Abstract
Objective:
Measurement of salivary biomarkers can provide important information regarding hypothalamic–pituitary–adrenal axis activity both under normal conditions as well as in response to psychological or physical stress. Our aim was to correlate salivary stress markers, such as cortisol, α-amylase and immunoglobulin A, with the Pediatric Risk Index Score of Mortality, underlying disease (pathologic, trauma and postoperative), need for mechanical ventilation/sedation and time lag between onset of illness and admission in children admitted in the pediatric intensive care unit.
Methods:
We enrolled 79 pediatric intensive care unit patients (2–14 years) over a 2-year period, which satisfy the including criteria, but finally salivary biomarkers were evaluated in 65 patients. Saliva samples were collected within 24 h of admission at 8 a.m., 2 p.m. and 8 p.m. to examine potential disruption of circadian rhythm.
Results:
Overall, the salivary biomarkers were increased; specifically, median values were (a) cortisol at 8 a.m.: 50.04 nmol/L, 2 p.m.: 30.69 nmol/L and 8 p.m.: 247.12 nmol/L; (b) α-amylase: at 8 a.m.: 22.567 U/L; 2 p.m.: 22.702 U/L and 8 p.m.: 21.484 U/L and (c) IgA at 8 a.m.: 95.10 mg/dL, 2 p.m.: 88.55 mg/dL and 8 p.m.: 80.80 mg/dL. Significantly higher levels were demonstrated in children younger than 6 years and those with Pediatric Risk Index Score of Mortality ⩾8 upon admission. Disturbances in circadian rhythm were observed. Cortisol circadian rhythm disturbance was observed only in children with Pediatric Risk Index Score of Mortality score ⩾8 upon admission while maintaining normal α-amylase circadian rhythm, which was associated with less than 3 days hospitalization in pediatric intensive care unit. No daily variance in IgA was observed.
Conclusion:
Salivary biomarkers may serve, in critically ill children, as a sensitive, non-invasive method, important for the early recognition of those at high risk and guiding intervention, before clinical deterioration, promoting the quality of health care in pediatric population.
Keywords: Salivary cortisol, amylase, IgA, stress, pediatric critical illness
Introduction
Critical illness may be induced by different underlying life-threatening diseases, such as infection, sepsis, trauma, respiratory insufficiency or hypoxia and severe neurological status. The associated endocrine, nervous, metabolic and immunological changes are defined as acute stress syndrome.1 These changes are normally adaptive and time limited and aim to improve survival.2 Unrecognized or untreated clinical deterioration can lead to serious adverse events, prolonged stay in the intensive care unit, poor outcome and even unexpected death.3,4
The neuroendocrine dysfunction (NED) induced by stress significantly differs between healthy5 and critically ill children6,7 and likely varies from that in adults. The extent of NED has not been well defined in pediatric critical illness.8,9 Relative or absolute adrenal insufficiency is a clinical condition, frequently met in intensive care unit patients, associated with fluid- and catecholamine-resistant hypotension, that leads to vasopressor-resistant shock and increases need of vasoactive support.10,11 Many studies have focused on adrenal insufficiency in pediatric shock and sepsis.12–14 Moreover, several studies have focused on stress induced by acute respiratory distress syndrome15 and more recently burns.16
Reported incidence rates of adrenal insufficiency widely range from 30% to 88%. This disparity is likely due to the variety of diagnostic strategies. Researchers have attempted to provide the diagnosis of acute stress syndrome based on the observation of high baseline cortisol levels in pediatric patients with serious illness, using adrenocorticotropic hormone stimulation tests and measurement of the adrenocorticotropic hormone. The results indicate variability in the use of low- or high-dose corticotropin, in measurement of baseline cortisol level as well as in the use of total or free cortisol levels.17,18
Initially, salivary cortisol and α-amylase measurement has been used to estimate perceived stress in healthy children and were measured in different age groups, gender, body mass index (ΒΜΙ) and pubertal development.19,20 Few studies have aimed to compare normal values in healthy children and those with critical illness (Table 1). Variations in cortisol and α-amylase reactivity in saliva have been observed with age, but not with BMI, sex and even menstruation in girls.27
Table 1.
Salivary biomarkers in healthy and critically ill children.
| Study | Population | Cortisol (nmol/L) | SAA (U/L) | SIgA (mg/dL) |
|---|---|---|---|---|
| Maguire et al.21 | N = 22 (5.1–18.5 years) Healthy children |
8 a.m: 0–25 (range) 12 mid-day: 0–10 8 p.m.: 0–4 |
||
| Gröschl et al.22 | N = 212 (2–15 years) Healthy children |
8 a.m.: 3–54.9 (range) 2 p.m.: 1.1–2.7 8 p.m.: 0.2–8.7 |
||
| Balbao et al.23a | N = 15 (49–187 months) Healthy children N = 32 (0.7–201 months) Critically ill children |
18.8 median (8–53.5) range 94.1 median (7.7–448.3) range |
||
| Granger et al.24
Review |
Healthy children | 400–900 (range) | ||
| Starzak et al.25 | N = 74 (10.05 ± 1.68 years) Healthy children |
8 a.m.: 79.83 (mean) ± 43.12 (SD) | 2.439 (mean) ± 1.192 (SD) | |
| Jafarzadeh et al.26 | N = 28 (1–10 years) Healthy children |
8 a.m.: 4.2 (mean) ± 3.85 (SD) | ||
| Our study | N = 65 (2–14 years) Critically ill children |
8 a.m.: 50.04 median (range 4.24–1.563) 2 p.m.: 30.69 median (range 4.76–854) 8 p.m.: 247.12 median (range 4.0–370) |
8 a.m.: 22.567 (range 4.46–261.25) 2 p.m.: 22.702 (range 4.32–380.79) 8 p.m.: 21.484 (range: 19.0–145.68) |
8 a.m.: 95.10 (range 1–4.84) 2 p.m.: 88.55 (range 3–1.99) 8 p.m.: 80.80 (range 3–2.200) |
No specific time for sample collection was mentioned in the daily pattern of salivary α-amylase and cortisol activity in children, which parallels with the previous findings in adults, but salivary α-amylase activity over the course of a day is opposite to that observed for cortisol, with lowest levels 1 h after awakening and increasing levels over the day.27–29
Salivary cortisol has been used as an alternative tool for hypothalamic–pituitary–adrenal (HPA) axis function and deregulation.30 Salivary α-amylase (sAA) is suggested to reflect catecholaminergic changes due to increased activation of the sympathetic–adrenal–medullary (SAM) system during physical and psychological stress.31 High levels of salivary cortisol and sAA have been related with psychological and physical stress.32,33 Importantly, sAA seems to be more sensitive in conditions of acute stress compared with salivary cortisol.34,35
Little is known about salivary innate and adaptive immune responses to stress especially in children.36 Neuroendocrine regulation of secretory IgA (SIgA) synthesis and secretion and potential implications in oral health have been studied.37 Moreover, it has been demonstrated that psychological and physical stress may alter SIgA concentrations.38,39 However, no published studies have been reported on the potential role of SIgA in pediatric patients with critical illness.
Over recent years, studies have shown the inability of early recognition of illness severity in acutely ill pediatric patients and recognition of those at risk for physiological deterioration.40–42 In order to improve outcome, studies have evaluated warning signs and clinical alert criteria.43–45
In the context of pediatric intensive care management, risk factors have been established for predicting mortality, prognosis and outcome by the application of different scoring systems (Pediatric Index of Mortality (PIM), PIM2, PIM3, PRISM and PRISM III).46–48 Through the years, the quality of patient care for children admitted to intensive care units (ICUs) has significantly improved, achieving high survival ratios and documented decline in pediatric intensive care unit (PICU) mortality.49,50 Moreover, the main goal should be long-term (health-related) quality of life.51
Over the past decade, research has focused on the identification of new biomarkers that might support diagnosis and prognosis of disease. More specifically, the need for the development of a rapid, validated, non-invasive and low cost method has led to extensive research on saliva as an ideal biological fluid.52–54 Indeed, if further research indicates that salivary biomarkers are able to early recognize children in severe stress, their measurement may be a useful tool in ICU patients but also in the emergency room.
In this study, we aimed to correlate salivary biomarkers, such as cortisol, α-amylase and SIgA, with disease severity in children admitted in the PICU. It was hypothesized that in case such a correlation was observed, these salivary biomarkers could be added to the existing scoring systems used for the early detection for critical illness.
Material and methods
Study population
A prospective study was conducted in the PICU of “P. & A. Kyriakou” Athens Children’s Hospital over a time period of 3 years (January 2011 to January 2014). This is an 8-bed tertiary PICU, in which critically ill children aged 1 month to 18 years with pediatric, oncologic, surgical disease as well as trauma are admitted. Since previous studies including stress biomarkers in PICU patients are lacking, no calculation of sample size was performed for this study. The protocol was approved by the hospitals Ethics committee.
All children admitted to the PICU during the study period were offered enrollment. Parents were informed about this observational study and asked to provide written informed consent. Exclusion criteria included age < 2 years, immunodeficiency, use of steroids, underlying malignancy, expected discharge within 24 h (usually children admitted for post-surgical observation) or imminent death. In addition, enrolled children in which we failed to collect all three samples at the pre-determined daily assessment were excluded from analysis. Assessment of patients enrolled included demographics (age at admission, gender and underlying disease) and clinical data (past medical history, underlying disease and symptoms and signs), while illness severity was assessed upon admission to PICU by calculating the Pediatric Risk Index Score of Mortality (PRISM III), according to the equation described by Pollack et al.55 The Pediatric Risk of Mortality (PRISM) score is one of the main predictors of outcome used in the PICUs. It uses clinical and laboratory parameters and an increased score is associated with higher mortality. Additional clinical data included the following: type of admission (medical, surgical or trauma), mechanical ventilation/sedation, treatment with vasoactive drugs, length of stay in the PICU and mortality. Salivary biomarkers were measured and compared in the groups of patients by sex and age (2–5 and 6–14 years). Also, in order to demonstrate the severity of illness, biomarkers were compared in patients with different types of admission, with PRISM score lower and equal/higher than 8, in those who are supported with mechanical ventilation and without mechanical ventilation, in those in need of vasoactive support and those who remains hospitalized in PICU above 3 days. To better describe how acutely ill our patients were, we arbitrarily selected to categorize our subjects in those admitted within less or more than 6 h from disease onset. By taking into account whether the child had a PRISM score equal/higher or lower than 8, whether he or she was supported with mechanical ventilation, whether he or she need vasoactive support at the first 24 h in PICU and whether he or she stayed for more or less than 3 days in the PICU, two severity groups were created, in order to compare salivary biomarkers in gravitated conditions. The results of saliva assessment were compared with those from reported studies in healthy and critical ill children, which provide values overall, without taking into account puberty, BMI, gender and other possible influencing factors like pain and sleep/sedation.
Sample collection/analysis
Saliva samples were obtained, without any pre-treatment, only during the first 24 h of hospitalization in the PICU using the Salivette saliva collection system. For sampling, the Salivette swab (Sarstedt Ag & Co., Nümbrecht, Germany) was placed into the patient’s mouth for 2–5 min by a specially trained nurse. The technique did not differ in intubated patients. A minimum of 0.2 mL of saliva was needed as per the manufacturer’s recommendations to saturate the swab. The samples were then stored at −70°C until analysis. To determine whether the circadian pattern of cortisol and α-amylase secretion was retained, three samples were collected (8.00 a.m., 2.00 p.m. and 8.00 p.m.).
Salivary cortisol concentrations were measured using an electrochemiluminescence immunoassay using the Elecsys/Cobas e411 immunochemistry analyzer (Roche Co., Basel, Switzerland). The intra- and inter-assay precision coefficients of variation for salivary cortisol ranged from 1.5% to 6.1% and 4.1% to 8%, respectively. The analytical sensitivity was between 1.00 and 1750 nmol/L or 0.036 and 63.4 μg/dL (defined by the Limit of Detection and the maximum of the master curve). Salivary α-amylase concentrations were determined with Siemens Advia 1800 Clinical Chemistry System (Siemens Healthcare Diagnostics, Tarrytown, NY, USA) using the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) method.
Salivary secretory IgA (SIgA) concentrations were determined by means of an immune-nephelometric technique using the BN ProSpec nephelometer (Siemens Healthineers, Erlangen, Germany). According to the manufacturer, the inter- and intr α-assay coefficients of variation ranged 3.9%–6.8% and 1.8%–5.9%, respectively.
Statistical analysis
The analysis was conducted with the software SPSS v.22. Descriptive statistics were calculated for all examined variables. Normality was evaluated using the Kolmogorov–Smirnov test. Since values had a non-normal distribution, non-parametric tests were used for the analysis. Excessive values were excluded. Chi-square analysis was used for the examination of categorical variables and Mann–Whitney’s, Kruskal–Wallis and Spearman’s Rho analyses for differences in the levels of categorical variables in relation to the continuous. Salivary cortisol values were log-transformed. Significance was set at p ⩽ 0.05.
Results
The study population consisted of 65 children, among 796 PICU admissions during the study period. Most children were excluded either because of age less than 2 years (n = 357), parental refusal to participate in the study (n = 56), admission for post-surgical observation (n = 112), malignancy (n = 136) or PICU hospitalization duration for less than 48 h (n = 56). Finally, 14 additional patients were enrolled, but later excluded because of no satisfactory saliva samples obtained, rendering salivary biomarkers immeasurable. Of 65 children for whom complete data were collected, 41 (64.1%) were males. Average age was 7.52 ± 3.91 years. The majority of patients were admitted due to medical illness (52.3%), required mechanical ventilation (70.8%) and stayed at PICU for more than 3 days (66.1%). Table 2 depicts demographic and clinical data of our cohort.
Table 2.
Characteristics of the cohort.
| Characteristic | N (%) |
|---|---|
| Total patients | 65 |
| Age (years) | |
| 2–5 | 23 (35.4) |
| 6–14 | 42 (64.6) |
| Gender | |
| Male | 41 (64.1) |
| Female | 24 (36.9) |
| Type of admission | |
| Medical | 34 (52.3) |
| Surgical | 12 (18.5) |
| Trauma | 19 (29.2) |
| Underlying disease | 21 (32.3) |
| PRISM | |
| Less than 8 | 36 (55.4) |
| More than 8 | 29 (44.6) |
| Mechanical ventilation (MV) | 46 (70.8) |
| Sedation | 38 (58.5) |
| Use of vasoactive drugs | 9 (14.1) |
| Time of admission | |
| Early (<6 h) | 40 (61.5) |
| Delayed | 25 (38.4) |
| Length of stay in ICU (days) | 4.9 (median) |
| Less than 3 days | 22 (33.8) |
| More than 3 days | 43 (66.1) |
| Deaths | 3 (4.6) |
ICU: intensive care unit; PRISM: Pediatric Risk Index Score of Mortality.
Salivary biomarkers in study population
Overall, salivary biomarkers (cortisol, amylase and SIgA) were elevated when compared to the reported normal values in healthy and critical ill children21–26 (Table 1) without variances concerning age, gender and BMI. Levels of the salivary biomarkers measured in our cohort are shown in Table 3.
Table 3.
Salivary biomarkers (cortisol (nmol/L), α-amylase (U/L) and IgA (mg/dL)).
| Cortisol 8 a.m. |
Cortisol 2 p.m. |
Cortisol 8 p.m. |
α-Amylase 8 a.m. |
α-Amylase 2 p.m. |
α-Amylase 8 p.m. |
IgA 8 a.m. |
IgA 2 p.m. |
IgA 8 p.m. |
|
|---|---|---|---|---|---|---|---|---|---|
| Mean | 133.72 | 102.76 | 130.38 | 45,412.98 | 52,117.11 | 68,765.15 | 396.82 | 342.07 | 274.49 |
| Median | 54.64 | 32.77 | 27.37 | 262.22 | 260.29 | 245.50 | 121.50 | 102 | 80.80 |
| SD | 272.13 | 182.15 | 344.75 | 158,682.4 | 194,724.5 | 254,564.0 | 767.41 | 562.35 | 538.25 |
| SE | 32.52 | 22.25 | 41.50 | 18,446.46 | 23,109.56 | 30,211.19 | 102.54 | 77.24 | 71.29 |
| 95% CI | 64.89 | 44.43 | 82.81 | 36,764.43 | 46,091.44 | 60,255.47 | 205.51 | 155 | 142.82 |
| 99% CI | 86.16 | 59.03 | 109.99 | 48,791.85 | 61,196.18 | 80,001.93 | 273.64 | 206.54 | 190.12 |
| Min | 4.24 | 4.76 | 5.21 | 5.21 | 23.45 | 36.56 | 1.26 | 1.26 | 1.26 |
| Max | 1563 | 1163.6 | 1750 | 1750.00 | 11,4591.0 | 1,456,810 | 4110 | 3120 | 2900 |
CI: confidence interval; SE: standard error; SD: standard deviation; IgA: immunoglobulin A.
Salivary biomarkers in different subgroups of patients
Children aged less than 2–5 years had significantly higher cortisol at 8 p.m. (73 ± 93.5) and higher SIgA at 8 a.m. (532.18 ± 1101.5) in comparison to children aged 6–14 years (47.17 ± 79.16, U = 197, p = 0.015 and 292.86 ± 790.25, U = 139, p = 0.008), respectively (Figure 1). When patients were compared according to PRISM score upon admission, those with equal or higher PRISM score (>8) were found to have significantly higher levels of saliva α-amylase at 8 a.m. (U = 328.5, p = 0.042). Also, a trend for higher levels of salivary cortisol at 8 p.m. (U = 228, p = 0.058) was noted although this difference was not statistically significant (Figure 2). Despite the high levels overall, no significant difference was found between cortisol, α-amylase and IgA levels when patients were categorized according to type for admission, PRISM score admission (lower/equal/higher than 8), immediate or delayed PICU hospitalization, length of PICU stay, mechanical ventilation and outcome.
Figure 1.
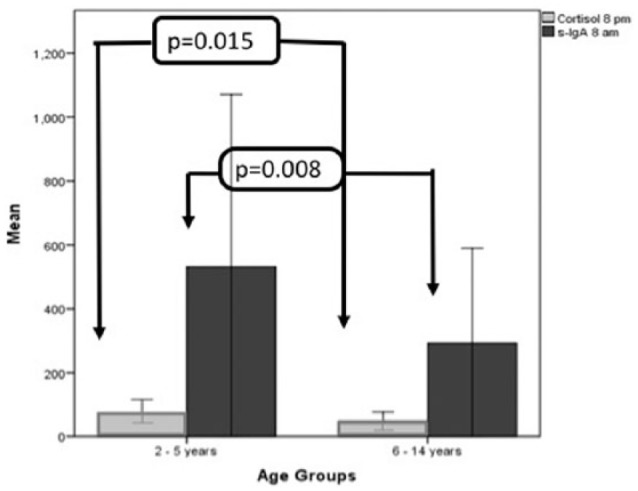
Salivary cortisol and SIgA between age groups. Evening salivary cortisol (8 p.m.) and morning SIgA (8 a.m.) were higher in younger children (2–5 years) when compared to the group of older children. Salivary cortisol levels were measured in nmol/L, while salivary IgA in mg/dL. Error bars: 95% CI.
Figure 2.
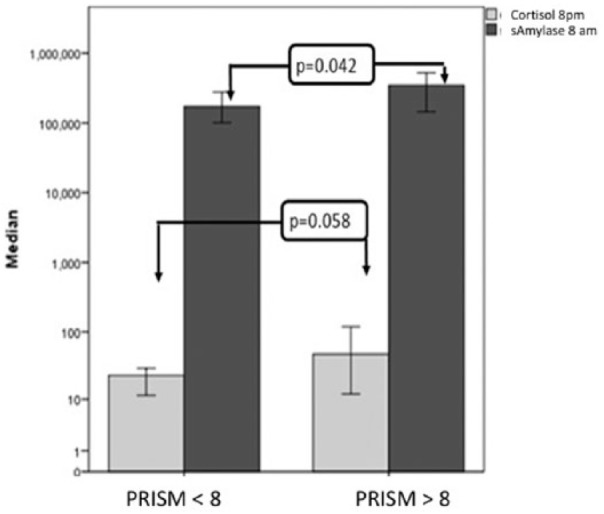
Salivary cortisol and α-amylase according to PRISM score upon admission. Evening salivary cortisol (8 p.m.) and morning amylase levels (8 a.m.) were higher in children with PRISM score greater than 8 upon PICU admission. Salivary cortisol levels were measured in nmol/L, while salivary amylase in mg/dL. Error bars: 95% CI.
Concerning the two severity groups, the results are shown in Table 4. The children in the high-gravity group had significantly higher cortisol at 2 p.m. (79.80 ± 88.21 vs 25.80 ± 24.71, U = 25, p = 0.036; Figure 3). Although older children (6–14 years) were more likely to belong to the high-severity group when compared to children 2–5 years old (69.2% vs 30.8%), this difference was not statistically significant.
Table 4.
High- and low-gravity group definition.
| Variable | High gravity | Low gravity |
|---|---|---|
| PRISM | More than 8(29) | Up to 8(36) |
| Mechanical ventilation | Yes (46) | No (19) |
| Total days in PICU | More than 3(43) | Up to 3(22) |
| Vasoactive drugs | Yes (9) | No (56) |
PICU: pediatric intensive care unit; PRISM: Pediatric Risk Index Score of Mortality.
Figure 3.
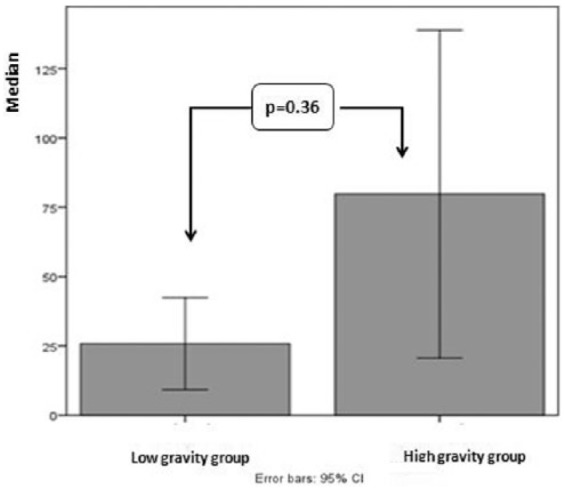
Difference in salivary cortisol at 2 p.m. in the gravity groups.
Circadian rhythm of salivary biomarkers
Cortisol and α-amylase normality was maintained by 49.2% and 35.4% of children, respectively. Salivary cortisol levels significantly changed during the first hospitalization day (Figure 4). Children maintaining normal cortisol circadian rhythm were more likely to have admission PRISM score <8 (p = 0.049) and significantly lower salivary cortisol at 8 p.m. (U = 217, p = 0.011; Figure 5). Children not maintaining normal cortisol circadian rhythm had significantly higher cortisol at 8 p.m. (U = 217, p = 0.011). Moreover, children who did not maintain normal α-amylase circadian rhythm had significantly lower α-amylase at 2 p.m. (U = 173.5, p = 0.000).
Figure 4.
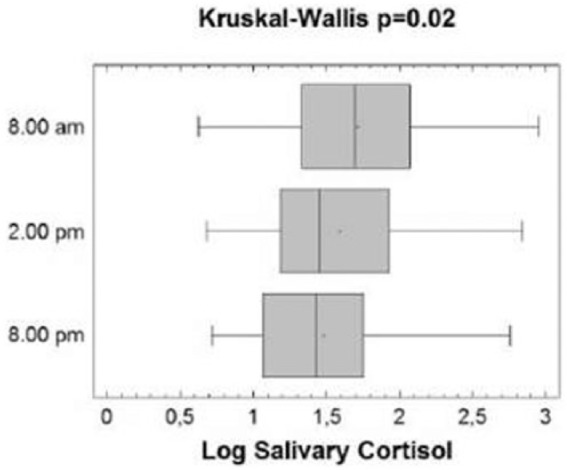
Salivary cortisol level changes during the first hospitalization day. Boxes represent the interquartile range, lines inside boxes represent the median value, cross represents mean marker and whiskers represent the lowest and highest observations. ANOVA repeat measures p = 0.035 (means) and Kruskal–Wallis p = 0.02 (medians).
Figure 5.
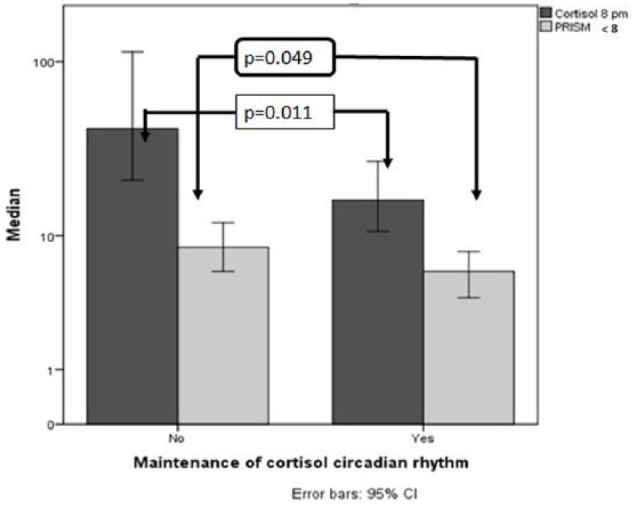
Characteristics of children maintaining normal cortisol circadian rhythm. Children maintaining normal cortisol circadian rhythm had lower levels of cortisol at 8 p.m. and had PRISM < 8 upon PICU admission. Error bars: 95% CI.
In addition, children who stayed in the PICU less than 3 days were more likely to maintain normal α-amylase circadian rhythm (p = 0.043). Finally, 71.4% of children aged 6–14 years were unable to maintain a normal α-amylase circadian rhythm as opposed to 28.6% of children aged 2–5 years (p = 0.068). Overall distribution of salivary biomarkers during the three different time points is shown in Figure 6.
Figure 6.
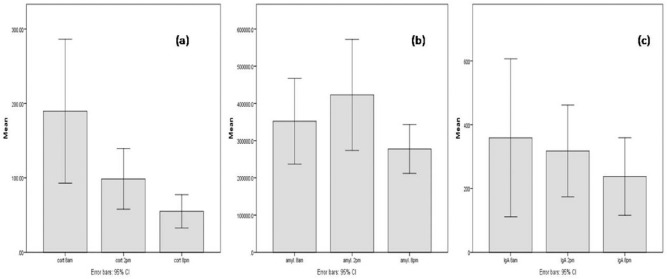
Daily distribution of salivary biomarkers: (a) cortisol (nmol/L), (b) α-amylase (U/L) and (c) IgA (mg/dL).
Discussion
NED in children with life-threatening conditions has been extensively studied over the past 10 years. Although there has been significant research, risk factors for the development of adrenal insufficiency, which aggravates critical illness, remain unclear. Moreover, at present, there are no available biomarkers which may assist clinicians in the early detection of impeding adrenal insufficiency.56,57 This is important since these data may increase our understanding of pathogenesis and guide corticosteroid replacement therapy.58–60
Recent studies have demonstrated a strong relationship between childhood trauma,61 stress, maltreatment, post-traumatic stress disorder (PTSD)62 and deregulation of the hypothalamic–pituitary axis. More recently, salivary biomarker research has focused on physical exercise, obesity,25 dentistry,20,54 psychiatric diseases,63 burns16 and critical illness.64
The majority of studies from sports medicine have shown that SIgA has been implicated in stress and antimicrobial defense.65,66 Moreover, recent studies have shown that prolonged exercise and intensified training can evoke decreases in saliva secretion of SIgA resulting to an increased risk of upper respiratory tract infections (URTI) in adolescents and young adults.39,67 Specifically, high levels of saliva SIgA are associated with low incidence of URTIs.
SIgA plays a significant role in the protection of URTI. SIgA exerts efficient microbial agglutination and virus neutralization and performs non-inflammatory extracellular and intracellular exclusion by inhibiting epithelial adherence and invasion.68 Numerous studies have attempted to correlate salivary IgA levels to a variety of oral systemic diseases such as carries, periodontal diseases, tonsillitis, adenoid hyperplasia, secretory otitis media and upper respiratory infections.69,70 Furthermore, it has been suggested that repeated antibiotic treatment may lead to persistently low salivary IgA levels. Sonesson71 reported lower concentration of salivary IgA in the saliva of children in comparison to adolescents and adults. Levels of SIgA vary widely between individuals.26
In our study, levels of daily distribution of salivary biomarkers, namely, cortisol, amylase and SIgA, in a cohort of critically ill children upon admission to PICU are described. Potential association between salivary biomarkers and disease severity established by PRISM upon admission and need for mechanical ventilation, acute stress determined by time between disease onset and PICU admission and outcome ascertained by PICU hospitalization duration and death were assessed.
Overall, salivary biomarkers were significantly elevated when compared to published values in healthy children22,24–26 (Table 1). As per Gröschl et al.,22 reference salivary median cortisol levels in healthy children aged 2–15 years are 8 a.m. 3.0–54.9, 2 p.m. 1.1–20.7 and 8 p.m. 0.2–8.7 (nmol/L). Moreover, Maguire et al.21 reported a range of salivary cortisol levels of 0–25 nmol/L at 8 a.m., 0–10 nmol/L at 2 p.m. and 0–4 nmol/L at 8 p.m. in children aged 5–18 years. In reference to sAA, Granger et al.24 have reported levels ranging between 400 and 900 U/mL in healthy children. Concerning SIgA, Jafarzadeh et al.26 reported median levels of 4.26 ± 3.85 mg/dL in a small number of healthy children aged 1–10 years. Most recently, Starzak et al.25 reported a median level of 2.439 ± 1.192 mg/dL. Regarding critically ill children, Balbao et al.23 reported values ranging between 7.7 and 448.3 nmol/L for salivary cortisol in children aged 0.5–8.3 years. Our results are in accordance with previous data indicating that critically ill children have higher salivary cortisol concentrations.23 In addition, in this cohort, extremely high concentrations of sAA were observed, although considerable variation in levels was noted (Table 3). Indeed, in our cohort, although levels of SIgA were significantly higher when compared to those reported in healthy children25,26 (Table 1), a significant variation was observed (Table 3). Therefore, no reliable postulation on the role of SIgA can be made from these data. In general, the significant increase in the examined biomarkers indicates the acute stress induced by critical illness influenced by other factors. Unfortunately, in the existing bibliography, concerning acute stress in pediatric emergency and intensive care is scarce. It has been hypothesized that biomarkers for diagnosing, monitoring and stratifying various forms of critical illness may be important and change the daily clinical practice in PICU.64
Importantly, to evaluate whether salivary biomarkers examined herein may be used as prognostic factors, possible association between salivary biomarkers and critical illness in children was examined. Younger children (<6 years old) and those with PRISM score equal and higher than 8 upon PICU admission were more likely to present with increased salivary biomarkers (Figures 1 and 2). Of note, we arbitrarily elected to compare children according to their PRISM score upon admission (<8 and ⩾8) following previous studies.72
Significant observations concerning the circadian rhythmicity were demonstrated. Most children had alteration of the circadian rhythm of both cortisol and α-amylase. Regarding salivary cortisol, children with a PRISM score equal and higher than 8 upon admission were more likely to present with disruption of cortisol circadian rhythm which was most likely due to an increase in their cortisol values at 8 p.m. Gonzalez et al.73 previously demonstrated abnormalities in cortisol regulation in children admitted to PICU lasting for 3–6 months post discharge. Moreover, putting together disease severity markers, namely, PRISM equal and >8 upon admission, mechanical ventilation, use of vasoactive support and hospitalization in the PICU for more than 3 days, a group of high-gravity children was formed. However, in this group of children, only higher salivary cortisol levels at 2 p.m. were observed, possibly due to the small number of children in our cohort. sAA rhythm was frequently disrupted, mainly due to decreased levels at 2 p.m., since only the 35.4% of the study population maintained normality. Moreover, since altered circadian rhythm of α-amylase was observed among children who remained hospitalized in the PICU for more than 3 days, an indirect marker of severity, one may postulate that decrease in sAA levels at 2 p.m. may serve as a surrogate marker of disease severity.
Overall, the development of biomarkers, especially in critically ill children, is important for the early diagnosis, guidance of treatment and monitoring.74 Τhe main goal is to discover specific associations between salivary biomarkers and NED or even outcome in pediatric critical care. These markers might be useful in understanding the pathogenesis and more importantly might assist in the early detection of the subgroup of patients in need of additional support.40–42 Salivary biomarkers are easy to obtain by non-invasive methods. Therefore, they could be used in combination with warning signs and clinical alert criteria,43–45 in order to improve quality of care for critically children. The major challenge to be resolved before salivary biomarkers may be used in clinical practice is the harmonization of measurements since to date reports use different methodologies and variable reference ranges.
This study has several limitations. No healthy controls were included. Saliva samples were not examined simultaneously with blood samples. Furthermore, samples were taken only on the first day, upon PICU admission, whereas more samples examined prospectively might have provided valuable data and address association of salivary biomarkers with prognosis. Notably, time between disease onset and PICU admission as well as between admission and first sample collection differ between patients. These are undoubtedly factors that may affect biomarker levels. However, in such prospective studies, such limitations are difficult to overcome. Moreover, other potential influencing factors including puberty and sleep destruction were not evaluated. Importantly, one may postulate that the number of children was small and therefore the study lacks power to reveal meaningful associations. Moreover, the variance of values was significant. Furthermore, since biomarkers may be influenced by multiple confounding factors including age, sex and underlying disease, the results of this study rather represent a first attempt to describe potential associations rather than providing evidence for use of salivary biomarkers as a tool to estimate stress and outcome. These limitations were mainly due to logistical and financial constraints. Finally, results on sAA and SIgA need to be confirmed in future studies since considerable variations in levels were noted limiting the interpretability of these biomarkers.
Conclusion
Despite aforementioned constraints, this study contributes to the literature by describing a pattern of salivary biomarkers in critically ill children. It is crucial to establish a practical and objective method which may provide clinical and/or laboratory criteria to identify children with severe stress and therefore poor prognosis upon admission to PICU. Ideally, this method should be easy to use, require no extensive experience of the observer, should be easy to reproduce, have a low cost and be minimally invasive and highly accurate. Salivary biomarkers may help identify children at high risk. Therefore, they may be easily added in existing scales used for evaluating children upon admission and monitoring critically ill children during hospitalization in the PICU.
However, inconsistent findings demonstrate the need to establish normative cortisol, α-amylase and SIgA salivary levels and further describe changes in acute stress. Thence, using such a non-invasive method, salivary stress markers may potentially be added in new scores and assist clinicians to early identify the subgroup of patients with critical illness and poor prognosis in need of additional support.
Acknowledgments
We thank the medical and nurse staff in PICU department at “P. & A. Kyriakou,” Children’s Hospital, Athens, Greece for data collection.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: We obtained ethical approval from the Ethics Committee of “P. & A. Kyriakou,” Children’s Hospital, Athens, Greece where the study took place. The approval was issued on 7 July 2010.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Geolocation information: This work took place in a tertiary pediatric hospital in Athens, Greece.
Informed consent: All participants were enrolled only after parents provided written informed consent.
Lay summary: This study aimed to quantify stress in acutely ill children hospitalized in intensive care by measuring molecules in saliva during the first 24 h after admission. Indeed, it was found that the stress hormone, namely cortisol, as well as salivary amylase and secretory immunoglobulin A were increased in acutely ill children. Moreover, higher salivary markers were found in children younger than 6 years and those admitted with a high severity score upon admission, implying that if these findings are confirmed, measuring these molecules in saliva may help identifying children at high risk needing additional support.
Trial registration: This was an observational study and therefore we did not proceed to obtain trial registration.
ORCID iD: Vassiliki Papaevangelou  https://orcid.org/0000-0001-6075-3002
https://orcid.org/0000-0001-6075-3002
References
- 1. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol 2009; 5: 374–381. [DOI] [PubMed] [Google Scholar]
- 2. Mc Ewen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007; 87(3): 873–904. [DOI] [PubMed] [Google Scholar]
- 3. Hassan-Smith Z. Overview of the endocrine response to critical illness: how to measure it and when to treat. Best Pract Res Clin Endocrinol Metab 2011; 25: 705–717. [DOI] [PubMed] [Google Scholar]
- 4. Shulman DI, Palmert MR, Kemp SF. Adrenal insufficiency: still a cause of morbidity and death in childhood. Pediatrics 2007; 119(2): e484–e494. [DOI] [PubMed] [Google Scholar]
- 5. Charmandari E, Achermann JC, Carel JC, et al. Stress response and child health. Sci Signal 2012; 5(248): mr1. [DOI] [PubMed] [Google Scholar]
- 6. Menon K, Ward RE, Lawson ML, et al. A prospective multicenter study of adrenal function in critically ill children. Am J Respir Crit Care Med 2010; 182(2): 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hebbar K, Rigby MR, Felner EI, et al. Neuroendocrine dysfunction in pediatric critical illness. Pediatr Crit Care Med 2009; 10(1): 35–40. [DOI] [PubMed] [Google Scholar]
- 8. Trimarchi T. Endocrine problems in critically ill children: an overview. AACN Clin Issues 2006; 17(1): 66–78. [DOI] [PubMed] [Google Scholar]
- 9. Langer M, Modi BP, Agus M. Adrenal insufficiency in the critically ill neonate and child. Curr Opin Pediatr 2006; 18(4): 448–453. [DOI] [PubMed] [Google Scholar]
- 10. Charmandari Ε, Nicolaides NC, Chrousos GP. Adrenal insufficiency, www.thelancet.com [DOI] [PubMed] [Google Scholar]
- 11. Hebbar KB, Stockwell JA, Leong T, et al. Incidence of adrenal insufficiency and impact of corticosteroid supplementation in critically ill children with systemic inflammatory syndrome and vasopressor-dependent shock. Crit Care Med 2011; 39(5): 1145–1150. [DOI] [PubMed] [Google Scholar]
- 12. Inwald DP, Butt W, Tasker RC. Fluid resuscitation of shock in children: what, whence and whither? Intensive Care Med 2015; 41(8): 1457–1459. [DOI] [PubMed] [Google Scholar]
- 13. Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med 2009; 37: 666–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Linck Júnior A, Rego Filho Ede A, Moriya LK, et al. Adrenal insufficiency in children with sepsis. Rev Bras Ter Intensiva 2011; 23(4): 478–483. [PubMed] [Google Scholar]
- 15. Samransamruajkit R, Jitchaiwat S, Deerojanawong J, et al. Adrenal insufficiency in early phase of pediatric acute lung injury/acute respiratory distress syndrome. J Crit Care 2007; 22(4): 314–318. [DOI] [PubMed] [Google Scholar]
- 16. Brown NJ, Kimble RM, Rodger S, et al. Biological markers of stress in pediatric acute burn injury. Burns 2014; 40: 887–895. [DOI] [PubMed] [Google Scholar]
- 17. Mushtaq T, Shakur F, Wales JK, et al. Reliability of the low dose synacthen test in children undergoing pituitary function testing. J Pediatr Endocrinol Metab 2008; 21: 1129–1132. [DOI] [PubMed] [Google Scholar]
- 18. Magnotti M, Shimshi M. Diagnosing adrenal insufficiency: which test is best? The 1μg or the 250μg cosyntropin stimulation test? Endocrine Pract 2008; 14: 233–238. [DOI] [PubMed] [Google Scholar]
- 19. Allen AP, Kennedy PJ, Cryan JF, et al. Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci Biobehav Rev 2014; 38: 94–124. [DOI] [PubMed] [Google Scholar]
- 20. Evans BE, Greaves-Lord K, Euser AS, et al. Determinants of physiological and perceived physiological stress reactivity in children and adolescents. PLoS ONE 2013; 8: e61724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maguire AM, Ambler GR, Moore B, et al. The clinical utility of alternative, less invasive sampling techniques in the assessment of oral hydrocortisone therapy in children and adolescents with hypopituitarism. Eur J Endocrinol 2007; 156(4): 471–476. [DOI] [PubMed] [Google Scholar]
- 22. Gröschl M, Rauh M, Dörr HG. Circadian rhythm of salivary cortisol, 17alpha-hydroxyprogesterone, and progesterone in healthy children. Clin Chem 2003; 49(10): 1688–1691. [DOI] [PubMed] [Google Scholar]
- 23. Balbao V, Costa M, Castro M, et al. Evaluation of adrenal function in critically ill children. Clin Endocrinol 2014; 81: 559–565. [DOI] [PubMed] [Google Scholar]
- 24. Granger DA, Kivlighan KT, el-Sheikh M, et al. Salivary alpha-amylase in biobehavioral research: recent developments and applications. Ann N Y Acad Sci 2007; 1098: 122–144. [DOI] [PubMed] [Google Scholar]
- 25. Starzak DE, Konkol KF, McKune AJ. Effects of cardiorespiratory fitness and obesity on salivary secretory IgA and alpha-amylase in South African children. Children 2016; 3(3): E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jafarzadeh A, Sadeghi M, Karam GA, et al. Salivary IgA and IgE levels in healthy subjects: relation to age and gender. Braz Oral Res 2010; 24(1): 21–27. [DOI] [PubMed] [Google Scholar]
- 27. Harris M, Cox S, Brett C, et al. Stress in childhood, adolescence and early adulthood, and cortisol levels in older age. Stress 2017; 20(2): 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirschbaum CL, Hellhammer D. Salivary cortisol. In: Encyclopedia of stress. Germany: University of Trier, Academic Press, 2000, vol. 3, pp. 379–383. [Google Scholar]
- 29. Adam E, Hoyt L, Granger D. Diurnal alpha amylase patterns in adolescents: associations with puberty and momentary mood states. Biol Psychol 2011; 88(2–3): 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jessop DS, Turner-Cobb JM. Measurement and meaning of salivary cortisol: a focus on health and disease in children. Stress 2008; 11(1): 1–14. [DOI] [PubMed] [Google Scholar]
- 31. Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology 2009; 34(4): 486–496. [DOI] [PubMed] [Google Scholar]
- 32. Fortunato CK, Dribin AE, Granger DA, et al. Salivary alpha-amylase and cortisol in toddlers: differential relations to affective behavior. Dev Psychobiol 2008; 50: 807–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009; 34(2): 163–171. [DOI] [PubMed] [Google Scholar]
- 34. Wolf MJ, Nicholls E, Chen E. Chronic stress, salivary cortisol, and a-amylase in children with asthma and healthy children. Biol Psychol 2008; 78: 20–28. [DOI] [PubMed] [Google Scholar]
- 35. Maruyama Y, Kawano A, Okamoto S, et al. Differences in salivary alpha-amylase and cortisol responsiveness following exposure to electrical stimulation versus the Trier Social Stress Tests. PLoS ONE 2012; 7(7): e39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bosch JA, Carroll D. Stress and mucosal secretory immunity. In: Fink E. (ed.) Encyclopedia of stress. 2nd ed. London: Elsevier, 2006, pp. 768–774. [Google Scholar]
- 37. Teeuw W, Bosch JA, Veerman EC, et al. Neuroendocrine regulation of salivary IgA synthesis and secretion: implications for oral health. Biol Chem 2004; 385: 1137–1146. [DOI] [PubMed] [Google Scholar]
- 38. Matos-Gomes N, Katsurayama M, Makimoto FH, et al. Psychological stress and its influence on salivary flow rate, total protein concentration and IgA, IgG and IgM titers. Neuroimmunomodulation 2010. 17(6): 396–404. [DOI] [PubMed] [Google Scholar]
- 39. Ring C, Carroll D, Hoving J, et al. Effects of competition, exercise, and mental stress on secretory immunity. J Sports Sci 2005; 23(5): 501–508. [DOI] [PubMed] [Google Scholar]
- 40. Wolfler A, Osello R, Gualino J. The importance of mortality risk assessment: validation of the pediatric index of mortality 3 score. Pediatr Crit Care Med 2016; 17: 251–256. [DOI] [PubMed] [Google Scholar]
- 41. Pollack MM, Holubkov R, Funai T, et al. The pediatric risk of mortality score: update 2015. Pediatr Crit Care Med 2016; 17: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Visser IH, Hazelzet JA, Albers MJ, et al. Mortality prediction models for pediatric intensive care: comparison of overall and subgroup specific performance. Intensive Care Med 2013; 39: 942–950. [DOI] [PubMed] [Google Scholar]
- 43. Namachivayam P, Shann F, Shekerdemian L, et al. Three decades of pediatric intensive care: who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med 2010; 11(5): 549–555. [DOI] [PubMed] [Google Scholar]
- 44. Pollack MM, Holubkov R, Funai T, et al. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med 2014; 15: 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aspesberro F, Mangione-Smith R, Zimmerman JJ. Health-related quality of life following pediatric critical illness. Intensive Care Med 2015; 41: 1235–1246. [DOI] [PubMed] [Google Scholar]
- 46. Tume L. The deterioration of children in ward areas in a specialist children’s hospital. Nurs Crit Care 2007; 12: 12–19. [DOI] [PubMed] [Google Scholar]
- 47. Pearson GA. (ed.). Why children die: a pilot study 2006, 2008, http://www.cemach.org.uk/ [Google Scholar]
- 48. Hodkinson P, Argent A, Wallis L, et al. Pathways to care for critically ill or injured children: a cohort study from first presentation to healthcare services through to admission to intensive care or death. PLoS ONE 2016; 11(1); e0145473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parshuram CS, Duncan HP, Joffe AR, et al. Multicentre validation of the bedside paediatric early warning system score: a severity of illness score to detect evolving critical illness in-hospitalised children. Crit Care 2011; 15: R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chapman S, Grocott M, Frank L. Systematic review of paediatric alert criteria for identifying hospitalised children at risk of critical deterioration. Intensive Care Med 2010; 36: 600–611. [DOI] [PubMed] [Google Scholar]
- 51. Seiger N, Maconochie I, Oostenbrink R, et al. Validity of different pediatric early warning scores in the emergency department. Pediatrics 2013; 132(4): e841–e850. [DOI] [PubMed] [Google Scholar]
- 52. Nunes LA, Mussavira S, Bindhu OS. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: a systematic review. Biochem Med 2015; 25(2): 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Helmerhorst EJ, Oppenheim FG. Saliva: a dynamic proteome. J Dent Res 2007; 86(8): 680–693. [DOI] [PubMed] [Google Scholar]
- 54. Zhang A, Sun H, Wang X. Saliva metabolomics opens door to biomarker discovery, disease diagnosis, and treatment. Appl Biochem Biotechnol 2012; 168(6): 1718–1727. [DOI] [PubMed] [Google Scholar]
- 55. Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med 1996; 24: 743–752. [DOI] [PubMed] [Google Scholar]
- 56. Uçar A, Baş F, Saka N. Diagnosis and management of pediatric adrenal insufficiency. World J Pediatr 2016; 12(3): 261–274. [DOI] [PubMed] [Google Scholar]
- 57. Nelson L, Markovitz B. Are we correctly diagnosing adrenal insufficiency or are we just spitting into the wind? Pediatr Crit Care Med 2015; 16(4): 385–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hanna W, Wong HR. Pediatric sepsis: challenges and adjunctive therapies. Crit Care Clin 2013; 29(2): 203–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Menon K, McNally JD, Choong K, et al. A cohort study of pediatric shock: frequency of corticosteriod use and association with clinical outcomes. Shock 2015; 44(5): 402–409. [DOI] [PubMed] [Google Scholar]
- 60. Markovitz BP, Goodman DM, Watson RS, et al. A retrospective cohort study of prognostic factors associated with outcome in pediatric severe sepsis: what is the role of steroids? Pediatr Crit Care Med 2005; 6(3): 270–274. [DOI] [PubMed] [Google Scholar]
- 61. Heim C, Nater UM, Maloney E, et al. Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Arch Gen Psychiatry 2009; 66(1): 72–80. [DOI] [PubMed] [Google Scholar]
- 62. Pervanidou P. Biology of post-traumatic stress disorder in childhood and adolescence. J Neuroendocrinol 2008; 20(5): 632–638. [DOI] [PubMed] [Google Scholar]
- 63. Guerry JD, Hastings PD. In search of HPA axis dysregulation in child and adolescent depression. Clin Child Fam Psychol Rev 2011; 14(2): 135–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kaplan JM, Wong HR. Biomarker discovery and development in pediatric critical care medicine. Pediatr Crit Care Med 2011; 12(2): 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev 2011; 17: 6–63. [PubMed] [Google Scholar]
- 66. Brandtzaeg P. Secretory IgA: designed for anti-microbial defense. Front Immunol 2013; 6: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nakamura D, Akimoto T, Suzuki S, et al. Daily changes of salivary secretory immunoglobulin A and appearance of upper respiratory symptoms during physical training. J Sports Med Phys Fitness 2006; 46(1): 152–157. [PubMed] [Google Scholar]
- 68. Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol 2009; 70: 505–515. [DOI] [PubMed] [Google Scholar]
- 69. Lehtonen OP, Tenovuo J, Aaltonen AS, et al. Immunoglobulins and innate factors of immunity in saliva of children prone to respiratory infections. Acta Pathol Microbiol Immunol Scand C 1987; 95(1): 35–40. [DOI] [PubMed] [Google Scholar]
- 70. Parisotto TM, King WF, Duque C, et al. Immunological and microbiologic changes during caries development in young children. Caries Res 2011; 45(4): 377–385. [DOI] [PubMed] [Google Scholar]
- 71. Sonesson M. On minor salivary gland secretion in children, adolescents and adults. Swed Dent J Suppl 2011; 215: 9–64. [PubMed] [Google Scholar]
- 72. Costa GA, Delgado AF, Ferraro A, et al. Application of the Pediatric Risk of Mortality Score (PRISM) score and determination of mortality risk factors in a tertiary pediatric intensive care unit. Clinics 2010; 65: 1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gonzalez MDP, Als L, Donnell KOA, et al. Salivary cortisol and α-amylase levels following admission to pediatric intensive care. Arch Dis Child 2010; 95(Suppl 1): A44. [Google Scholar]
- 74. Goldman J, Becker ML, Jones B, et al. Development of biomarkers to optimize pediatric patient management: what makes children different? Biomark Med 2011; 5: 781–794. [DOI] [PMC free article] [PubMed] [Google Scholar]