Abstract
Introduction:
Brain metastases (BM) are common in advanced non-small cell lung cancer (NSCLC), and the prognosis is poor with few therapeutic options. This study evaluated the efficacy of three epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs) in preventing and treating BM in patients with EGFR mutation-positive advanced NSCLC.
Methods:
Patients with EGFR mutation-positive advanced NSCLC who visited a tertiary referral center from 1 December 2013 to 30 November 2017 were analyzed retrospectively. They received gefitinib, erlotinib, or afatinib until disease progression, death, or intolerable adverse events. The cumulative incidence of subsequent BM of initial non-BM patients, progression-free survival (PFS), and overall survival (OS) of the BM and non-BM patients were estimated and compared using the Kaplan–Meier and log-rank tests.
Results:
306 NSCLC patients were enrolled, with 116, 75, and 115 receiving first-line gefitinib, erlotinib, and afatinib, respectively. The afatinib group had a better PFS [12.7 versus 9.8 months; hazard ratio (HR) 0.59, p = 0.001] and OS (39.1 versus 22.0 months; HR 0.64, p = 0.035) than the gefitinib group. Afatinib tended to provide better BM prevention than gefitinib (BM cumulative incidence, HR 0.49; 95% confidence interval 0.34–0.71, p < 0.001) according to a Cox model adjusted for possible confounders. Patients with initial BM had a shorter PFS (p < 0.001) and OS (p = 0.015) than those without initial BM. Among the former, there were no differences in median PFS (p = 0.34) and median OS (p = 0.46) in the three EGFR-TKI groups.
Conclusions:
Our data suggested that, compared with gefitinib, afatinib provided better benefits significantly in terms of PFS and OS. Both had the same effectiveness in preventing subsequent BM.
Keywords: brain metastasis, EGFR mutation, non-small cell lung cancer, tyrosine kinase inhibitor
Introduction
Brain metastases (BM) have a poor prognosis and typically result in the deterioration of a patient’s quality of life, and about 25–45% of patients with lung cancer develop BM during the course of their disease.1 According to our own recent study and another study, epidermal growth factor receptor (EGFR) mutation is one of the predictors for subsequent BM.2,3 The previous standard management for BM included irradiation (whole-brain radiation therapy and stereotactic radiosurgery) and surgical resection. Traditional cytotoxic agents usually do not penetrate the blood–brain barrier (BBB) and show a suboptimal intracranial response rate of 30% and a median overall survival (OS) of 7.7 months.4 Owing to their good penetration through the BBB, the use of EGFR–tyrosine kinase inhibitors (TKIs) to treat BM in patients with EGFR mutation-positive non-small cell lung cancer (NSCLC) has drawn increasing attention. In a phase II study of EGFR mutation-positive NSCLC patients with BM, treatment with a first-line reversible EGFR-TKI, erlotinib or gefitinib, showed a disease control rate of 93%, while the median progression-free survival (PFS) and OS were 6.6 and 15.9 months, respectively.5 Afatinib is a second-generation TKI that binds irreversibly to EGFR, and is the first TKI with significant overall survival benefits in comparison with traditional chemotherapy. Although afatinib is less validated for the treatment of BM than gefitinib or erlotinib, a combined analysis of the BM subgroups in the LUX-Lung 3 and LUX-Lung 6 trials revealed a better PFS for the afatinib group than the chemotherapy group.6
Despite better survival outcomes in BM patients treated with EGFR-TKIs, brain recurrence after a good response to first-line EGFR-TKIs remains a major problem. Given the severe effect of BM on survival, the prevention of such metastases by an effective treatment is crucial. It has been reported that central nervous system (CNS) recurrence after an initial response to an EGFR-TKI was observed in 25–33% of patients treated with gefitinib and 1–8% of patients treated with erlotinib, respectively.7–10 Although another recent study also reported a difference between erlotinib and gefitinib with respect to the prevention of CNS recurrence and the treatment of CNS metastases,11 there has been no report directly comparing all three first-line EGFR-TKIs, that is, gefitinib, erlotinib, and afatinib, in terms of their effectiveness in preventing and controlling BM in NSCLC patients harboring EGFR mutations. This retrospective study thus compared the effects of gefitinib, erlotinib, and afatinib in the prevention and control of BM in patients with EGFR mutation-positive advanced NSCLC. Moreover, we performed Cox proportional hazards regression for the predictors of subsequent BM and determinants of PFS and OS after BM.
Materials and methods
This study was reviewed and approved by the Review Board and Ethics Committee of National Cheng Kung University Hospital (NCKUH B-ER-106-212). All data were anonymized, and, given the retrospective nature of the study, the need for written informed consent was waived. This research was carried out in accordance with approved guidelines and the Declaration of Helsinki. All of the EGFR mutation-positive patients with newly diagnosed or recurrent advanced NSCLC who visited the National Cheng Kung University Hospital from 1 December 2013 to 30 November 2017 were enrolled in the study. The patients all underwent a chest computed tomography (CT) scan, bone scan, and brain imaging [CT or magnetic resonance imaging (MRI)] for staging, based on the tumor, node, metastasis (TNM) system proposed by the American Joint Committee on Cancer, 7th edition. Stage I–IIIA patients were excluded, leaving only stage IIIB–IV patients in the analysis set.
We recorded the baseline characteristics of these patients, including age, sex, mutation subtype, performance status, initial BM, and TNM staging. All of the patients took gefitinib, erlotinib, or afatinib at the discretion of the treating providers and underwent brain imaging at the initial diagnosis or the recognition of advanced disease. Follow-up brain MRI or CTs were arranged by the doctors according to CNS signs or symptoms. CNS metastases included parenchymal BM and radiographically diagnosed leptomeningeal disease. The treatment modalities, including TKIs and radiotherapy, were recorded. Disease progression was determined based on the radiographic evidence according to Response Evaluation Criteria in Solid Tumors version 1.1.12
Epidermal growth factor receptor mutation analysis
Tumor tissues from primary lung tumors or metastatic lesions were obtained for EGFR mutation analysis. Tissue samples that consisted of >80% tumor content, as determined via microscopy with hematoxylin and eosin staining, were selected for the study. Deoxyribonucleic acid (DNA) was extracted using the QIAcube automated extractor (Qiagen Hilden, Germany) with the QIAamp DNA FFPE tissue kit (Qiagen) and eluted in ATE (QIAmp Tissue Elution) buffer (Qiagen) according to the manufacturer’s instructions. The presence of EGFR mutations was determined using the EGFR polymerase chain reaction (PCR) Kit (EGFR RUO Kit) and therascreen® EGFR RGQ PCR Kit (EGFR IVD Kit, Qiagen, Manchester, UK). These kits combine Scorpion’s and the amplification-refractory mutation system (ARMS) technologies to detect the mutations using real-time quantitative PCR.12
Statistical analysis
The frequencies and descriptive statistics of the demographic and clinical variables were calculated. Categorical variables were compared using the Chi-square test or Fisher’s exact test, whereas continuous variables were compared using Student’s t test or the Wilcoxon rank-sum test. The cumulative incidence of BM13 and the PFS and OS of the initial BM patients were estimated by the Kaplan–Meier method and compared using the log-rank test. We also performed Cox proportional hazards regression for the predictors of subsequent BM and determinants of PFS and OS. The selection of possible predictors and determinants was based on prior studies investigating the risk factors for BM and the prognostic factors of survival in early-stage lung cancer.14,15 Age, sex, smoking status, tumor size, nodal stage and EGFR mutation subtypes were chosen as the predictors and prognostic factors. Statistical Analysis System® software version 9.4 (SAS Institute, Cary, NC, USA) was used to perform the analyses. All the reported p values are two sided.
Results
Characteristics of patients
A total of 306 patients who visited the hospital from 1 December 2013 to 30 November 2017 were enrolled. Of those patients, 263 had newly diagnosed and 43 had recurrent EGFR mutation-positive advanced NSCLC. Figure 1 details the inclusion of subjects for analysis. Of the included patients, 116 (37.9%) patients received gefitinib, 75 (24.5%) patients received erlotinib, and 115 (37.6%) received afatinib as first-line therapy. Higher proportions of the patients who received afatinib had a better performance status (Table 1) and exon 19 deletions. The proportion of patients with initial BM was higher among the patients who received erlotinib. The distributions of EGFR mutation subtypes among three TKI treatment groups are summarized in Supplementary Table 1.
Figure 1.

Flow chart describing enrollment of patients in the study.
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; TKI, tyrosine kinase inhibitor.
Table 1.
Demographic and clinical characteristics of patients.
| Gefitinib (n = 116) | Erlotinib (n = 75) | Afatinib (n = 115) | p value | |
|---|---|---|---|---|
| Age, n (%) | 0.16 | |||
| ≥60 | 92 (79.3%) | 59 (78.7%) | 80 (69.6%) | |
| <60 | 24 (20.7%) | 16 (21.3%) | 35 (30.4%) | |
| Sex, n (%) | 0.63 | |||
| Male | 43 (37.1%) | 33 (44.0%) | 45 (39.1%) | |
| Female | 73 (62.9%) | 42 (56.0%) | 70 (60.9%) | |
| Smoking, n (%) | 0.38 | |||
| Positive | 90 (77.6%) | 57 (76.0%) | 96 (83.5%) | |
| Negative | 26 (22.4%) | 18 (24.0%) | 19 (16.5%) | |
| Tumor size, n (%) | 0.68 | |||
| ≥3 cm | 23 (19.8%) | 14 (18.7%) | 24 (20.9%) | |
| <3 cm | 80 (69.0%) | 51 (68.0%) | 83 (72.2%) | |
| NA | 13 (11.2%) | 10 (13.3%) | 8 (6.9%) | |
| Nodal involvement, n (%) | 0.85 | |||
| N0 | 17 (14.7%) | 13 (17.3%) | 19 (16.5%) | |
| N1/N2/N3 | 99 (85.3%) | 62 (82.7%) | 96 (83.5%) | |
| Stage, n (%) | 0.44 | |||
| Recurrence | 19 (16.4%) | 7 (9.3%) | 17 (14.8%) | |
| Stage IIIB | 91 (79.4%) | 67 (89.3%) | 96 (82.5%) | |
| Stage IV | 5 (4.2%) | 1 (1.4%) | 3 (2.7%) | |
| ECOG PS, n (%) | 0.01 | |||
| 0–1 | 99 (85.3%) | 58 (77.3%) | 102 (88.7%) | |
| ≥2 | 14 (12.1%) | 17 (22.7%) | 9 (7.8%) | |
| NA | 3 (2.6%) | 0 | 4 (3.5%) | |
| EGFR mutation, n (%) | <0.001 | |||
| Del 19 | 32 (27.6%) | 26 (34.7%) | 70 (60.9%) | |
| L858R | 76 (65.5%) | 45 (60.0%) | 28 (24.3%) | |
| Others | 6 (5.2%) | 3 (4.0%) | 11 (9.6%) | |
| Multiple | 2 (1.7%) | 1 (1.3%) | 6 (5.2%) | |
| Initial BM, n (%) | 23 (19.8%) | 34 (45.3%) | 30 (26.1%) | <0.001 |
BM, brain metastases; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PS, performance status; NA, not applicable.
Progression-free survival and overall survival of all patients
A comparison of PFS and OS of all the patients stratified by the different EGFR-TKIs is shown in Figure 2. PFS was significantly longer in the patients who received afatinib compared with those who received erlotinib or gefitinib [log-rank test, p = 0.007; Figure 2(a)]. OS was also longer in the patients who received afatinib compared with those who received erlotinib or gefitinib, although the difference was not statistically significant [log-rank test, p = 0.053; Figure 2(b)]. Using Cox proportional hazards regression to adjust for possible confounders, we found that the hazard ratio (HR) of PFS for afatinib versus gefitinib was 0.59 [95% confidence interval (CI) 0.43–0.81, p = 0.001], whereas the HR of OS for afatinib versus gefitinib was 0.64 (95% CI 0.42–0.97, p = 0.035). Male sex, poor performance status, and BM were poor prognostic factors for both PFS and OS (Table 2). Since T790M mutation accounts for 50–60% of all resistance mechanisms to first- and second-generation TKIs, and osimertinib is the standard treatment for T790M-mutant NSCLC,16 the frequency of osimertinib administration might affect the OS. We further analyzed the patients with disease progression after the use of first-line EGFR-TKIs and found that the proportion of patients who received osimertinib as a subsequent therapy were similar among the three groups (Supplementary Table 2).
Figure 2.
Progression-free survival (a) and overall survival (b) in patients with non-small cell lung cancer and epidermal growth factor receptor gene mutations treated with gefitinib, erlotinib, or afatinib.
CI, confidence interval; NR, not reached.
Table 2.
Cox proportional hazards regression of all patients for progression-free survival and overall survival.
| Progression-free survival |
Overall survival |
||||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age | >60 versus <60 | 0.95 (0.69–1.31) | 0.745 | 1.07 (0.72–1.59) | 0.751 |
| Sex | Male versus female | 1.47 (1.06–2.03) | 0.021 | 1.88 (1.26–2.80) | 0.002 |
| ECOG PS | ⩾2 versus <2 | 1.72 (1.14–2.59) | 0.010 | 3.18 (1.97–5.14) | <0.001 |
| Tumor size | >3 cm versus <3 cm | 1.38 (0.95–2.00) | 0.093 | 1.86 (1.14–3.05) | 0.013 |
| Nodal involvement | Positive versus negative | 1.72 (1.14–2.60) | 0.010 | 1.51 (0.90–2.52) | 0.120 |
| Smoking | Positive versus negative | 0.97 (0.64–1.46) | 0.873 | 0.89 (0.55–1.44) | 0.626 |
| EGFR mutation | Del 19 versus other mutation | 1.07 (0.81–1.42) | 0.630 | 0.87 (0.60–1.25) | 0.445 |
| Recurrence | Recurrence versus new diagnosis | 1.17 (0.75–1.83) | 0.479 | 1.07 (0.59–1.92) | 0.830 |
| Initial BM | Presence versus absence | 1.62 (1.18–2.23) | 0.003 | 1.37 (0.94–2.01) | 0.106 |
| Treatment | Erlotinib versus gefitinib | 0.77 (0.54–1.10) | 0.151 | 0.84 (0.55–1.28) | 0.422 |
| Afatinib versus gefitinib | 0.59 (0.43–0.81) | 0.001 | 0.64 (0.42–0.97) | 0.035 | |
BM, brain metastases; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; HR, hazard ratio; PS, performance status.
The prevention and treatment of brain metastases by the three first-line epidermal growth factor receptor–tyrosine kinase inhibitors in patients with epidermal growth factor receptor-mutated non-small cell lung cancer
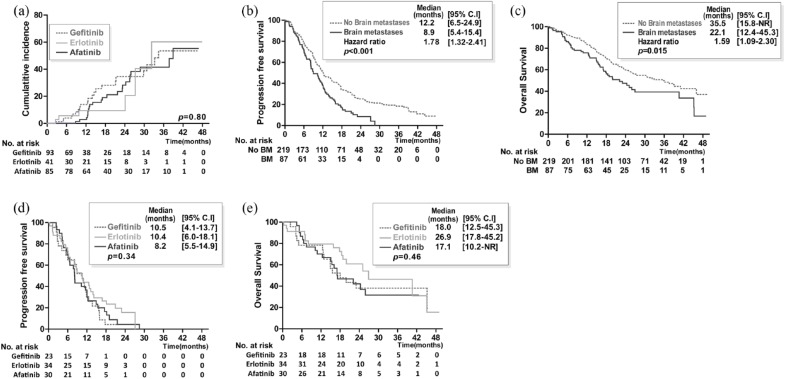
At the initiation of treatment, there were 93, 41, and 85 patients in the gefitinib, erlotinib, and afatinib groups, respectively, who were free of BM. The cumulative incidences of subsequent BM at 6, 12, 24, and 36 months were 3.8%, 13.9%, 34.6%, and 53.6%, respectively, in the gefitinib group; 5.6%, 9.3%, 9.3%, and 60.3%, respectively, in the erlotinib group; and 0%, 2.8%, 28.3%, and 41.5%, respectively, in the afatinib group, indicating no significant difference [p = 0.80; Figure 3(a)]. After using Cox proportional hazards regression to adjust for possible confounders, we found that the patients in the afatinib group had a lower HR of subsequent BM than those in the gefitinib group (0.49; 95% CI 0.34–0.71, p < 0.001; Supplementary Table 3). Lymph node involvement was found to be the predictor of subsequent BM (Supplementary Table 3).
Figure 3.
The prevention and treatment for brain metastases by three first-line epidermal growth factor receptor–tyrosine kinase inhibitors in patients with epidermal growth factor receptor mutation-positive advanced non-small cell lung cancer.
Cumulative incidence (a) of subsequent BM in patients without prior CNS involvement treated with gefitinib, erlotinib, or afatinib. Progression-free survival (b) and overall survival (c) of all patients with EGFR mutation-positive advanced non-small cell lung cancer and brain metastases. Progression-free survival (d) and overall survival (e) in patients with EGFR mutation-positive advanced non-small cell lung cancer and BM treated with gefitinib, erlotinib, or afatinib.
BM, brain metastases; CNS, central nervous system; EGFR, epidermal growth factor receptor; NR, not reached.
The analysis results of PFS stratified by the initial diagnosis of BM are shown in Figure 3(b). Patients with initial BM were associated with a shorter median PFS than those without initial BM [8.9 versus 12.2 months, HR 1.78 (95% CI 1.32–2.41); p < 0.001, Figure 3(b)]. The OS of the patients with BM at the initial diagnosis was also significantly shorter than that of those without initial BM [35.5 versus 22.1 months, HR 1.59 (95% CI 1.09–2.30); p = 0.015, Figure 3(c)]. Of the patients with initial BM, 34 received whole-brain radiotherapy and the proportions of patients receiving radiotherapy were similar among the three groups of patients (Supplementary Table 4). The overall response rate seemed similar among the three groups. There was also no significant difference in terms of PFS and OS among the patients who received the three kinds of treatment [Figure 3(d), (e)]. Using Cox proportional hazards regression, we found that tumor size was a poor prognostic factor for PFS and OS in patients with initial BM (Table 3), whereas male sex and poor performance status were poor prognostic factors for OS.
Table 3.
Cox proportional hazards regression for progression-free survival and overall survival of patients with initial brain metastases.
| Progression-free survival |
Overall survival |
||||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age | >60 versus <60 | 1.13 (0.59–2.15) | 0.709 | 1.04 (0.50–2.19) | 0.910 |
| Sex | Male versus female | 1.70 (0.94–3.09) | 0.081 | 2.48 (1.20–5.16) | 0.015 |
| ECOG PS | ⩾2 versus <2 | 1.81 (0.93–3.52) | 0.082 | 2.59 (1.18–5.71) | 0.018 |
| Tumor size | >3 cm versus <3 cm | 3.16 (1.17–8.53) | 0.023 | 5.52 (1.12–27.1) | 0.035 |
| Nodal involvement | Positive versus negative | 0.80 (0.25–2.51) | 0.698 | 0.69 (0.17–2.89) | 0.616 |
| Smoking | Positive versus negative | 1.59 (0.68–3.73) | 0.283 | 1.42 (0.57–3.57) | 0.452 |
| EGFR mutation | Del 19 versus others | 1.63 (0.89–3.00) | 0.114 | 0.91 (0.45–1.84) | 0.784 |
| Recurrence | Recurrence versus new diagnosis | 1.61 (0.30–8.62) | 0.577 | 1.18 (0.17–8.09) | 0.865 |
| Treatment | Erlotinib versus gefitinib | 0.56 (0.27–1.14) | 0.108 | 0.92 (0.39–2.14) | 0.840 |
| Afatinib versus gefitnib | 0.61 (0.30–1.23) | 0.168 | 1.16 (0.50–2.71) | 0.726 | |
ECOG, Eastern Cooperative Oncology Group; CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio; PS, performance status.
Discussion
To the best of our knowledge, this is the first real-world study evaluating and comparing the impact of three first-line EGFR-TKIs in the prevention and treatment of BM in patients with EGFR mutation-positive advanced NSCLC. The PFS and OS were better for afatinib than for gefitinib in all the patients, and among those without BM. Afatinib also provided the same effectiveness in preventing and treating BM as gefitinib and erlotinib, as shown in Figures 2 and 3(a). BM remains a major complication in lung cancer patients due to the limited penetration of the BBB by chemotherapy. Recent studies have demonstrated that EGFR-TKIs have better intracranial efficacy than chemotherapy.6,17 However, a real-world study comparing the efficacy of different EGFR-TKIs in preventing or treating BM has not previously been published. Li and colleagues showed that the time to neurological progression was effectively extended in pre-existing BM patients with EGFR-sensitizing mutations initially treated with erlotinib compared with gefitinib (30 versus 15.8 months, p = 0.024).11 However, from the diagnosis of CNS progression, the median OS in the erlotinib group and gefitinib group did not differ significantly (16 versus 12.6 months, p = 0.793). In a retrospective study enrolling 148 EGFR mutation-positive patients with BM, Bai and colleagues showed that EGFR-TKIs (gefitinib and erlotinib) have a promising antitumor effect against BM, with a disease control rate of 87.2%, as well as a median PFS and OS of 11.2 and 13.6 months, respectively.18 Furthermore, there was no statistically significant difference in terms of PFS (11.3 versus 10.8 months, p = 0.203) and OS (13.8 versus 13.5 months, p = 0.319) between the gefitinib and erlotinib. Similar to the previous two studies, there was no statistically significant difference in PFS between the patients with BM who received gefitinib and those who received erlotinib in our study. Meanwhile, a recent study analyzing the BM subgroups of the LUX-Lung 3 and LUX-Lung 6 trials revealed a significant improvement in PFS for the afatinib group compared with the chemotherapy group.4 Although the effects of afatinib have been compared with those of other EGFR-TKIs, including one observational study that compared three agents in terms of PFS19 and one randomized trial that compared afatinib with gefitinib,20 there has been no previous study comparing three TKIs in terms of the prevention and treatment of BM. In our study, the median PFS (8.2 months) of the BM patients who received afatinib was similar to that (8.2 months) reported for the results of the LUX-Lung 3 and LUX-Lung 6 trials,6 and to that reported for real-world practice (9.2 months).21
Since many studies have found that lung cancer patients with EGFR mutations tend to develop BM, choosing a treatment that can help to prevent BM is also critically important. Heon and colleagues demonstrated that lower rates of CNS progression were noted in EGFR-mutant advanced NSCLC patients initially treated with an EGFR-TKI than in such patients treated with chemotherapy.17 In that study, the cumulative incidences of subsequent BM at 6, 12, and 24 months were 1%, 6%, and 21%, respectively, in the EGFR-TKI group; findings that partially validate our results. Our study further disclosed that mediastinal lymph node involvement was a poor prognostic factor for subsequent BM, a finding which also corroborates the results of other studies.14,22,23
Unlike many retrospective studies reporting that erlotinib is more effective than gefitinib in preventing and treating BM,11,24–26 our study showed similar effects among the three EGFR-TKIs. The mechanism underlying a difference in the treatment effects of erlotinib and gefitinib has been considered attributable to the difference in maximum tolerated dose (MTD); the MTD of gefitinib is one third that of erlotinib.27 Therefore, the concentration of erlotinib in the CNS would be relatively higher than that of gefitinib. In addition, several investigations have also shown that the concentration of erlotinib in cerebrospinal fluid (CSF) is higher than that of gefitinib. However, the integrity of the BBB has been reported to be disrupted by the tumors themselves, usually at the later stages of the disease.28 In addition, the tumors themselves facilitate angiogenesis without a normal BBB. These findings can be radiologically observed in terms of brain edema around BM and via the enhancement of contrast agents with respect to BM size ⩾ 5 mm.29 Therefore, gefitinib at the standard dose does not sufficiently penetrate the BBB in the absence of CNS involvement, whereas when BM are evident, they probably improve the CNS concentration with a consequent improvement in central activity.6,30 In our study, the cumulative incidences of subsequent BM in the three EGFR-TKI groups were not significantly different. However, after using Cox proportional hazards regression to adjust for possible confounders and to compare the three EGFR-TKI treatment groups, we found that the patients in the afatinib group had a lower HR of subsequent BM than those in the gefitinib group. Similarly, other recent studies have demonstrated that afatinib has the potential to treat CNS metastases effectively and that the median CSF penetration rate of afatinib was, in fact, higher than the rate previously reported.30,31 The regression of CNS metastases observed during afatinib treatment has provided evidence that afatinib concentration in the CSF is sufficient to inhibit tumor growth due to its potency at relatively low concentrations.30 Another case report also showed that BM refractory to erlotinib responded substantially to afatinib despite a nonresponse in extra-CNS lesions.32 In Supplementary Figure 1, we provide the brain scans for three patients with EGFR-mutant NSCLC whose BM markedly responded to gefitinib, erlotinib, and afatinib, respectively (please note that the agreement of these patients was obtained). To date, the superiority of erlotinib over gefitinib or afatinib in treating EGFR-mutant NSCLC with BM has not been proven in any prospective study. There is thus a need to conduct clinical trials with specific CNS endpoints to evaluate candidate EGFR-TKIs in terms of their CNS penetration and in terms of efficacy in treating established CNS metastases and preventing them from occurring.
Several limitations of the current study must be acknowledged. First, it was a single-center retrospective study, and there were significant differences in the characteristics of the three groups. The participants who received afatinib included higher proportions with better performance and exon 19 deletions, whereas those who received erlotinib included a higher proportion with BM. These differences have also been noted in some real-world studies,21 and one possible explanation is that physicians usually prescribe afatinib as a first-line treatment based on the favorable OS of patients with exon 19 deletions.33 Furthermore, because many studies have reported that erlotinib is more effective in treating BM than gefitinib,11,24–26 physicians may tend to prescribe erlotinib for patients with initial BM.11 Although we had tried to control for possible confounders using the Cox model, a subgroup analysis comparing three groups of patients with exon 19 deletions and Eastern Cooperative Oncology Group performance statuses of 0–1 showed that the PFS and OS were similar among patients receiving different kinds of EGFR-TKIs (Supplementary Figure 2 and Supplementary Table 5). Given that there were more BM in the erlotinib group, no statistical difference in the proportion of patients receiving brain radiotherapy (p = 0.87; Supplementary Table 4) was observed. We further calculated Cox models for PFS and OS by using the erlotinib group as the reference. It was interesting to find that there was also no significant difference between afatinib and erlotinib in PFS and OS after adjusting possible confounders (Supple-mentary Table 6). Second, we did not survey all possible driver mutations that may make patients prone to the development of BM, such as mutations or amplifications of Mesenchymal epithelial transition (Met) and Anaplastic lymphoma kinase (ALK) translocations.34,35 However, the frequency of concurrent genes in EGFR-mutant NSCLC patients is only around 6%. Therefore, the impact of such genes may be minimal.36 Third, although the risk of CNS progression was not significantly different among the three EGFR-TKI treatment groups, the quality of life (QoL) for BM patients using these therapies remains undetermined. BM cause deterioration in the QoL of patients, and our own recent study also showed that the QoL scores in patients receiving afatinib were lower than those of patients receiving gefitinib.37 As such, the QoL in patients with BM receiving the three EGFR-TKIs requires further investigation. Fourth, some data were not available for comparing the overall response rate of BM to the three TKIs (Supplementary Table 3). Finally, though all the patients underwent brain imaging at the time of the initial diagnosis or at recurrence, the brain imaging was conducted based on the occurrence of symptoms rather than according to a predefined period to document the subsequent metastases. As a result, we might have missed asymptomatic BM, which would have caused us to underestimate the incidence of BM. However, as the follow-up schedule was applied to each group of patients, differential bias would not be generated.
In conclusion, our study revealed a better PFS in patients treated with afatinib in comparison with patients treated with gefitinib. Furthermore, compared with the other two EGFR-TKIs, afatinib provided similar intracranial efficacy in patients with or without pre-existing BM. This is the first study to directly compare first- and second-generation TKIs in terms of their effectiveness in preventing and treating CNS metastases. Prospective studies with patients of matched characteristics and regular brain images would be worthwhile for future research.
Supplemental Material
Supplemental material, Supp_Figure_1 for Preventing and treating brain metastases with three first-line EGFR-tyrosine kinase inhibitors in patients with EGFR mutation-positive advanced non-small cell lung cancer by Po-Lan Su, Yi-Lin Wu, Wei-Yuan Chang, Chung-Liang Ho, Yau-Lin Tseng, Wu-Wei Lai, Wu-Chou Su, Chien-Chung Lin and Szu-Chun Yang in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, Supp_Figure_2AB for Preventing and treating brain metastases with three first-line EGFR-tyrosine kinase inhibitors in patients with EGFR mutation-positive advanced non-small cell lung cancer by Po-Lan Su, Yi-Lin Wu, Wei-Yuan Chang, Chung-Liang Ho, Yau-Lin Tseng, Wu-Wei Lai, Wu-Chou Su, Chien-Chung Lin and Szu-Chun Yang in Therapeutic Advances in Medical Oncology
Acknowledgments
Po-Lan Su and Yi-Lin Wu contributed equally to this work. We are indebted to Yi-Ting Yen, Wen-Ping Su, Shang-Yin Wu, Yu-Ming Yeh, and Cheng-Hung Lee for their generous support with the recruitment of subjects. This study is based in part on data from the Cancer Data Bank of National Cheng Kung University Hospital.
Footnotes
Funding: This study was supported by the grant MOHW106-TDU-B-211-144-004 from the Ministry of Health and Welfare, Taipei, Taiwan; the grants MOST 104-2314-B-006-046-MY3 and MOST105-2314-B-076- MY2 from the Ministry of Science and Technology, Taipei, Taiwan; and the grant NCKUH-10503002 from National Cheng Kung University Hospital, Tainan, Taiwan.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Po-Lan Su, Department of Internal Medicine, National Cheng Kung University Hospital, Tainan, Taiwan; College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Yi-Lin Wu, Department of Nursing, National Cheng Kung University Hospital, Tainan, Taiwan; College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Wei-Yuan Chang, Department of Internal Medicine and Institute of Clinical Medicine, National Cheng Kung University Hospital, Tainan, Taiwan; College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Chung-Liang Ho, Department of Pathology, National Cheng Kung University Hospital, Tainan, Taiwan; College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Yau-Lin Tseng, Department of Surgery, National Cheng Kung University Hospital, Tainan, Taiwan; College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Wu-Wei Lai, Department of Surgery, National Cheng Kung University Hospital, Tainan, Taiwan; College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Wu-Chou Su, Department of Internal Medicine and Institute of Clinical Medicine, National Cheng Kung University Hospital, Tainan, Taiwan; College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Chien-Chung Lin, Department of Internal Medicine, National Cheng Kung University Hospital, 138 Sheng-Li Road, Tainan 704, Taiwan.
Szu-Chun Yang, Department of Internal Medicine, National Cheng Kung University Hospital, Tainan, Taiwan; Department of Public Health, National Cheng Kung University, Tainan, Taiwan; College of Medicine, National Cheng Kung University, Tainan, Taiwan.
References
- 1. D’Antonio C, Passaro A, Gori B, et al. Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol 2014; 6(3): 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li L, Luo S, Lin H, et al. Correlation between EGFR mutation status and the incidence of brain metastases in patients with non-small cell lung cancer. J Thorac Dis 2017; 9(8): 2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang WY, Wu YL, Su PL, et al. The impact of EGFR mutations on the incidence and survival of stages I to III NSCLC patients with subsequent brain metastasis. PLoS One 2018; 13(2): e0192161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edelman MJ, Belani CP, Socinski MA, et al. Outcomes associated with brain metastases in a three-arm phase III trial of gemcitabine-containing regimens versus paclitaxel plus carboplatin for advanced non-small cell lung cancer. J Thorac Oncol 2010; 5(1): 110–116. [DOI] [PubMed] [Google Scholar]
- 5. Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012; 77: 556–560. [DOI] [PubMed] [Google Scholar]
- 6. Schuler M, Wu YL, Hirsh V, et al. First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol 2016; 11: 380–390. [DOI] [PubMed] [Google Scholar]
- 7. Atagi S, Goto K, Seto T, et al. Erlotinib for Japanese patients with activating EGFR mutation-positive non-small-cell lung cancer: combined analyses from two phase II studies. Future Oncol 2016; 12: 2117–2126. [DOI] [PubMed] [Google Scholar]
- 8. Omuro AM, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 2005; 103: 2344–2348. [DOI] [PubMed] [Google Scholar]
- 9. Park K, Yu CJ, Kim SW, et al. First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: the ASPIRATION study. JAMA Oncology 2016; 2: 305–312. [DOI] [PubMed] [Google Scholar]
- 10. Yamamoto N, Goto K, Nishio M, et al. Final overall survival in JO22903, a phase II, open-label study of first-line erlotinib for Japanese patients with EGFR mutation-positive non-small-cell lung cancer. Int J Clin Oncol 2017; 22(1): 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li MX, He H, Ruan ZH, et al. Central nervous system progression in advanced non-small cell lung cancer patients with EGFR mutations in response to first-line treatment with two EGFR-TKIs, gefitinib and erlotinib: a comparative study. BMC Cancer 2017; 17: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen YL, Lu CC, Yang SC, et al. Verification of wild-type EGFR status in non-small cell lung carcinomas using a mutant-enriched PCR on selected cases. Mol Diagn 2014; 16: 486–494. [DOI] [PubMed] [Google Scholar]
- 13. Stish BJ, Pisansky TM, Harmsen WS, et al. Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J Clin Oncol 2016; 34(32): 3864–3871. [DOI] [PubMed] [Google Scholar]
- 14. Bajard A, Westeel V, Dubiez A, et al. Multivariate analysis of factors predictive of brain metastases in localised non-small cell lung carcinoma. Lung Cancer 2004; 45: 317–323. [DOI] [PubMed] [Google Scholar]
- 15. Batevik R, Grong K, Segadal L, et al. The female gender has a positive effect on survival independent of background life expectancy following surgical resection of primary non-small cell lung cancer: a study of absolute and relative survival over 15 years. Lung Cancer 2005; 47: 173–181. [DOI] [PubMed] [Google Scholar]
- 16. Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 2017; 17: 637–658. [DOI] [PubMed] [Google Scholar]
- 17. Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res 2012; 18(16): 4406–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bai H, Xiong L, Han B. The effectiveness of EGFR-TKIs against brain metastases in EGFR mutation-positive non-small-cell lung cancer. OncoTargets Ther 2017; 10: 2335–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuan FC, Li SH, Wang CL, et al. Analysis of progression-free survival of first-line tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring leu858Arg or exon 19 deletions. Oncotarget 2017; 8: 1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016; 17(5): 577–589. [DOI] [PubMed] [Google Scholar]
- 21. Liang SK, Hsieh MS, Lee MR, et al. Real-world experience of afatinib as a first-line therapy for advanced EGFR mutation-positive lung adenocarcinoma. Oncotarget 2017; 8: 90430–90443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hubbs JL, Boyd JA, Hollis D, et al. Factors associated with the development of brain metastases: analysis of 975 patients with early stage nonsmall cell lung cancer. Cancer 2010; 116: 5038–5046. [DOI] [PubMed] [Google Scholar]
- 23. Gaspar LE, Chansky K, Albain KS, et al. Time from treatment to subsequent diagnosis of brain metastases in stage III non-small-cell lung cancer: a retrospective review by the Southwest Oncology Group. J Clin Oncol 2005; 23(13): 2955–2961. [DOI] [PubMed] [Google Scholar]
- 24. Bai H, Han B. The effectiveness of erlotinib against brain metastases in non-small cell lung cancer patients. Am J Clin Oncol 2013; 36(2): 110–115. [DOI] [PubMed] [Google Scholar]
- 25. Deng Y, Feng W, Wu J, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol 2014; 2(1): 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sekine A, Satoh H, Iwasawa T, et al. Prognostic factors for brain metastases from non-small cell lung cancer with EGFR mutation: influence of stable extracranial disease and erlotinib therapy. Med Oncol 2014; 31(10): 228. [DOI] [PubMed] [Google Scholar]
- 27. Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol 2003; 21(12): 2237–2246. [DOI] [PubMed] [Google Scholar]
- 28. Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 2010; 16(23): 5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev 2014; 40(6): 716–722. [DOI] [PubMed] [Google Scholar]
- 30. Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol 2015; 10(1): 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tamiya A, Tamiya M, Nishihara T, et al. Cerebrospinal fluid penetration rate and efficacy of afatinib in patients with EGFR mutation-positive non-small cell lung cancer with leptomeningeal carcinomatosis: a multicenter prospective study. Anticancer Res 2017; 37(8): 4177–4182. [DOI] [PubMed] [Google Scholar]
- 32. Hata A, Katakami N. Afatinib for erlotinib refractory brain metastases in a patient with EGFR-mutant non-small-cell lung cancer: can high-affinity TKI substitute for high-dose TKI? J Thorac Oncol 2015; 10(7): e65–e66. [DOI] [PubMed] [Google Scholar]
- 33. Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16(2): 141–151. [DOI] [PubMed] [Google Scholar]
- 34. Benedettini E, Sholl LM, Peyton M, et al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am J Pathol 2010; 177(1): 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Preusser M, Berghoff AS, Ilhan-Mutlu A, et al. ALK gene translocations and amplifications in brain metastases of non-small cell lung cancer. Lung Cancer 2013; 80(3): 278–283. [DOI] [PubMed] [Google Scholar]
- 36. Hu W, Liu Y, Chen J. Concurrent gene alterations with EGFR mutation and treatment efficacy of EGFR-TKIs in Chinese patients with non-small cell lung cancer. Oncotarget 2017; 8(15): 25046–25054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang SC, Lin CC, Lai WW, et al. Dynamic changes in quality of life after three first-line therapies for EGFR mutation-positive advanced non-small-cell lung cancer. Ther Adv Med Oncol. Epub ahead of print 5 February 2018. DOI: 10.1177/1758834018755072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supp_Figure_1 for Preventing and treating brain metastases with three first-line EGFR-tyrosine kinase inhibitors in patients with EGFR mutation-positive advanced non-small cell lung cancer by Po-Lan Su, Yi-Lin Wu, Wei-Yuan Chang, Chung-Liang Ho, Yau-Lin Tseng, Wu-Wei Lai, Wu-Chou Su, Chien-Chung Lin and Szu-Chun Yang in Therapeutic Advances in Medical Oncology
Supplemental material, Supp_Figure_2AB for Preventing and treating brain metastases with three first-line EGFR-tyrosine kinase inhibitors in patients with EGFR mutation-positive advanced non-small cell lung cancer by Po-Lan Su, Yi-Lin Wu, Wei-Yuan Chang, Chung-Liang Ho, Yau-Lin Tseng, Wu-Wei Lai, Wu-Chou Su, Chien-Chung Lin and Szu-Chun Yang in Therapeutic Advances in Medical Oncology