dermatologic adverse events. Curr Oncol 2015;22:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen KL, Lin CC, Cho YT et al. Comparison of skin toxic effects associated with gefitinib, erlotinib, or afatinib treatment for non‐small cell lung cancer. JAMA Dermatol 2016;152:340–342. [DOI] [PubMed] [Google Scholar]
- 29. Melosky B, Hirsh V. Management of common toxicities in metastatic NSCLC related to anti‐lung cancer therapies with EGFR‐TKIs. Front Oncol 2014;4:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Douillard JY, Ostoros G, Cobo M et al. First‐line gefitinib in Caucasian EGFR mutation‐positive NSCLC patients: A phase‐IV, open‐label, single‐arm study. Br J Cancer 2014;110:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thatcher N, Chang A, Parikh P et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non‐small‐cell lung cancer: Results from a randomised, placebo‐controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527–1537. [DOI] [PubMed] [Google Scholar]
- 32. Jia Y, Lacouture ME, Su X et al. Risk of skin rash associated with erlotinib in cancer patients: A meta‐analysis. J Support Oncol 2009;7:211–217. [PubMed] [Google Scholar]
- 33. Ensslin CJ, Rosen AC, Wu S et al. Pruritus in patients treated with targeted cancer therapies: Systematic review and meta‐analysis. J Am Acad Dermatol 2013;69:708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: A systematic review of the literature and meta‐analysis. J Am Acad Dermatol 2012;67:400–408. [DOI] [PubMed] [Google Scholar]
- 35. Mok T, Cheng Y, Zhou X et al. Dacomitinib versus gefitinib for the first‐line treatment of advanced EGFR mutation positive non‐small cell lung cancer (ARCHER 1050): A randomized, open‐label phase III trial. J Clin Oncol 2017;35(suppl 18):LBA9007. [DOI] [PubMed] [Google Scholar]
- 36. Lacouture ME, Keefe DM, Sonis S et al. A phase II study (ARCHER 1042) to evaluate prophylactic treatment of dacomitinib‐induced dermatologic and gastrointestinal adverse events in advanced non‐small‐cell lung cancer. Ann Oncol 2016;27:1712–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Non‐small cell lung cancer. Version 8.2017. July 14, 2017. Available at www.nccn.org. Accessed September 25, 2017.
- 38. Yu HA, Arcila ME, Rekhtman N et al. Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res 2013;19:2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oxnard GR, Arcila ME, Sima CS et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR‐mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sequist LV, Waltman BA, Dias‐Santagata D et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kobayashi S, Boggon TJ, Dayaram T et al. EGFR mutation and resistance of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2005;352:786–792. [DOI] [PubMed] [Google Scholar]
- 42.AstraZeneca. TAGRISSO (osimertinib). Highlights of prescribing information. November 2015. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208065s000lbl.pdf. Accessed September 25, 2017.
- 43. Mok TS, Wu YL, Ahn MJ et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med 2017;376:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goss G, Tsai CM, Shepherd FA et al. Osimertinib for pretreated EGFR Thr790Met‐positive advanced non‐small‐cell lung cancer (AURA2): A multicentre, open‐label, single‐arm, phase 2 study. Lancet Oncol 2016;17:1643–1652. [DOI] [PubMed] [Google Scholar]
- 45. Soria JC, Ohe Y, Vansteenkiste J et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2017;378:113–125. [DOI] [PubMed] [Google Scholar]
- 46. Yang JCH, Cho BC, Kim DW et al. Osimertinib for patients with leptomeningeal metastases (LM) from EGFR‐mutant non‐small cell lung cancer (NSCLC): Updated results from the BLOOM study. J Clin Oncol 2017;35(suppl 15):2020A. [Google Scholar]
- 47. Chu CY, Chen KY, Wen‐Cheng Chang J et al. Taiwanese Dermatological Association consensus for the prevention and management of epidermal growth factor receptor tyrosine kinase inhibitor‐related skin toxicities. J Formos Med Assoc 2017;116:413–423. [DOI] [PubMed] [Google Scholar]
- 48. Desai NS, Poindexter GB, Monthrope YM et al. A pilot quality‐of‐life instrument for pruritus. J Am Acad Dermatol 2008;59:234–244. [DOI] [PubMed] [Google Scholar]
- 49. Love EM, Marrazzo GA, Kini S et al. ItchyQoL bands: Pilot clinical interpretation of scores. Acta Derm Venereol 2015;95:114–115. [DOI] [PubMed] [Google Scholar]
- 50. Lacouture ME, Mitchell EP, Piperdi B et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open‐label, randomized trial evaluating the impact of a pre‐emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:1351–1357. [DOI] [PubMed] [Google Scholar]
- 51. Park K, Lee JS, Lee KH et al. BI 1482694 (HM61713), an EGFR mutant‐specific inhibitor, in T790M+ NSCLC: Efficacy and safety at the RP2D. J Clin Oncol 2016;34(suppl 15):9055A. [Google Scholar]
- 52. Tan DSW, Yang JCH, Leighl NB et al. Updated results of a phase 1 study of EGF816, a third‐generation, mutant‐selective EGFR tyrosine kinase inhibitor (TKI), in advanced non‐small cell lung cancer (NSCLC) harboring T790M. J Clin Oncol 2016;34(suppl 15):9044A. [Google Scholar]
- 53. Husain H, Martins R, Goldberg S et al. P3.02b‐001 phase 1 dose escalation of PF‐06747775 (EGFR‐T790M inhibitor) in patients with advanced EGFRm (del 19 or L858R+/‐T790M) NSCLC. J Thorac Oncol 2017;12(suppl 1):S1185. [Google Scholar]
- 54. Kim ES. Olmutinib: First global approval. Drugs 2016;76:1153–1157. [DOI] [PubMed] [Google Scholar]
- 55. Carroll J. Following lethal tox report, Boehringer scraps plans for high‐speed development, kills $730M Hanmi deal. Endpoints News; October 1, 2016. Available at https://endpts.com/following-lethal-tox-report-boehringer-scraps-plans-for-high-speed-development-kills-730m-hanmi-deal/. Accessed August 3, 2017.
- 56.Astellas announces decision to discontinue ASP8273 treatment arm and close randomization for clinical study protocol 8273‐CL‐0302. Astellas News Releases; May 10, 2017. Available at http://newsroom.astellas.us/2017-05-10-Astellas-Announces-Decision-to-Discontinue-ASP8273-Treatment-and-Close-Randomization-for-Clinical-Study-Protocol-8273-CL-0302. Accessed September 25, 2017.
- 57. Broderick JM. Clovis ends development of rociletinib in lung cancer. OncLive; May 6, 2016. Available at http://www.onclive.com/web-exclusives/clovis-ends-development-of-rociletinib-in-lung-cancer. Accessed September 25, 2017.
- 58. Cho YT, Chen KL, Chu CY. Treatment strategies of epidermal growth factor receptor inhibitor‐induced skin toxicities: Pre‐emptive or reactive? Ann Transl Med 2016;4:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.