Abstract
Objective:
Tert-butylhydroquinone (t-BHQ) protective effect against oxidative damage in thymus from malnourished pops-rats was evaluated.
Methods:
Malnutrition in pops-rats was induced during the lactation period and first-, second-, and third-degree malnourished rats were studied (MN1, MN2, and MN3). To determine t-BHQ protective effect, lipid peroxidation (LPx) was assessed, as well as the carbonyl content. The reduced glutathione and glutathione disulfide content were determined and antioxidant enzyme activities were measured.
Results:
Oxidative protein damage, LPx, and Nuclear Factor-κB (NF-κB) content, increased in the MN2 and MN3 compared to well-nourished rats, associated with lower protein content and antioxidant activity of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase. Tert-butylhydroquinone treatment induced a protective effect against lipids and proteins oxidative damage, as well as decrease in NF-κB in MN rats and restored the antioxidant mechanisms, mostly GPx and SOD. No differences were found between male and female animals.
Conclusions:
Results show that higher body weight deficit leads to increased oxidative damage and probably inflammation, attributable to alterations in antioxidant mechanisms. These effects were reversed by the t-BHQ-treatment, which restores the antioxidant response. Our findings suggest that t-BHQ could be an interesting pharmacological intervention, but it needs to be studied further.
Keywords: malnutrition, oxidative stress, antioxidants, t-BHQ, NF-κB
Introduction
Childhood malnutrition is defined as an imbalance between nutrient requirements and intake, which results in cumulative deficits of macro or micronutrients that negatively affect children growth and development.1 Malnutrition affects people all over the world, being the children younger than 5 years the most vulnerable. It has been estimated that 159 million children are stunted (low height for their age) and 50 million are wasted (low weight for their height).2 MN malnutrition also causes higher susceptibility to infections, increasing their frequency and severity. At the same time, infections also contribute to malnutrition, creating a vicious cycle between infection and nutritional status. Therefore, it is considered the leading cause of immunodeficiency3 and places malnourished children at greater risk of dying from infectious diseases. Nearly half of all deaths in children younger than 5 years are attributable to this condition,4 meaning that there are about 3 million unnecessary and unacceptable losses of lives every year.2
One common target organ in malnutrition and infection is the thymus.5 Alterations in this organ have been observed, both in children and in malnourished experimental models, mainly increased apoptosis, reduced thymic hormone activity in serum, mature thymocytes depletion, and decreased cell proliferation.6,7 Besides, MN has been linked to a deficient antioxidant defense system8 and increased DNA and lipid oxidative damage.9 It has been reported that in children with severe malnutrition have lower blood concentrations of Reduced glutathione (GSH)10 along with increased malondialdehyde (MDA) levels.8
Accordingly, there is considerable interest in developing therapeutic strategies to prevent oxidative damage and restoring antioxidant mechanisms in malnourished organisms. Hence, it has been proposed that these effects might be minimized with antioxidants supplementation.11 A synthetic antioxidant that has received great attention in the last years is the tert-butylhydroquinone (t-BHQ) that induces Nrf2 transcriptional activity, increases antioxidant enzymes expression, thus stimulating a protective effect against oxidative stress (OS).12 Reactive oxygen species can induce activation of transcription factors including related to OS protection13 and NF-κB, this latter involved in inflammatory response.14
Moreover, it is well established that the suckling period is critical for development of lymphoid system15,16; for this reason, we considered of uppermost importance to determine t-BHQ protective effect against oxidative damage in thymus from malnourished pops-rats.
Materials and Methods
Animals
Wistar albino nursing rats, from the closed breeding colony at the Universidad Autónoma Metropolitana (UAM)-Xochimilco, were maintained in the UAM-Iztapalapa under standard conditions (12-hour light/dark, temperature 22°C ± 3°C, with 45% Relative humidity [RH]). Nursing rats were fed with a rodent’s balanced diet (PMI 5001; Richmond, English River, Iowa) and water ad libitum. All procedures with animals were strictly carried out according to the NIH Guide for the care and use of laboratory animals, and the NOM 062-ZOO-1999.
Experimental Malnutrition
Experimental MN was induced during lactation by food competition, based on reducing the quantity of milk/pup by increasing the number of pups per nursing mother,17 as we described previously.9 All experimental groups (Well-nourished [WN] and MN) received 3 t-BHQ (75 mg/kg/d) doses dissolved in saline solution and administrated intraperitoneally (IP) from day 18 until day 20. Control (nontreated; NT) groups (WN and MN) received only saline solution. At the end of the weaning period, MN rats (NT and treated) were subclassified depending on the malnourish degree: MN1 body weight deficit between 10% and 24%; MN2 deficit between 25% and 39%; and MN3 body weight deficit >40%. All MN groups were compared to the age-matched WN rats’ body weight. At day 21 pups were euthanized by cervical dislocation; the thymus was removed and stored at −70°C until analysis.
Tert-Butylhydroquinone Treatment
To select an appropriate experimental t-BHQ dose to be used in the MN animals, a dose–response curve using different t-BHQ concentrations was performed (0, 25, 50, 75, and 100 mg/kg body weight). Tert-butylhydroquinone was administered via IP, from day 18 to 20 after birth, and thymus redox status Reduced/Oxidized glutathione (GSH/GSSG) was determined by high-performance liquid chromatography (HPLC).
GSH/GSSG Ratio Determination
The GSH and GSSG content were determined by the HPLC technique with some modifications.18
Lipid Peroxidation Assay
Lipid peroxidation (LPx) was assessed by the MDA formation, according to a previous report.19 The optical density was determined at 532 nm. Protein concentration was determined using a commercial Bradford reagent (Bio-Rad, Hercules, California), and a 1.41 mg/mL standard of Bovine serum albumin (BSA). The concentrations of MDA, expressed as µmol of MDA/mg of protein.
Protein Oxidation
One hundred milligrams of thymus tissue were processed. Proteins were isolated using the Tissue Protein Extraction Reagent (Thermo Scientific, Rockford, Illinois), with the protease inhibitor Complete-Mini (Roche Diagnostics, Mannheim, Germany). Total protein concentration was determined spectrophotometrically at 595 nm.
Carbonyl content was measured using an Oxyblot Protein Oxidation Detection Kit (Chemicon International, Temecula, California) following the manufacturer instructions. The quantification and densitometry analysis was done using a Kodak Molecular Imaging Software (version 4.5.1).
Western Blot
Proteins (30 μg) were separated on 12% Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Polyvinylidene difluoride (PVDF) membranes and probed with anti-superoxide dismutase (SOD), anti-glutathione peroxidase (GPx), anti-Catalase (CAT), anti-NF-κB p50 and p65 (Santa Cruz Biotechnology, Santa Cruz, California) dilution (1:1000). Membranes were washed 3 times with Tris-buffered solution (TBS)–Tween and incubated with a horseradish peroxidase-conjugated secondary antibody for 1 hour. The blots were developed using a chemiluminescence reagent. The proportion of proteins was quantified by densitometric analysis.
Antioxidant Enzymatic Activity
Protein was isolated from 100 mg of thymus as described. Superoxide dismutase activity was evaluated spectrophotometrically at 560 nm, through the xanthine/xantine oxidase system, based on protocols proposed.20,21 Glutathione peroxidase activity was assessed at 340 nm through Nicotinamide adenine dinucleotide phosphate (NADPH) monitoring of protocol described.22 CAT activity was quantified using an established protocol that evaluates H2O2 decrease at 240 nm.23
Statistical Analysis
Statistically significant differences among groups were established using the Duncan test. P values ≤.05 were considered as significant differences. Data are presented as mean ± standard error, and samples were evaluated in triplicates for each treatment (n = 3 female; and n = 3 male).
Results
Body and Thymus Weight in Rats
In all MN groups (treated and NT), rats exhibited a significant body weight deficit in comparison with WN ones MN rats’ weight deficits were established in comparison to the mean body weight of their respective WN group. No differences were found among the body and thymus weights obtained for the MN-t-BHQ with their respective NT groups, neither between male and female animals (Table 1).
Table 1.
Body and Thymus Weight Determined for WN and MN Male and Female Nontreated and t-BHQ-Treated Rats.
| n = 15 | WN | MN1 | MN2 | MN3 |
|---|---|---|---|---|
| Body weight (g) | ||||
| Group | 54.34 ± 0.61 | 45.39 ± 0.36a | 37.07 ± 0.4a | 29.39 ± 0.6a |
| Male (n = 8) | 54.50 ± 0.96 | 45.63 ± 0.41 | 37.22 ± 0.58 | 30.04 ± 0.58 |
| Female (n = 7) | 54.15 ± 0.89 | 45.14 ± 0.71 | 36.86 ± 0.68 | 29.03 ± 1.19 |
| Weight deficit (%) | – | 16 | 31 | 45 |
| Thymic weight (g) | ||||
| Group | 0.23 ± 0.006 | 0.20 ± 0.006a | 0.17 ± 0.009a | 0.11 ± 0.004a |
| Male (n = 8) | 0.247 ± 0.01 | 0.19 ± 0.01 | 0.18 ± 0.018 | 0.12 ± 0.004 |
| Female (n = 7) | 0.23 ± 0.007 | 0.20 ± 0.007 | 0.17 ± 0.009 | 0.11 ± 0.009 |
| Thymic deficit (%) | – | 11 | 21 | 49 |
| n = 21 | WN-t-BHQ | MN1-t-BHQ | MN2-t-BHQ | MN3-t-BHQ |
| Body weight (g) | ||||
| Group | 56.7 ± 0.68 | 44.52 ± 0.22a | 36.18 ± 0.37a | 27.51 ± 0.89a |
| Male (n = 11) | 56.14 ± 0.94 | 44.78 ± 0.35 | 35.56 ± 0.61 | 27.76 ± 0.28 |
| Female (n = 10) | 57.32 ± 0.95 | 44.25 ± 0.25 | 36.91 ± 0.38 | 27.42 ± 0.42 |
| Weight deficit (%) | – | 21 | 36 | 54 |
| Thymic weight (g) | ||||
| Group | 0.22 ± 0.04 | 0.17 ± 0.03a | 0.14 ± 0.09a | 0.098 ± 0.04a |
| Male (n = 11) | 0.23 ± 0.005 | 0.17 ± 0.009 | 0.14 ± 0.01 | 0.09 ± 0.002 |
| Female (n = 10) | 0.22 ± 0.003 | 0.17 ± 0.004 | 0.14 ± 0.01 | 0.09 ± 0.005 |
| Thymic deficit (%) | – | 18 | 35 | 54 |
Abbreviation: t-BHQ, tert-butylhydroquinone.
a Statistical significance based on their respective group WN (P < .05). The mean ± standard error is represented.
GSH/GSSG Ratio
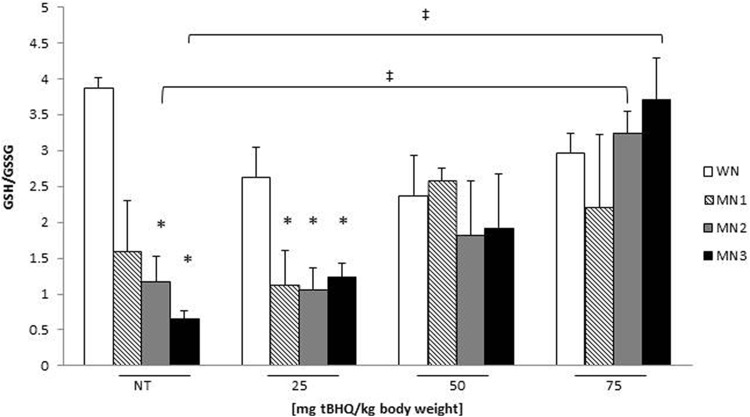
To select the optimal t-BHQ dose that modifies redox state (RS) in MN animals, redox status (GSH/GSSG ratio) was determined in WN (n = 12), MN1 (n = 12), MN2 (n = 12), and MN3 (n = 12) rats. Accordingly, doses of 25, 50, and 75 mg t-BHQ/kg body weight were administered to WN, MN1, MN2, and MN3 rats for 3 days. Figure 1 shows that MN2-NT and MN3-NT groups decreased the GSH/GSSG ratio 58% and 77% respectively, when compared to WN-NT group, demonstrating that MN modifies RS, shifting it to a more oxidized state. MN1, MN2, and MN3 groups treated with 25 mg t-BHQ showed 50% decreased in ratio compared to their WN-t-BHQ. Interestingly, MN2 and MN3 treated with 50 or 75 mg t-BHQ did not present changes compared to their WN-t-BHQ and maintained their redox status. However MN2 and MN3 treated 75 mg t-BHQ increased 2.8 and 5.8 times the ratio with respect to the MN2-NT and MN3-NT and even with similar values to WN-NT. Therefore, the t-BHQ 75 mg dose was chosen for further experiments. No significant differences were found among the t-BHQ-treated WN groups and WN-NT group.
Figure 1.
Thymus redox status in WN and MN rats treated with different t-BHQ doses 25, 50, and 75 mg t-BHQ/kg body weight. Bars represent the mean ± SE. *P < .05 significant differences between MN groups with different treatments in relation to their WN group. ‡ P < .05 statistical differences between treated groups with (75 mg t-BHQ) in relative to corresponding NT-MN group. NT indicates nontreated; SE, standard error; t-BHQ, tert-butylhydroquinone.
Oxidative Damage
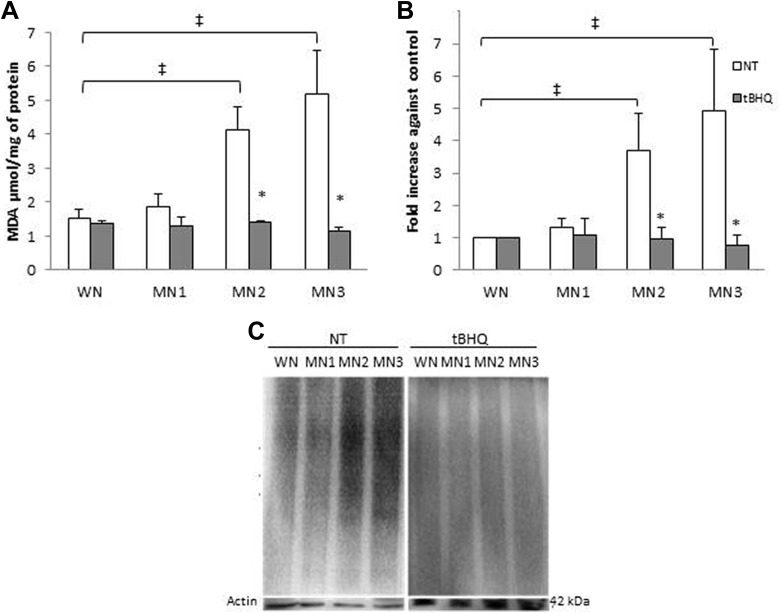
Lipid peroxidation and protein oxidation was determined in NT and t-BHQ treated rats. Figure 2A shows an increase for the MN2-NT and MN3-NT in more than 60%, suggesting that malnourishment promote LPx. When the NT and t-BHQ-treated groups were compared, the MN2-t-BHQ and MN3-t-BHQ showed less LPx, 66% and 80%, compared to their respective MN-NT group. MN-t-BHQ groups did not present differences with respect to the WN-t-BHQ and WN-NT, suggesting that t-BHQ treatment protects against LPx. Figure 2B shows the densitometric analysis normalized against the WN. Interestingly MN2-NT and MN3-NT groups presented up to 2.6 and 3.9 times oxidation compared to the WN-N, suggesting that the weight decrease might be associated with higher values of protein oxidative damage. The MN-t-BHQ groups did not present differences with respect to WN-t-BHQ and to WN-NT. That is to say, protein oxidative damage in those groups was prevented by t-BHQ treatment (73% and 84%, respectively). Figure 2C shows an Oxyblot representative blot. No differences in respect to oxidative damage were found between male and female rats (Table 2).
Figure 2.
Lipid peroxidation and protein oxidation obtained from t-BHQ and NT, WN, and MN rats. A, Lipid peroxidation. B, Protein oxidative damage, densitometric analysis. C, Representative Oxyblot; actin was used as an internal control. Each point represents the mean ± SE. *P < .05 statistical significance between MN-t-BHQ groups in relation to their MN-NT group. ‡ P < .05 statistical differences between MN-NT groups versus WN-NT. NT indicates nontreated; SE, standard error; t-BHQ, tert-butylhydroquinone.
Table 2.
Comparison Between WN and MN Female (F) and Male (M) Nontreated and t-BHQ Treated of the Determinations Different.
| Group/Determination | WN | WN-t-BHQ | MN1 | MN1-t-BHQ | MN2 | MN2-t-BHQ | MN3 | MN3-t-BHQ |
|---|---|---|---|---|---|---|---|---|
| LPx | ||||||||
| M* | 1.55 ± 0.4 | 1.2 ± 0.13 | 1.7 ± 0.4 | 1.2 ± 0.1 | 4.2 ± 1.1 | 1.3 ± 0.1 | 5.0 ± 2.2 | 1.06 ± 0.1 |
| F | 1.47 ± 0.2 | 1.43 ± 0.2 | 2.0 ± 0.3 | 1.3 ± 0.16 | 3.8 ± 0.9 | 1.42 ± 0.1 | 5.4 ± 1.7 | 1.1 ± 0.2 |
| Oxyblot | ||||||||
| M* | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.3 | 1.3 ± 0.7 | 3.9 ± 1.6 | 0.9 ± 0.4 | 4.3 ± 1.7 | 0.5 ± 0.2 |
| F | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.4 ± 0.4 | 0.7 ± 0.2 | 3.3 ± 0.7 | 1.0 ± 0.4 | 5.5 ± 2.3 | 0.9 ± 0.6 |
| SOD content | ||||||||
| M* | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.7 ± 0.2 | 1.0 ± 0.1 | 0.5 ± 0.1 | 1.3 ± 0.3 | 0.3 ± 0.0 | 0.6 ± 0.0 |
| F | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.91 ± 0.3 | 0.9 ± 0.2 | 0.6 ± 0.1 | 1.1 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.1 |
| GPx content | ||||||||
| M* | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.9 ± 0.5 | 1.0 ± 0.5 | 1.0 ± 0.5 | 0.5 ± 0.2 | 0.1 ± 0.0 | 1.1 ± 0.3 |
| F | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.6 ± 0.1 | 0.7 ± 0.0 | 0.7 ± 0.3 | 1.2 ± 0.4 | 0.2 ± 0.1 | 1.0 ± 0.1 |
| CAT content | ||||||||
| M* | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.3 | 0.7 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.2 |
| F | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.9 ± 0.1 | 1.0 ± 0.2 | 0.6 ± 0.0 | 0.7 ± 0.5 | 0.5 ± 0.0 | 0.5 ± 0.1 |
| NF-κb p50 content | ||||||||
| M* | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.2 ± 0.3 | 1.1 ± 0.1 | 1.1 ± 0.4 | 1.0 ± 0.3 | 1.3 ± 0.6 | 1.2 ± 0.4 |
| F | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.0 | 1.2 ± 0.0 | 1.0 ± 0.1 | 1.1 ± 0.3 |
| NF-κb p65 content | ||||||||
| M* | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.3 | 1.0 ± 0.5 | 1.2 ± 0.5 | 0.2 ± 0.1 | 1.1 ± 0.5 | 0.3 ± 0.1 |
| F | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.2 ± 0.3 | 1.0 ± 0.2 | 1.4 ± 0.3 | 0.2 ± 0.0 | 1.0 ± 0.2 | 0.5 ± 0.0 |
| Antioxidant activity (SOD) | ||||||||
| M* | 31.0 ± 7.1 | 17.9 ± 13.6 | 27.9 ± 9.8 | 99.4 ± 25.5 | 16.2 ± 2.5 | 85.9 ± 19 | 5.5 ± 2.3 | 80.5 ± 12 |
| F | 27.7 ± 4.9 | 26.3 ± 13.5 | 21.5 ± 3.6 | 88.2 ± 16.7 | 17.3 ± 1.9 | 72.7 ± 13 | 3.16 ± 1.2 | 77.2 ± 8.7 |
| Antioxidant activity (GPx) | ||||||||
| M* | 20.7 ± 6.4 | 11.4 ± 2.5 | 11.9 ± 3.3 | 27.5 ± 5.7 | 10.5 ± 2.2 | 25.9 ± 2.3 | 3.3 ± 2.48 | 26.4 ± 2.5 |
| F | 24.2 ± 6.6 | 16.5 ± 1.7 | 16.6 ± 5.9 | 35.0 ± 2.8 | 13.7 ± 3.3 | 28.6 ± 1.8 | 5.25 ± 2.3 | 29.8 ± 0.6 |
| Antioxidant activity (CAT) | ||||||||
| M* | 40.7 ± 12.1 | 41.3 ± 1.8 | 21.0 ± 6.8 | 34.0 ± 4.4 | 20.2 ± 3.1 | 33.3 ± 3.9 | 11.7 ± 3.8 | 39.0 ± 4.9 |
| F | 42.2 ± 9.1 | 40.6 ± 1.1 | 26.5 ± 9.0 | 32.8 ± 2.9 | 18.0 ± 2.9 | 31.3 ± 2.2 | 10.0 ± 1.5 | 35.9 ± 3.2 |
Abbreviations: GPx, glutathione peroxidase; LPx, lipid peroxidation; SOD, superoxide dismutase; t-BHQ, tert-butylhydroquinone.
*No differences were found between male and female rats of the same group.
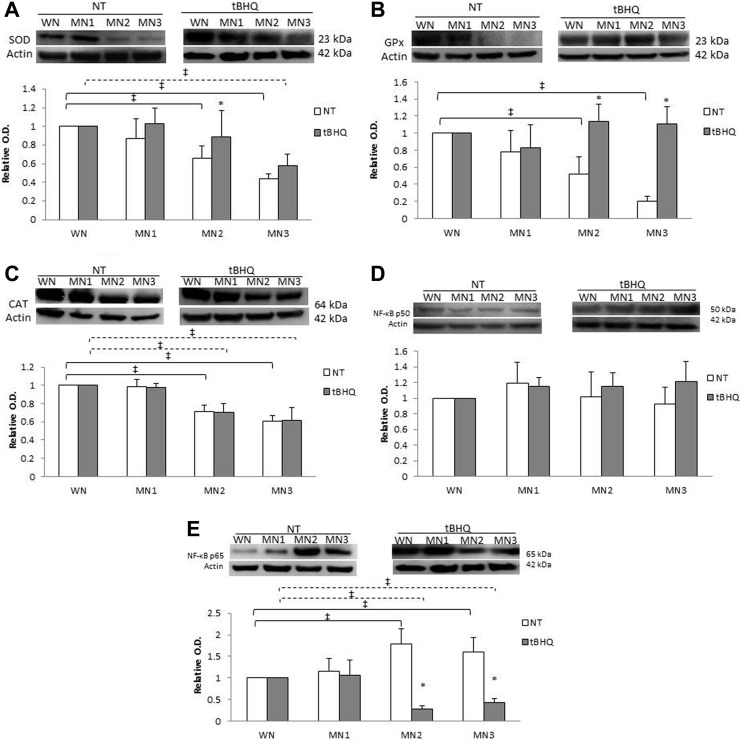
Antioxidant Enzymes and NF-κB Content
The data were normalized against WN-t-BHQ or WN-NT. Superoxide dismutase content (Figure 3A) exhibited differences between the MN2-NT and MN3-NT groups with a 35% and 57% reduction, respectively, compared to WN-NT. Comparing between NT and t-BHQ-treated groups, we observed that the MN2-t-BHQ increased SOD levels (27%) compared to MN2-NT. Glutathione peroxidase levels (Figure 3B) decreased in MN2-NT and MN3-NT in comparison to WN-NT (49% and 79%, respectively). No differences were found among the different t-BHQ-treated groups. However, when NT and t-BHQ-treated groups were compared, MN2-t-BHQ and MN3-t-BHQ increased GPx levels (54% and 82%, respectively) compared to their MN-NT group. Interestingly, CAT expression (Figure 3C) showed a decrease in MN2-NT and MN3-NT as well as the MN2-t-BHQ and MN3-t-BHQ groups (30% and 40%, respectively), compared to the WN-NT and WN-t-BHQ groups, suggesting that t-BHQ treatment did not have any effect on CAT content, unlike SOD and GPx. The content of NF-κB p50 (Figure 3D) does not show differences between the different t-BHQ-treated groups and NT. The NF-κB p65 content (Figure 3E) showed an increase in MN2-NT and MN3-NT of 77% and 60%, respectively, in comparison to WN-NT. For its part MN2-t-BHQ and MN3-t-BHQ had decrease of 73% and 58%, respectively, compared to WN-t-BHQ. Comparing NT and t-BHQ-treated groups, it was observed that NF-κB p65 expression showed a decrease in MN2-t-BHQ and MN3-t-BHQ by 85% and 74%, respectively, compared to their MN groups, suggesting probably that t-BHQ treatment has an effect against the inflammatory process. No differences in the antioxidant enzymes and NF-κB content were found between male and female animals (Table 2).
Figure 3.
Antioxidant enzymes, NF-κB p50 and p65 subunits. Representative blots performed for (A) SOD, (B) GPx, (C) CAT, (D) p50, and (E) p65. Relative optic density was normalized against WN-t-BHQ or WN-NT. Each point represents the mean ± SE. *P < .05 statistical significance between MN-t-BHQ in relation to their MN-NT group. ‡ P < .05 significant differences between MN-NT groups (black line) or MN-t-BHQ (dotted line) with respect to their WN. GPx indicates glutathione peroxidase; NT, nontreated; SE, standard error; SOD, superoxide dismutase; t-BHQ, tert-butylhydroquinone.
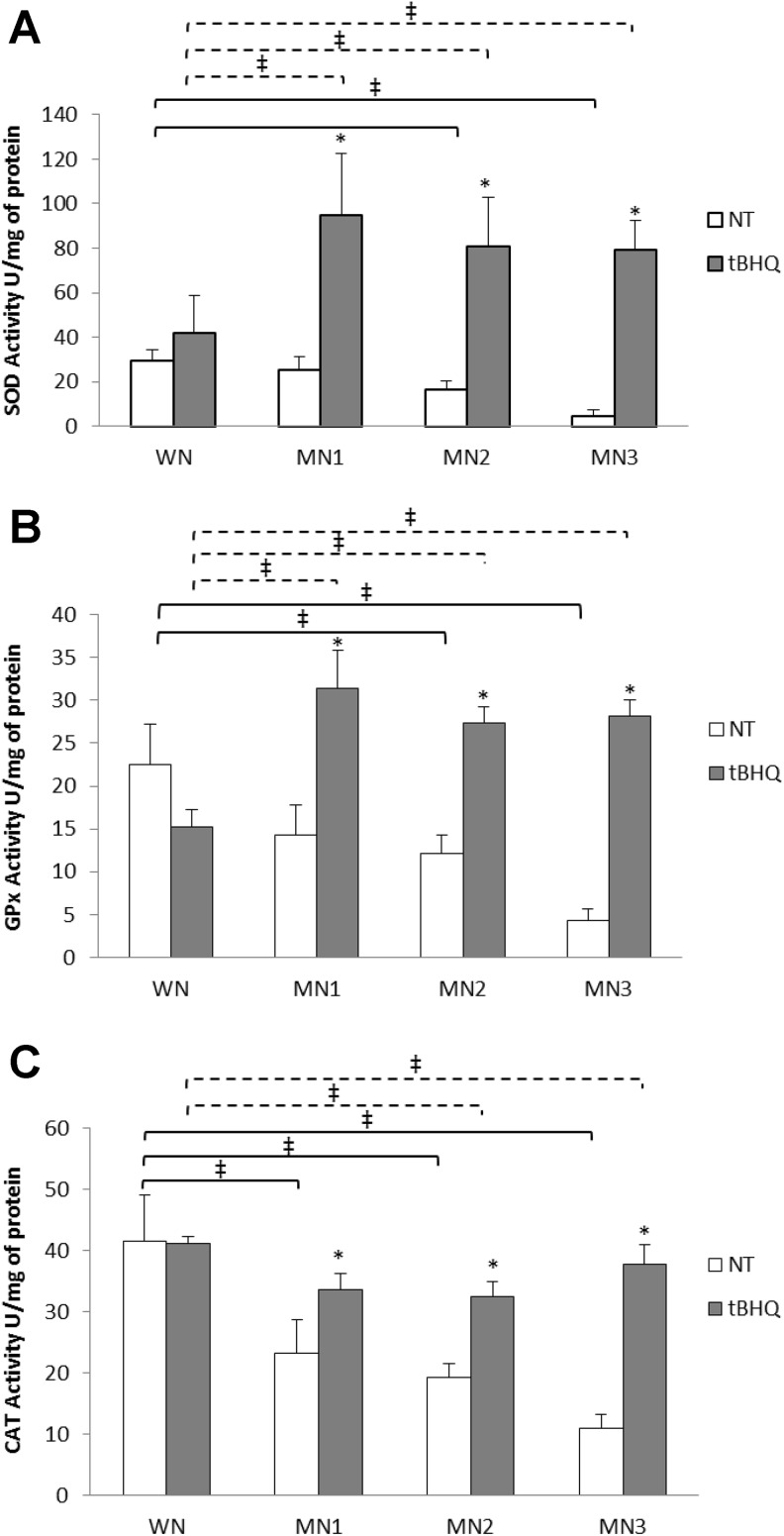
Antioxidant Enzyme Activity
Superoxide dismutase, GPx, and CAT enzymatic activity was quantified in WN, MN1, MN2, and MN3 t-BHQ-treated and NT groups (Figure 4). The SOD (Figure 4A) and GPx enzymatic activity (Figure 4B) exhibited differences for the MN2-NT and MN3-NT, which decreased 50% and 80%, respectively, compared to the WN-NT. The MN1-t-BHQ and MN2-t-BHQ groups increased 80% SOD activity, whereas MN3-t-BHQ increased 95% compared to their MN-NT groups. Besides, MN-t-BHQ groups augmented GPx activity more than 100% compared to their respective MN-NT group.
Figure 4.
Figure shows the enzymatic activity for (A) SOD, (B) GPx, and (C) CAT. Each point represents the mean ± SE. *P < .05 statistical significance between MN-t-BHQ groups in relation to their MN-NT group. ‡ P < .05 significant differences between MN-NT groups (black line) or MN-t-BHQ (dotted line) with respect to their WN. GPx indicates glutathione peroxidase; NT, nontreated; SE, standard error; SOD, superoxide dismutase; t-BHQ, tert-butylhydroquinone.
Additionally, CAT activity (Figure 4C) decreased 50% in the MN1-NT and MN2-NT, and MN3 70% in comparison to WN-NT. Although MN2-t-BHQ and MN3-t-BHQ decreased 20% and 10%, respectively, in comparison with WN-t-BHQ. Comparing NT and t-BHQ-treated groups, it was observed that MN2-t-BHQ and MN3-t-BHQ had an increase in CAT activity (56% and 85%, respectively) compared to their MN-NT groups. However, although CAT activity was enhanced with t-BHQ, this was not enough to reach the values observed in the WN groups. Interestingly, CAT was the most affected enzyme. No differences in antioxidant enzymatic activities were found between male and female rats (Table 2).
Discussion
It is well-documented that a decreased food intake, either in quantity or quality, mainly affects immunological defense mechanisms, favoring the increased sensitivity to infection.24 These mechanisms are regulated mostly by cells that differentiate and mature in lymphoid organs and that are highly susceptible to the effects MN, in particular the thymus.7
Of the various lymphoid tissues, thymus is histologically perhaps the most consistent across species and descriptions for one species generally substitute for that of others.25 Malnutrition impairs host immunity and is particularly detrimental to lymphoid organs, like the thymus.5 The thymus reacts to nutritional disorders more rapidly than other organs do, due to the rapid cell turnover that characterizes the lymphoid tissues. Menkel first described the relationship between the atrophy of the thymus and malnutrition in 1810. Then, Simon proposed the thymus as an early critical barometer of malnutrition and Vint and Watts described the characteristics of the thymus obtained in autopsies of children who died of kwashiorkor and marasmus malnutrition.26–29
Although thymic atrophy is one of the most notorious features in malnourished organisms, there are other alterations in this organ, such as decreased cell proliferation, increased apoptosis, together with alterations in antioxidant defense mechanisms.6,9 Here, a thymus deficit and body weight in MN rats were observed in the NT and t-BHQ-treated groups compared to their respective WN groups. These weight deficits were notable in MN3 independently of treatment. This indicates that the diminution in an organism body weight can be reflected on the weight of this particular organ.
The relative weight of the thymus reaches its maximal at about the time of puberty, after which it gradually decreases.30 The age-associated thymus involution follows a regular pattern for all individuals, although clear differences between the sexes have been described.31,32 In a study accomplished in monkeys, the thymic delineation of the cortex and the medulla was significantly decreased in the 7 to 15 years of age-group for males compared to female monkeys,33 whereas in mouse, the difference in the female and male thymus weight was more pronounced after 2 to 2.5 months of age, being the female mouse thymus heavier.30 Similarly, it was evidenced that thymic atrophy occurs at a differential rate in female and male mice, with a greater number of cells in thymus of female compared with male mice, up to the age of 9 months.34 This suggests better thymic function in females.35 It is very likely that the causes for these differences are a result of the hormonal milieu and, specifically, the effects of endogenous and/or exogenous sex steroids, as supported by findings in laboratory animals,36 from the onset puberty. However, other studies have shown that thymic size was similar in both male and female mice from birth to adulthood.37 In our study, no significant differences could be observed between females and males, probably due to the age of the offspring (21 days), where the endocrine changes are not so evident between both sexes, and the thymus has not yet reached its maximal size. However, it is very important to carry out studies in later stages of life, to identify whether malnutrition can affect differentially each gender.
It has been reported that food supplementation for 32 days with t-BHQ 0.001% (wt/wt in high-fat diet mice) and that t-BHQ oral administration reduced body weight gain in normal diet fed mice.38 In relation to this, in this study no differences were found in the body and thymus weight in the t-BHQ-treated groups compared to the NT. This suggests that the concentration and time period of t-BHQ administration are determinant factors that can impact on the organism body weight.
The evaluation of the GSH/GSSG ratio provides a reliable estimate of the cellular RS and is often measured as an OS indicator. Likewise, it has been shown that several pathological conditions where OS plays an important role are characterized by an imbalance in the GSH/GSSG ratio. So it is of great importance to maintain a strict regulation of this system because GSH deficiency puts cell at risk of oxidative damage.39 Due to the above and since there is no information about the possible protective effect that t-BHQ might induce in lactating organisms, a dose–response curve was done to determine the optimal experimental concentration at which the t-BHQ improves and/or maintain the RS in malnourished organisms. Our data showed that the GSH/GSSG ratio decrease in the MN2 and MN3 treated with t-BHQ 25 mg/kg and NT compared to their respective WN group; meaning that MN is linked with alterations in RS and that this dose did not prevent OS. MN groups treated with t-BHQ 50 mg/kg showed no difference with respect to the WN maintain the normal GSH/GSSG ratio. Although the t-BHQ 75 mg/kg dose caused an increase in the values in MN2 and MN3. Since the t-BHQ 75 mg/kg dose improved GSH/GSSG ratio that dose was chosen for the rest of the experiments in the rationale that it could probably lead to an improvement in antioxidant protection mechanisms and a decrease in OS. However, t-BHQ 100 mg/kg generated a toxic effect and caused cellular death (50%), probably modifying the cellular RS and consequently exerting a prooxidant role40 due to a reduction in the intracellular GSH levels and/or an increase in the electrophiles such as tBBQ that could deplete cellular GSH increasing cellular susceptibility to electrophilic attack and causing cell death.41
It has been argued that OS can lead to structural and functional modifications in different molecules that alter cells physiology and compromise the organism42; it is known that childhood MN increases LPx in blood and serum. Our results show that MN is clearly related to LPx rise in MN2-NT and MN3-NT, compared to the WN-NT group. Lipid peroxidation can bring serious consequences to MN organisms because it is known that LPx products such as MDA can modify cell membrane ionic permeability and augment protein damage,43 which are associated with edema pathogenesis in kwashiorkor MN.44 When MN rats were treated with t-BHQ, LPx levels were similar to the ones observed in the WN-NT group. The t-BHQ protective effect in experimental models has also been evidenced in other studies, where LPx decrease was observed when was exposed to cisplatin, a chemotherapeutic drug that causes nephrotoxicity.45
In regard to proteins oxidative damage, it has been reported that oxidized proteins in MN organisms increase in cerebellum, hippocampus, and cerebral cortex.46 In addition, in children with kwashiorkor MN, oxidized amino acids such as o,o′-dityrosine and ortho-tyrosine has been found in urinary.44 In this study, we observed an increase in protein oxidation in function of malnutrition degree MN3>MN2>MN1. Suggesting that body deficits greater than 25% may predispose to increased oxidative damage. Protein oxidation can promote proteolysis, denaturation, aggregation, loss of function, and even cell death.47 It should be noted that this damage decreased in MN2-t-BHQ and MN3-t-BHQ, compared to their MN-NT group. Hence, t-BHQ protected the animals also against protein oxidative damage. In other studies have shown similar results regarding t-BHQ protective in kidney of pretreated animals that were exposed to cisplatin or to ischemia–reperfusion.45,48
Several studies have determined the effect of MN on antioxidant defense mechanisms.46 In these studies, a decrease in the nonenzymatic antioxidant molecule levels has been reported; mainly in particular trace elements involved in oxidative damage protection such as Se and Cu.49
It has been shown that SOD serum activity and GPx activity in whole blood are reduced in MN3 children.50,51 Hence, another aim of this study was to determine the protective effect of t-BHQ on antioxidant mechanisms by assessing SOD, GPx, and CAT protein content and enzymatic activity. Our results showed a decrease in SOD content in the group MN3-t-BHQ compared to the WN-t-BHQ; interestingly SOD enzymatic activity was increased in all MN degrees t-BHQ-treated rats. In contrast, no changes in GPx content were found in MN t-BHQ-treated groups when compared to WN-t-BHQ group. This could be related in turn, with an improvement in their enzymatic activity in the same MN groups. Likewise, it has been shown that t-BHQ pretreatment in rats avoids nephrotoxicity by increasing GPx enzymatic activity.45 The increase in these antioxidant protection mechanisms may be related to the protective effect observed in proteins and lipids against oxidative damage in MN-t-BHQ-treated animals, due to a hormetic response generated by t-BHQ, which is capable of inducing a preconditioning cellular response. That is, when cells are subjected to low concentrations of a toxic agent they might generate an adaptive response, which protects them against the toxicity of that same agent at higher concentrations.52
In regard to CAT, its content decreased in the MN2-t-BHQ and MN3-t-BHQ and its activity was the most committed in all MN groups. This decrease is probably caused by iron deficiency, an element indispensable in their active site, which in turn could be associated with a ferritin deficiency, iron storage protein that is diminished in MN children.53 Another explanation may be due to a reduction in CAT synthesis rate as result of dietary restrictions in MN organisms.54
As mentioned above, t-BHQ is a known Nrf2 inductor, however, contrary to what was expected, no significant differences were found in this transcription factor content in the subcellular fractions analyzed (data not show), probably because Nrf2 distribution in the different cytoplasmic compartments is a fast temporal and spatial event,55 which did not coincide with our experimental determinations, that were carried out at the end of the treatment.
It has been shown that MN itself induces a low-grade inflammatory state,56 thus it is worthy to explore the transcription factor NF-κB implicated in this inflammatory response. Some studies have confirmed that NF-κB increased transcriptional activity is associated with the increase in the p65 subunit, both gene and protein expression. That reports coincide with the results obtained in this study. The p65 content increased in MN2-NT and MN3-NT organisms. Suggesting that NF-κB carried out its transcriptional activity57 and activated gene expression, including those involved in the inflammatory response such as TNF-α.58 In addition, it has been evidenced that in MN mice, peritoneal macrophages increased NF-κB activity compared to cells of WN.59 In contrast to p65 increase in the MN2-NT and MN3-NT, animals treated with t-BHQ showed a decrease in NF-κB. This in turn could indicate a decrease in the pro-inflammatory protein content.60 As proposed previously, 1% t-BHQ pretreatment for a week prior to traumatic brain injury markedly decreased NF-κB activation, inflammatory cytokines production, and even attenuated apoptosis frequency. For this reason, it has been suggested that inflammatory cytokines induction, such as TNF-α or IL-6, are inhibited by phenolic antioxidants, including t-BHQ. Thus, NF-κB is proposed as target for inhibition, specifically by blocking to formation of NF-κB-binding complex with DNA.61 Likewise, in the present study it can be suggested that t-BHQ may have anti-inflammatory effects in MN organisms and also be related to the oxidative damage reduction observed here, although this fact must be confirmed.
In summary, our study shows that MN in early stages in life is strongly associated with increased oxidative damage, which can directly contribute to the thymus dysfunction and consequently can potentially modify the immunologic response against infectious stimuli in these organisms. Interestingly, t-BHQ induced a protective effect against lipids and proteins oxidative damage in MN rats, by restoring their antioxidant protection mechanisms, mostly GPx and SOD. However, more experiments are needed before suggesting that t-BHQ might be a helpful molecule to help restoring MN dysfunctions. Likewise, we consider that the effects at the long run might be different between sexes, so a new study must be designed in order to evaluate these differences in stages after breastfeeding, where there should be more evident.
Acknowledgments
The authors wish to thank M.V.Z. Rocío González and Dra. Yvonne Heuze for their animal facilities. Rosas-Trejo and Toledo-Pérez are CONACYT scholarship holders.
Authors’ Note: A.L-.L., M.K., and G.G-.G. were involved in the design of the study, generation, collection, interpretation of data, and revision of the manuscript. M.d.l.Á.R-.T., E.G-.M., and R.T-.P. assisted in the collection and assembly of data. O.N-.M. was involved in analysis and interpretation of data, and revision of the manuscript. M.C.G-.T. was involved in design of the study, analysis of data, revision of the manuscript, and supervision of the investigation. All authors approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Rosas-Trejo and Toledo-Pérez are CONACYT scholarship holders.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Mehta NM, Corkins MR, Lyman B, et al. ; American Society for Parenteral and Enteral Nutrition Board of Directors. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37(4):460–481. [DOI] [PubMed] [Google Scholar]
- 2. United Nations Children’s Fund, World Health Organization, World Bank Group. Joint Child Malnutrition Estimates—Levels and Trends. New York, NY: UNICEF; Geneva, GE, Switzerland: WHO; Washington, WA: WBG; 2016. [Google Scholar]
- 3. Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46(10):1582–1588. [DOI] [PubMed] [Google Scholar]
- 4. Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition—a systematic review. PLoS One. 2014;9(8):e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Savino W, Dardenne M, Velloso LA, Dayse Silva-Barbosa S. The thymus is a common target in malnutrition and infection. Br J Nutr. 2007;98(S1):S11–S16. [DOI] [PubMed] [Google Scholar]
- 6. Ortiz R, Cortés E, Medina H. Malnutrition alters the rates of apoptosis in splenocytes and thymocyte subpopulations of rats. Clin Exp Immunol. 2008;155(1):96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Savino W, Dardenne M. Nutritional imbalances and infections affect the thymus: consequences on T-cell-mediated immune responses. Proc Nutr Soc. 2010;69(4):636–643. [DOI] [PubMed] [Google Scholar]
- 8. Aly GS, Shaalan AH, Mattar MK, et al. Oxidative stress status in nutritionally stunted children. Egypt Paediatr Assoc Gaz. 2014;62(1):28–33. [Google Scholar]
- 9. Gavia-García G, González-Martínez H, Miliar-García Á, et al. Oxidative damage and antioxidant defense in thymus of malnourished lactating rats. Nutrition. 2015;31(11-12):1408–1415. [DOI] [PubMed] [Google Scholar]
- 10. Becker K, Leichsenring M, Gana L, et al. Glutathione and associated antioxidant systems in protein energy malnutrition: results of a study in Nigeria. Free Radic Biol Med. 1995;18(2):257–263. [DOI] [PubMed] [Google Scholar]
- 11. Ghone RA, Suryakar AN, Kulhalli PM, et al. A study of oxidative stress biomarkers and effect of oral antioxidant supplementation in severe acute malnutrition. J Clin Diagn Res. 2013;7(10):2146–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varady J, Gessner DK, Most E, Eder K, Ringseis R. Dietary moderately oxidized oil activates the Nrf2 signaling pathway in the liver of pigs. Lipids Health Dis. 2012;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mann GE, Bonacasa B, Ishii T, Siow RC. Targeting the redox sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: protection afforded by dietary isoflavones. Curr Opin Pharmacol. 2009;9(2):139–145. [DOI] [PubMed] [Google Scholar]
- 14. Birben E, Sahiner UM, Sackeson C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pérez-Cano FJ, Castellote C, Marín-Gallén S, González-Castro A, Franch A, Castell M. Phenotypic and functional characteristics of rat spleen lymphocytes during suckling. Dev Comp Immunol. 2007;31(12):1264–1277. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. Infant and Young Child Feeding: Model Chapter for Textbooks for Medical Students and Allied Health Professionals. Geneva, GE, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 17. Ortiz R, Cortés E, Pérez L, et al. Assessment of an experimental method to induce malnutrition by food competition during lactation. Med Sci Res. 1996;24:843–846. [Google Scholar]
- 18. Gómez-Quiroz LE, Factor VM, Kaposi-Novak P, Coulouarn C, Conner EA, Thorgeirsson SS. Hepatocyte-specific c-Met deletion disrupts redox homeostasis and sensitizes to Fas-mediated apoptosis. J Biol Chem. 2008;283(21):14581–14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alarcón-Aguilar A, González-Puertos VY, Luna-López A, et al. Comparing the effects of two neurotoxins in cortical astrocytes obtained from rats of different ages: involvement of oxidative damage. J Appl Toxicol. 2014;34(2):127–138. [DOI] [PubMed] [Google Scholar]
- 20. Paoletti F, Aldinucci D, Mocali A, Caparrini A. A sensitive spectrophotometric method for the determination of superoxide dismutase in tissue extracts. Anal Biochem. 1986;154(2):536–541. [DOI] [PubMed] [Google Scholar]
- 21. Stirpe F, Della-Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem. 1969;244(14):3855–3863. [PubMed] [Google Scholar]
- 22. Ahmad S, Pardini RS. Evidence for the presence of glutathione peroxidase activity towards an organic hydroperoxidase in larvae of the cabbage looper moth, Trichoplusia ni. Insect Biochem. 1998;18(8):861–866. [Google Scholar]
- 23. Aebi H. Catalase in vitro. Method Enzymol. 1984;105:121–126. [DOI] [PubMed] [Google Scholar]
- 24. Rodríguez L, González C, Flores L, Jiménez-Zamudio L, Graniel J, Ortiz R. Assessment by flow cytometry of cytokine production in malnourished children. Clin Diagn Lab Immunol. 2005;12(4):502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haley PJ. Species differences in the structure and function of the immune system. Toxicology. 2003;188(1):49–71. [DOI] [PubMed] [Google Scholar]
- 26. Vint FW. Post-mortem findings in natives of Kenya. East Afr Med J. 1937;13:352. [Google Scholar]
- 27. Beisel WR. History of nutritional immunology: introduction and over view. J Nutr. 1992;122(suppl 3):591–596. [DOI] [PubMed] [Google Scholar]
- 28. Prentice A. The thymys: a barometer of malnutrition. Br J Nutr. 1999:81(5):345–347. [PubMed] [Google Scholar]
- 29. Pallaro AN, Roux ME, Slobodianik NH. Nutrition disorders and immunologic parameters: study of the thymus in growing rats. Nutrition. 2001;17(9):724–728. [DOI] [PubMed] [Google Scholar]
- 30. Pepper FJ. The effect of age, pregnancy and lactation on the thymus gland and lymph nodes of the mouse. J Endocrinol. 1961;22:335–348. [DOI] [PubMed] [Google Scholar]
- 31. Domínguez-Gerpe L, Rey-Méndez M. Evolution of the thymus size in response to physiological and random events throughout life. Microsc Res Tech. 2003;62(6):464–476. [DOI] [PubMed] [Google Scholar]
- 32. Dumont-Lagacé M, St-Pierre C, Perreault C. Sex hormones have pervasive effects on thymic epithelial cells. Sci Rep. 2015;5:12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spoor MS, Radi ZA, Dunstan RW. Characterization of age-and gender related changes in the spleen and thymus from control cynomolgus macaques used in toxicity studies. Toxicol Pathol. 2008;36(5):695–704. [DOI] [PubMed] [Google Scholar]
- 34. Aspinall R, Andrew D. Age-associated thymic atrophy is not associated with a deficiency in the CD44(+)CD25(-)CD3(-)CD4(-)CD8(-) thymocyte population. Cell Immunol. 2001;212(2):150–157. [DOI] [PubMed] [Google Scholar]
- 35. Pido-Lopez J, Imami N, Aspinall R. Both age and gender affect thymic output: more recent thymic migrants in females than males as they age. Clin Exp Immunol. 2001;125(3):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ackman JB, Kovacina B, Carter BW, et al. Sex difference in normal thymic appearance in adults 20-30 years of age. Radiology. 2013;268(1):245–253. [DOI] [PubMed] [Google Scholar]
- 37. Yellayi S, Teuscher C, Woods JA, et al. Normal development of thymus in male and female mice requires estrogen/estrogen receptor-α signaling pathway. Endocrine. 2000;12(3):207–213. [DOI] [PubMed] [Google Scholar]
- 38. Nam KW, Kim YH, Kwon HJ, Rhee SK, Kim WJ, Han MD. Tert-butylhydroquinone reduces lipid accumulation in C57BL/6 mice with lower body weight gain. Arch Pharm Res. 2013;36(7):897–904. [DOI] [PubMed] [Google Scholar]
- 39. Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57(3-4):145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gharavi N, Haggarty S, El-Kadi A. Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr Drug Metab. 2007;8(1):1–7. [DOI] [PubMed] [Google Scholar]
- 41. Okubo T, Yokoyama Y, Kano K, Kano I. Cell death induced by the phenolic antioxidante tert-butylhydroquinone and its metabolite tert-butylquinone in human monocytic leukemia U937 cells. Food Chem Toxicol. 2003;41(5):679–688. [DOI] [PubMed] [Google Scholar]
- 42. González-Torres MC, Betancourt-Rule M, Ortiz-Muñiz R. Daño oxidativo y antioxidantes. Bioquimia. 2000;25(1):3–9. [Google Scholar]
- 43. Okunade WG, Olorunsogo OO. Effect of reactive oxygen species on the erythrocyte calcium-pump function in protein-energy malnutrition. Biosci Rep. 1992;12(6):433–443. [DOI] [PubMed] [Google Scholar]
- 44. Manary MJ, Leeuwenburgh C, Heinecke JW. Increased oxidative stress in kwashiorkor. J Pediatr. 2000;137(3):421–424. [DOI] [PubMed] [Google Scholar]
- 45. Pérez-Rojas JM, Guerrero-Beltrán CE, Cruz C, Sánchez-González DJ, Martínez-Martínez CM, Pedraza-Chaverri J. Preventive effect of tert-butylhydroquinone on cisplatin-induced nephrotoxicity in rats. Food Chem Toxicol. 2011;49(10):2631–2637. [DOI] [PubMed] [Google Scholar]
- 46. Feoli AM, Siqueira IR, Almeida L, et al. Effects of protein malnutrition on oxidative status in rat brain. Nutrition. 2006;22(2):160–165. [DOI] [PubMed] [Google Scholar]
- 47. Burton GJ, Jauniaux E. Oxidative Stress. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guerrero-Beltrán CE, Tapia E, Sánchez-González DJ, Martínez-Martínez CM, Cristobal-García M, Pedraza-Chaverri J. Tert-butylhydroquinone pretreatment protects kidney from ischemia-reperfusion injury. J Nephrol. 2012;25(1):84–89. [DOI] [PubMed] [Google Scholar]
- 49. Ashour MN, Salem SI, El-Gabdan HM, Elwan NM, Basu TK. Antioxidant status in children with protein-energy malnutrition (PEM) living in Cairo, Egypt. Eur J Clin Nutr. 1999;53(8):669–673. [DOI] [PubMed] [Google Scholar]
- 50. Thakur S, Gupta N, Kakkar P. Serum cooper and zinc concentrations and their relation to superoxide dismutase in severe malnutrition. Eur J Pediatr. 2004;163(12):742–744. [DOI] [PubMed] [Google Scholar]
- 51. Akinola F, Oguntibeju O, Alabi O. Effects of severe malnutrition on oxidative stress in Wistar rats. Sci Res Essays. 2010;5(10):1145–1149. [Google Scholar]
- 52. López-Diazguerrero NE, González PV, Hernández-Bautista R, et al. Hormesis: lo que no mata, fortalice [in Spanish]. Gac Med Mex. 2013;149(4):438–447. [PubMed] [Google Scholar]
- 53. Gutiérrez-Rodríguez C, Trujillo-Hernández B, Martínez-Contreras A, Pineda-Lucatero A, Millán-Guerrero RO. Frecuencia de helmintiasis intestinal y su asociación con deficiencia de hierro y desnutrición en niños de la región occidente de México [in Spanish]. Gac Med Méx. 2007;143(4):297–300. [PubMed] [Google Scholar]
- 54. Albretch R, Pelissier MA. About the oxidative stress status in children with kwashiorkor. Food Chem Toxic. 1995;33(12):1081–1083. [DOI] [PubMed] [Google Scholar]
- 55. Srivastava S, Alfieri A, Siow RC, Mann GE, Fraser PA. Temporal and spatial distribution of Nrf2 in rat brain following stroke: quantitation of nuclear to cytoplasmic Nrf2 content using a novel immunohistochemical technique. J Physiol. 2013;591(14):3525–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ling PR, Smith R, Kie S, Boyce P, Bistrian BR. Effects of protein malnutrition on IL-6 mediated signaling in the liver and the systemic acute-phase response in rats. Am J Physiol Integer Comp Physiol. 2004;287(4):R801–R808. [DOI] [PubMed] [Google Scholar]
- 57. Visconti R, Ceruttil J, Battista S, et al. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFkB p65 protein expression. Oncogene. 1997;15(16):1987–1994. [DOI] [PubMed] [Google Scholar]
- 58. Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. [DOI] [PubMed] [Google Scholar]
- 59. Fock RA, Rogero MM, Vinolo MA, Curi R, Borges MC, Borelli P. Effects of protein-energy malnutrition on NF-kappaB signalling in murine peritoneal macrophages. Inflammation. 2010;33(2):101–109. [DOI] [PubMed] [Google Scholar]
- 60. Jin W, Ni H, Dai Y, et al. Effects of tert-butylhydroquinone on intestinal inflammatory response and apoptosis following traumatic brain injury in mice. Mediators Inflamm. 2010;2010:502564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ma Q, Kinner K, Ye J, Chen BJ. Inhibition of nuclear factor kappaB by phenolic antioxidants: interplay between antioxidant signaling and inflammatory cytokine expression. Mol Pharmacol. 2003;64(2):211–219. [DOI] [PubMed] [Google Scholar]