Abstract
Arachidonic acid (C20:4) is related to a wide range of biological effects including lipid homeostasis. The fatty acid desaturase-2 (FADS2) gene encodes for the delta-6-desaturase, which is involved in the biosynthesis of C20:4 from linoleic acid (C18:2). The purpose of this study was to characterise mutations in the promoter of the porcine FADS2, evaluating in particular the effect of one haplotype tagging polymorphism (rs321384923A > G) on the biosynthesis pathway of C20:4. A total of 1,192 Duroc barrows with records on fatty acid composition in muscle and subcutaneous fat were genotyped. Pigs carrying the A allele showed, irrespective of fat content, both enhanced FADS2 expression and higher C20:4 in muscle and exhibited increased ratios of C20:4 to C18:2 and of C20:4 to eicosadienoic acid (C20:2) in both muscle and adipose tissue. Despite the inverse relationship observed between C20:4 and fat content, the rs321384923 polymorphism had no impact on lean weight. It is concluded that the haplotype encompassing the rs321384923 polymorphism at the porcine FADS2 affects the n-6 fatty acid profile by specifically modifying the desaturation efficiency of C18:2 to C20:4 rather than by concomitant variations in C18:2 following changes in fat content.
Introduction
Arachidonic acid (C20:4, all-cis-5,8,11,14–20:4) is the precursor of several bioactive lipid mediators of the eicosanoid family related to a wide range of biological effects including lipid homeostasis and inflammatory response. C20:4 is essential in many organs such as liver and brain, where it is one of the most abundant fatty acids. In skeletal muscle, C20:4 promotes myocyte growth both in vitro1 and in vivo2 through the Akt/mTOR pathway. Mainly esterified into phospholipids, it also exerts a substantial contribution to maintaining membrane fluidity and in cell signalling. The C20:4 content differs between lipid classes, tissues and muscles and is influenced by both the diet and the individual’s genetic background3,4. Diets rich in C20:4 or diets producing relatively high levels of linoleic acid (C18:2, all-cis-9,12–18:2) result in enhanced levels of C20:4 in plasma2,5 and muscle2,6. Although C20:4 can be taken up from the diet, it can also be synthesised in the animal. There is evidence indicating that the biosynthesis of C20:4 is genetically mediated, notably in pigs, where substantial genetic variation between4 and within genetic types7 has been reported.
The delta-6-desaturase enzyme, encoded by the fatty acid desaturase-2 (FADS2) gene, is responsible for the first and rate-limiting step in the biosynthesis of C20:4 (Fig. 1), where C18:2 is desaturated to γ-linolenic acid (all-cis-6,9,12–18:3)8. The lack of FADS2 leads to obesity resistance and, as reported by Stoffel et al.9 using auxotrophic mice mutants, it may activate a surrogate reaction in which C18:2 is elongated to eicosadienoic acid (C20:2, all-cis-11,14–20:2) and then to eicosatrienoic acid (all-cis-5,11,14–20:3) but not to C20:4. In pigs, FADS2 is located in chromosome 2 (2:9632454–9667044:-1) as a part of a cluster including FADS1 and FADS3. The genomic structure of the gene is comprised of 12 exons and 11 introns (assembly Sscrofa11.1), which produce three protein-coding splice variants. The activity of FADS2 in the synthesis of long chain fatty acid is enhanced by an alternative transcript of pig FADS110, as also reported in baboons and humans11. In a recent genome-wide association study with five divergent pig populations, Zhang et al.12 provide evidence that in Erhualian pigs the region containing FADS2 was associated with the C20:4 content in muscle. Although an uncharacterised polymorphism in exon 3 of the pig FADS2 has been associated with C20:4 and intramuscular fat (IMF) content13, the sequence variation of FADS2 has not been otherwise investigated. FADS2 is a TATA-less gene and such genes are often subjected to complex transcription mechanisms. This makes the promoter region of FADS2 a sensible location for screening for DNA polymorphisms. The aim of our study was to describe genetic variants in the promoter of the porcine FADS2 and then to further investigate their association with C20:4 and fat content in the main lipogenic tissues. To this end, we made use of a biorepository of fat, muscle and liver specimens from a high-fat Duroc pig line where at least two relevant genes for fatty acid composition are also segregating14,15.
Figure 1.
The role of FADS2 in the biosynthesis of arachidonic acid from linoleic acid. The fatty acid desaturase-2 (FADS2, Δ-6 desaturase) catalyses the first step for the biosynthesis of arachidonic acid (all-cis-5,8,11,14–20:4), in which linoleic acid (all-cis-9-12-18:2) is desaturated to γ-linolenic acid (all-cis-6,9,12–18:3) and then elongated into dihomo-γ-linolenic acid (all-cis-8,11,14–20:3). Alternatively, linoleic acid is elongated into eicosadienoic acid (all-cis-11,14–20:2), which in turn can be either desaturated to eicosatrenoic acid (all-cis-5,11,14–20:3) via fatty acid desaturase-1 (FADS1, Δ-5 desaturase) or to dihomo-γ-linolenic acid (all-cis-8,11,14–20:3) via FADS2 (Δ-8 desaturase). The arachidonic acid is finally synthetized by desaturating dihomo-γ-linolenic acid via FADS1.
Materials and Methods
Ethics Statement
All pigs used in the study were raised and slaughtered in commercial units following applicable regulations and good practice guidelines on the protection of animals kept for farming purposes, during transport and slaughter. The experimental protocol was approved by the Ethical Committee on Animal Experimentation of the University of Lleida.
Animals and phenotypes
A total of 1,192 barrows from 159 sires and 590 dams of the same Duroc line were used in this experiment. Pigs were raised in 20 batches between 2002 and 2016 following a similar standard protocol for data recording and tissue sampling16. In each batch, pigs were raised from 75 days of age until slaughter at 210 days in the same farm under identical conditions. Pigs had ad libitum access to commercial feed (Esporc, Riudarenes, Girona, Spain). From 160 days of age onwards they were fed a finishing diet including around 6.0% fat (27% C18:2 and 0.3% C20:4 of total fatty acids). All pigs were slaughtered in the same abattoir, where carcass weight and carcass backfat and loin thickness at 6 cm off the midline between the third and fourth last ribs were measured by an on-line ultrasound automatic scanner (AutoFOM, SFK-Technology, Denmark). Immediately after slaughter, samples of semimembranosus (SM, n = 187) muscle, subcutaneous adipose tissue (n = 388) and liver (n = 118) were collected, snap-frozen, and stored at −80 °C. After chilling for about 24 h at 2 °C, samples of the muscles gluteus medius (GM, n = 1,179) and longissimus thoracis (LM, n = 548) were collected, vacuum packaged, and stored at −20 °C. Intramuscular and liver fat content, as well as fatty acid composition, were determined in duplicate by quantitative gas chromatography17. The proportion of C18:2, C20:2 and C20:4 were expressed as percentages relative to total fatty acid content (14:0; 16:0; cis-9–16:1; 18:0; cis-11–18:1; cis-9–18:1; C18:2, all-cis-9,12,15–18:3; 20:0; cis-13–20:1; C20:2; and C20:4) and their ratios calculated as indicators of FADS2 activity.
Genotyping
Genomic DNA was isolated from GM muscle samples using a standard protocol. The proximal promoter of the FADS2 (~1 Kb) was amplified and sequenced in a subset of 14 pigs with high or low C20:4 content in SM (Supplementary Table S1) with primers and conditions detailed in Supplementary Table S2. An RFLP-PCR genotyping protocol was set up to genotype the rs321384923A > G substitution. PCRs were carried out in 13 μL reactions containing 60 ng of genomic DNA, 1x buffer, 200 nM of dNTP mix, 2 mM of MgCl2, 500 nM of each primer and 1 U of Taq polymerase (Bioline). Thermocycling conditions were 95 °C 10 min, 35 cycles of 95 °C 20 sec, 56 °C 20 sec and 72 °C 20 sec finishing with 72 °C 5 min. Ten μl of PCR were digested with AvaI (37 °C × 3 h) and solved by electrophoresis in agarose gels. Additionally, two other SNPs known to influence fat content and composition in our resource Duroc line (the AY487830:g.2228 T > C SNP at the stearoyl-CoA desaturase (SCD) gene on chromosome 14 and the NM_001024587:g.1987C > T SNP at the leptin receptor (LEPR) gene on chromosome 6) were genotyped as described in Ros-Freixedes et al.15
FADS2 expression
Total RNA from 70 SM samples from two batches (AA, n = 14; AG, n = 26; GG, n = 30) and 31 livers from one batch (AA, n = 2; AG, n = 15; GG, n = 14) was isolated with TRI-Reagent (Sigma-Aldrich) following the manufacturer’s indications. Purity of the RNA was assessed by spectrophotometry with a Nanodrop-1000 and the integrity was tested by electrophoresis in agarose gels. FADS2 and two reference genes, YWHAZ and RPL32, were analysed by a quantitative PCR assay (Supplementary Table S3). Briefly, 2 μg of total RNA were reverse transcribed using SuperScript IV Reverse Transcriptase (Invitrogen) with oligo-dT and random primers. Real-time PCR assays were carried out in triplicate in 8 μl reactions, containing 1x iTaq Universal SYBR Green Supermix (Bio-Rad), 200 nM of each primer and 3 μl cDNA template diluted 1:30 in water. Cycling parameters were 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min, followed by melt curve analysis. To quantify and normalise the FADS2 expression data, we used the ΔΔCt method against the geometrical mean of the two reference genes18.
Statistical analyses
The association analysis of FADS2 genotypes with C18:2, C20:2, C20:4 and their ratios was performed using a mixed model including the batch (20 levels for fatty acids), the FADS2 genotype (3 levels), the SCD genotype (3 levels) and the LEPR genotype (3 levels) as fixed effects and the sire and the dam as random effects, with fat content as a covariate (IMF for muscle, backfat thickness for subcutaneous fat and fat content for liver). The same model was used for gene expression (without the covariate) and for carcass traits (with the age at slaughter as a covariate instead of fat content). Additivity was tested replacing the genotype effect by the covariate [1, 0, −1] for the AA, AG, and GG genotypes, respectively. The effects of the FADS2 genotype and additivity were tested using the F-statistic while the pairwise differences among FADS2 genotypes were contrasted with the Tukey-HSD test. Results are presented as least-square means ± standard error and were considered statistically significant at P < 0.05. The non-linear relationship of IMF with C20:4 was assessed regressing the reciprocal term of IMF on C20:4. All models were solved using the JMP Pro 12 package (SAS Institute Inc., Cary, NC).
Results
Sequence variability at the FADS2 promoter
A total of 5 SNP polymorphisms and one 12 bp insertion were segregating in the proximal promoter of the pig FADS2 gene (Supplementary Fig. S1). Among them, three SNPs (rs336076510, rs321384923 and rs331050552 at positions -676, -706 and -798 bp upstream the ATG codon, respectively) were fully linked forming two haplotypes (AAT and GGC). The stability of the two haplotypes was confirmed by genotyping the three SNPs in a subset of 51 pigs evenly distributed across haplotypes (data not shown). The middle SNP, rs321384923A > G substitution, was selected for further analysis as it modified a potential retinoic acid/oestrogen related receptors (TGCCCG) binding site while no potential transcription factor binding sites were detected in the other two SNP sites. While human and rat FADS2 expression responds to oestrogen hormone19 and vitamin A20, identification of causal mutations has been hindered by the presence of clusters of polymorphisms in strong linkage disequilibrium both upstream and downstream of the translation start site21.
FADS2 rs321384923 genotype frequencies
The frequencies of the FADS2 rs321384923 genotypes by SCD and LEPR genotypes are given in Supplementary Table S4. The A allele was the minor allele (frequency of 30.9%). The g.2228 T > C SCD and the g.1987C > T LEPR SNPs were both segregating at intermediate frequencies (46.1% and 43.5% for the T allele, respectively). All possible genotypes for the three SNPs were observed and, as expected for genes in different chromosomes, they were in linkage equilibrium (r2 < 0.005, for all pairwise linkage disequilibrium between SNPs).
FADS2 genotype and FADS2 expression
FADS2 expression was determined in SM on pigs of the three FADS2 rs321384923 genotypes (Fig. 2). The expression analysis was only performed in SM, since it was not possible to obtain GM and LM samples immediately after slaughter. The relative gene expression of FADS2 in muscle was 2-fold higher in the AA genotype as compared to the GG genotype (2.34 vs 1.10, P < 0.01). As evidenced by the allele substitution effect (0.63 ± 0.18, P < 0.01), heterozygous pigs displayed intermediate levels of gene expression. Results of FADS2 expression in liver confirmed the same trend, with pigs carrying the A allele (AA and AG) showing higher expression than the GG pigs (2.83 vs 1.38, P < 0.05). From this foundation, we proceed to explore the possible functional consequences caused by the higher expression of the A allele at the porcine FADS2.
Figure 2.
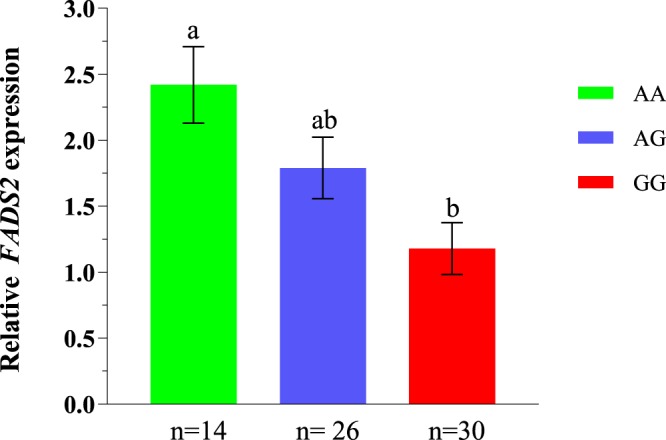
Relative FADS2 mRNA expression in muscle by rs321384923 genotype. The FADS2 gene expression in the semimembranosus muscle was around two-fold higher for the AA genotype as compared to the GG genotype. The number of pigs (n) per genotype ranged from 14 to 30. Error bars represent standard errors. Means with different superscripts differ significantly (P < 0.05).
FADS2 genotype and arachidonic acid
The effect of the FADS2 rs321384923 was first assessed in GM (Table 1). The results are presented adjusted for IMF, but those unadjusted led to similar conclusions. AA pigs had 12.5% more C20:4 and 6.1% less C20:2 in GM than GG pigs. As suggested by the expression data, the FADS2 SNP displayed an additive behaviour, with a G to A allele substitution effect of 0.09 ± 0.02, for C20:4 (P < 0.001), and -0.13 ± 0.03, for C20:2 (values x 10; P < 0.001). The same trend was observed when fatty acids were quantitatively expressed in mg/g of muscle. These values, although only accounting for about 2% of the total variance of these two fatty acids (2.2% for C20:4 and 1.7% for C20:2), provide support to the hypothesis that there exists genetic variation in the promoter region of the FADS2 that impacts n-6 fatty acid biosynthesis in pigs. This was confirmed by analysing the indicator ratios of FADS2 activity (Table 1). Regarding the C20:4 to C18:2 ratio, which can be interpreted as the overall efficiency of transforming C18:2 to C20:4, AA pigs were 12.0% more efficient than GG pigs. Interestingly, for the alternative route that converts C18:2 into C20:2 (C20:2/C18:2 ratio), AA pigs were 2.9% less efficient than GG pigs. However, since FADS2 also acts in the desaturation pathway from C20:2 to C20:4, a supplementary effect of the FADS2 SNP on C20:4 is expected to occur over this route, with the A allele further enhancing the synthesis of C20:4 and the G allele accumulating more C20:2. This effect was highlighted by the relatively greater differences by genotype for the C20:4/C20:2 ratio, which was 17.8% higher in AA pigs than in GG pigs, explaining up to 5.8% of the total variance of the ratio. On the other hand, C18:2, the primary substrate in the endogenous metabolism of C20:4, should decrease with increased FADS2 activity. We only were able to detect this effect when C18:2 was expressed in mg/g of muscle instead of as a percentage of total fatty acids. Then, as expected, AA pigs showed the lowest value of C18:2 (15.4 mg, 16.0 mg and 16.0 mg for AA, AG and GG, respectively, P < 0.05).
Table 1.
Effect of the FADS2 rs321384923 genotype on n6-fatty acid composition.
| Trait | P-valuea | FADS2 b | ||
|---|---|---|---|---|
| AA | AG | GG | ||
| Backfat, mm | 0.99 | 26.3 ± 0.4 | 26.3 ± 0.3 | 26.3 ± 0.2 |
| IMF, % dry matter | 0.10 | 18.0 ± 0.5 | 18.7 ± 0.3 | 19.1 ± 0.3 |
| C18:2, % | 0.13 | 10.06 ± 0.13 | 10.28 ± 0.07 | 10.33 ± 0.07 |
| C20:2, % (x10) | <0.001 | 4.65 ± 0.07a | 4.85 ± 0.04b | 4.95 ± 0.04b |
| C20:4, % | <0.001 | 1.62 ± 0.04a | 1.55 ± 0.02a | 1.44 ± 0.02b |
| C20:4/C18:2 (x10) | <0.001 | 1.59 ± 0.03a | 1.52 ± 0.02a | 1.42 ± 0.02b |
| C20:2/C18:2 (x100) | 0.004 | 4.70 ± 0.04a | 4.77 ± 0.02a | 4.84 ± 0.02b |
| C20:4/C20:2 | <0.001 | 3.51 ± 0.07a | 3.25 ± 0.04b | 2.98 ± 0.04c |
As compared to the GG pigs, the AA pigs showed a higher content of arachidonic acid (C20:4) and a lower content of eicosadienoic acid (C20:2) in muscle because they were more efficient transforming linoleic acid (C18:2) into C20:4. Subcutaneous fat was measured in terms of backfat thickness and intramuscular fat was determined in gluteus medius muscle. The proportion of each fatty acid is expressed as a percentage relative to total fatty acid content and, as well as ratios, adjusted for intramuscular fat (IMF) content. aP-value associated with the effect of the FADS2 genotype; bPairwise comparisons of FADS2 genotypes. cWithin row, means with different superscripts differ significantly (P < 0.05).
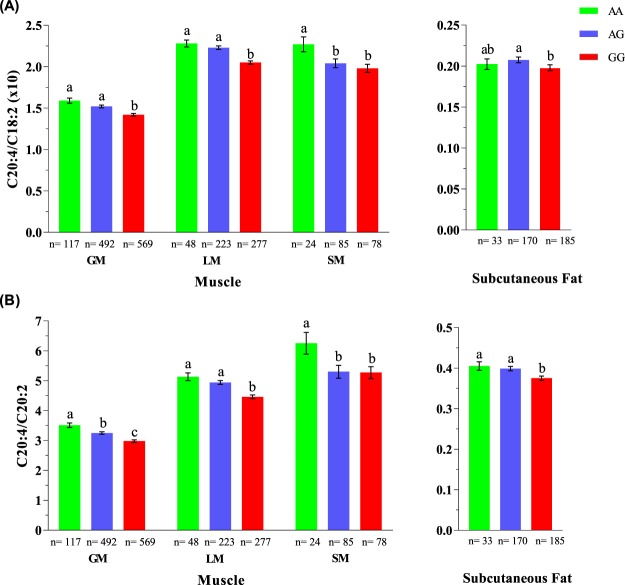
The association of FADS2 rs321384923 genotypes with fatty acid composition was investigated in two other muscles (LM and SM), subcutaneous fat and liver. The effect of FADS2 SNP in LM and SM were in line with those observed in GM, particularly for the C20:4 to C18:2 and C20:4 to C20:2 ratios (Fig. 3). As compared to GG pigs, AA pigs had a greater proportion of C20:4 in relation to C18:2, both in LM (2.28 vs 2.05, values x10) and in SM (2.27 vs 1.98, values x10; Fig. 3A) and to C20:2, also both in LM (5.13 vs 4.46) and in SM (6.25 vs 5.27, Fig. 3B). The C20:4 to C18:2 and the C20:4 to C20:2 ratios gave similar results in subcutaneous fat, albeit around twenty times smaller in magnitude (Fig. 3B). However, we did not find differences across genotypes for these two ratios in liver (Supplementary Fig. S2). Regarding C20:4 content, the effect of the FADS2 genotype in LM was fully consistent with the results in GM, with AA pigs performing better than GG pigs (1.84% vs 1.62%, with a substitution effect of 0.12 ± 0.02, P < 0.001). Likely due to the limited data size, this effect was not evident in SM (3.12%, for AA, and 2.74%, for GG, with a substitution effect of 0.13 ± 0.11, P = 0.24). No difference between FADS2 genotypes was detected for C18:2 and C20:2 in both LM and SM. The results in LM and SM were the same when individual fatty acids were expressed in mg/g of muscle.
Figure 3.
Efficiency of arachidonic acid biosynthesis by FADS2 rs321384923 genotype in muscle and subcutaneous fat. (A) The AA genotype of FADS2 was more efficient than the GG genotype in transforming linoleic acid (C18:2) into arachidonic acid (C20:4) both in muscle (GM: m. gluteus medius; LM: m. longissimus thoracis muscle; and SM: m. semimembranosus muscle) and in subcutaneous fat, around 12% and 2%, respectively. As a result, (B) C20:4 to eicosadienoic acid (C20:2) ratio in muscle and in subcutaneous fat was, respectively, 18% and 8% greater in AA pigs as compared to GG pigs. The number of pigs (n) genotyped per tissue and genotype ranged from 24 to 569. Error bars represent standard errors. Within tissue, means with different superscripts differ significantly (P < 0.05).
Relationship of FADS2 and LEPR genotypes with fat content
We did not find evidence of association of FADS2 rs321384923 with carcass weight and lean percentage and, as a result, with lean weight (Table S5). The FADS2 genotype was not associated with IMF either (Table S5), although the A allele showed a negative trend towards decreasing IMF in GM, where the allele substitution effect was −0.49 ± 0.23 (P < 0.05). This finding contrasts with the clear-cut negative relationship between C20:4 and muscle fat content (Fig. 4). However, two overlapping phenomena should be considered when accounting for C20:4 in relation to total fatty acid content: the efficiency in transforming C18:2 into C20:4 and the dilution of C20:4 as overall endogenous fat synthesis progresses, two effects which in turn are not fully independent. As indicated by the covariate adjustment, IMF showed a negative relationship not only with all investigated n-6 fatty acids (beta: −0.07% ± 0.01, P < 0.001, for C20:4) but also with all proxy ratios (beta: −0.04 ± 0.01, P < 0.001, for C20:4/C18:2, values x10) except C20:2/C18:2 (beta: 0.06 ± 0.01, P < 0.001, values x 100), thereby indicating that increased fat content, in addition to diluting C20:4, involves a decline in the biosynthesis efficiency of C20:4. Both effects are taken into account by adjusting FADS2 genotype comparisons for fat content. Therefore, the differences in C20:4 between FADS2 genotypes should be attributed to differential efficiency performance.
Figure 4.
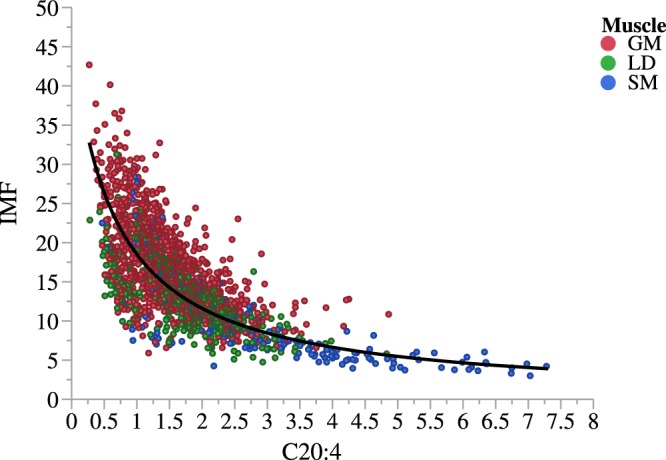
Relationship of arachidonic acid in muscle with intramuscular fat content. The arachidonic acid (C20:4) content, expressed as a percentage of total fatty acids, is negatively related to intramuscular fat (IMF-1 = 0.0218 + 0.0323*C20:4; R2:0.66). The regression was obtained across muscles using 1,912 datapoints from gluteus medius (GM, n = 1,177), longissimus thoracis (LM; n = 548) and semimembranosus (SM; n = 187) muscles. The GM showed the lowest C20:4 content (raw mean of 1.47%, 1.72% and 2.95%, for GM, LM and SM, respectively) and the highest level of IMF (raw mean of 17.2%, 13.3% and 9.6% for GM, LM and SM, respectively).
To validate the specificity of the FADS2 genotypes on C20:4 biosynthesis efficiency we made use of the LEPR g.1987C > T SNP as an internal control gene for fatness, provided that this polymorphism, which co-segregates with FADS2 SNP in this population, is known to affect lipid accumulation. The LEPR TT pigs used here produced around 5% and 11% more backfat and IMF, respectively, than LEPR CC pigs (Table 2) at no significant change in carcass weight. Whether adjusted for IMF or not, the results for the LEPR SNP on C20:4 were in line with the expected from Fig. 4, with the C allele affecting negatively IMF and positively C20:4 (Table 2). However, in contrast to the FADS2 SNP, the favourable effect of the LEPR C allele on C20:4 was accompanied by concomitant increases in C18:2 and C20:2. Hence, the LEPR SNP had no effect on the ratios associated with FADS2 activity (C20:4/C182, C20:2/C18:2 and C20:4/C20:2) when adjusting for IMF (Table 2). The dissimilar behaviour of FADS2 and LEPR SNPs in relation to these ratios substantiates the two paths by which C20:4 can be modified. Thus, while the effect of the LEPR SNP on C20:4 is to a great extent a matter of scale, a result of variations in fat content, the effect of the FADS2 polymorphism is based on changes in efficiency, which does not necessarily mean variations in fat content. No interaction between LEPR and FADS2 genotypes was observed for these fatty acids. The effect of the SCD genotype was neutral for n-6 fatty acid composition and fat content.
Table 2.
Effect of the LEPR g.1987C > T SNP genotype on n6-fatty acid composition.
| Trait | P-valuea | LEPR b | ||
|---|---|---|---|---|
| CC | CT | TT | ||
| Backfat, mm | 0.003 | 25.8 ± 0.3a | 26.1 ± 0.3a | 27.0 ± 0.3b |
| IMF, % dry matter | <0.001 | 17.9 ± 0.3a | 18.1 ± 0.3a | 19.8 ± 0.4b |
| C18:2, % | <0.001 | 10.49 ± 0.09a | 10.29 ± 0.08a | 9.89 ± 0.10b |
| C20:2, % (x10) | <0.001 | 4.94 ± 0.05a | 4.84 ± 0.04a | 4.65 ± 0.06b |
| C20:4, % | 0.01 | 1.59 ± 0.03a | 1.54 ± 0.02ab | 1.48 ± 0.03b |
| C20:4/C18:2 (x10) | 0.44 | 1.53 ± 0.02 | 1.50 ± 0.02 | 1.49 ± 0.02 |
| C20:2/C18:2 (x100) | 0.85 | 4.77 ± 0.03 | 4.76 ± 0.03 | 4.78 ± 0.03 |
| C20:4/C20:2 | 0.64 | 3.28 ± 0.05 | 3.24 ± 0.04 | 3.21 ± 0.06 |
As compared to TT pigs, the CC pigs showed a higher content of arachidonic acid (C20:4) and eicosadienoic acid (C20:2) in muscle because they were fatter and not because they were more efficient transforming linoleic acid (C18:2) into C20:4 and C20:2. Subcutaneous fat was measured in terms of backfat thickness and intramuscular fat was determined in gluteus medius muscle. The proportion of each fatty acid is expressed as a percentage relative to total fatty acid content and, as well as ratios, adjusted for intramuscular fat (IMF) content. aP-value associated with the effect of the LEPR genotype; bPairwise comparisons of LEPR genotypes. Within row, means with different superscripts differ significantly (P < 0.05).
Discussion
We report here a SNP in the promoter region of the pig FADS2 (rs321384923) with effect on the desaturation pathways leading to C20:4 biosynthesis. The polymorphism is a tagging SNP for a 3-SNP haplotype in the FADS2 promoter and it is situated -706 bp upstream from the ATG codon and -294 bp upstream from the start of transcription of the closest FADS2 transcript (ENSSSCT00000014289.3). Two previous genome-wide association studies have identified markers around this haplotype region associated with FADS2 expression and fatty acid metabolic traits. In the first one, in an Iberian x Landrace backcross, Revilla et al.22 found that the most significant cis-SNP for FADS2 gene expression (rs81474400) was located 230 Kb upstream of the FADS2. In this study, the three SNPs of the haplotype were found to be segregating in the Iberian founders, but no association was observed between the most proximal of them (rs331050552) and FADS2 mRNA expression in the backcrossed pigs. The low frequency of the minor allele and/or different haplotype structure in the backcrossed individuals could have interfered with the expected results in an experimental population of limited size. In the second genome-wide association study, a SNP (rs81360272) located in the fifth intron of FADS2, was reported to be associated in Erhualian pigs with proxy ratios of FADS2 activity12. SNPs from both studies are from the Illumina’s pig genotyping array and encompass a region containing at least four additional SNPs from this array. Using data on 272 Duroc pigs from our line genotyped with this chip, we found that these two SNPs were in low linkage disequilibrium with our tag SNP (r2 = 0.10–0.15) and had no effect on C20:4 and associated ratios. In contrast, the two nearest upstream SNPs to our haplotype (rs343441264 and rs81360470) were almost fully linked with our tag SNP (r2 = 0.88–0.92) and parallel their effects. Overall, this genomic pattern would confirm that our tag SNP is capturing a functional variant in the promoter region of FADS2 influencing C20:4 content in pigs.
The presence of the A allele of the rs321384923 SNP additively enhances FADS2 expression and, as a result, the desaturase activity in both muscle and subcutaneous fat. FADS2 is the rate limiting enzyme in the conversion of essential fatty acids C18:2 and α-linolenic to long-chain polyunsaturated fatty acids. The knock out of this gene results in mice lacking polyunsaturated fatty acids beyond eicosatrienoic acid23 (all-cis-5,11,14–20:3; Fig. 1), indicating that there are no other enzymes with a redundant activity. FADS2 participates in two well-characterized steps in the biosynthesis of n-6 polyunsaturated fatty acids: the desaturation of (i) C18:2 to γ-linolenic acid (all-cis-6,9,12–18:3) and (ii) C20:2 to dihomo-γ-linolenic acid (all-cis-8,11,14–18:3). These are critical steps to produce C20:4. Thus, the presence of the more active A allele accelerates the production of C20:4 through both routes, as seen by significant changes in the C20:4/C18:2 and C20:4/C20:2 ratios, resulting in 12–14% more C20:4 in the three muscles tested (GM, LM and SM) regardless of IMF. Furthermore, this was a consistent additive effect, paralleling the gene expression results. In agreement with this, there was a small correlated decrease in the amount of C18:2 in the genotypes carrying the A allele. These results are in line with previous findings9 indicating that a lack of FADS2 triggers an alternate reaction where C18:2 is diverted to C20:2 instead of all-cis-6,9,12–18:3, the first intermediate fatty acid in the canonical endogenous synthesis of C20:4. In the absence of FADS2, C20:2 cannot be transformed to all-cis-8,11,14–20:3, the last precursor of C20:4, and thus it is further desaturated via FADS1 to all-cis-8,11,14–20:3, an aberrant fatty acid which is incorporated as a surrogate of C20:4 in the diacylglycerol-backbone of membrane phospholipids. Unfortunately, we have no available data to test whether all-cis-8,11,14–20:3 declines as expected with the presence of the A allele.
Despite the fact that, in pigs, liver and subcutaneous fat express 8–10 times more FADS2 than muscle10, the effect of the FADS2 rs321384923 on the accumulation of C20:4 is more evident in muscle than in subcutaneous fat and liver. Apart from having more statistical power in muscle, the functional approach in this tissue can be more accurate for the reason that it shows a relatively higher de novo fatty acid synthesis24. In this regard, the case of the Duroc pigs is particularly interesting because they present a higher proportion of C18:2 in IMF relative to other breeds3. Moreover, an alternative transcript of FADS1, particularly enriched in subcutaneous fat and liver10, has been shown to regulate FADS2 activity in humans11. In this line, in a previous expression genome-wide association study22, the correlation between FADS1 and FADS2 mRNA expression was higher in liver than in subcutaneous fat (r = 0.92 and 0.63, respectively) while FADS2 expression showed the lowest correlation (r = 0.23) between these two tissues. Thus, the interaction between these two genes and tissue-specific mechanisms of regulation is a question worth exploring in the future.
Many human and mouse studies positively correlates Δ-6 activity with metabolic syndrome, insulin resistance and obesity (reviewed in Naughton et al.25). Diets rich in C18:2 result in an increase in C20:4, which can then be converted into prostacyclins and endocannabinoids, both of them with a strong pro-adipogenic activity25. Indeed, although many of these studies report weight gain and increased adipose inflammation, the final outcome is highly dependent on the whole diet and other external factors such age or physical activity. Some research even showed that dietary supplements with C18:2 increase lean mass26. In our study with pigs none of the carcass traits analyzed were affected by the higher FADS2 activity of the A rs321384923 allele. Even though C20:4 was negatively correlated with carcass weight (r = −0.39, p < 0.01, for GM) and positively with lean content (r = 0.22, p < 0.01, for GM), the effect of the FADS2 SNP on n6-fatty acid composition did not per se modify these traits. Our results also indicate that, although unevenly across muscles, the tag SNP could exert some influence on IMF, especially in muscles displaying lower C20:4 content (i.e. GM, with 15% and 50% less C20:4 than LM and SM, respectively; Fig. 4). Using data from four commercial genetic types, Renaville et al.13 found that a SNP in the exon 3 of FADS2 was associated with contents of C20:4 and IMF, but only in LM and not in muscle biceps femoris. In line with our results, the favorable effect on IMF was associated with the negative allele for C20:4 and evidenced in the muscle with the lowest C20:4. Moreover, the potential distinct effect of the FADS2 SNP on IMF in different muscles could be attributed to the differential partitioning of C20:4 between neutral lipids and phospholipids across muscles. Thus, Wood et al.3 found that the proportion of C20:4 in phospholipids as compared to neutral lipids was higher in LM (around 50 times) than in psoas major (around 10 times), even when both muscles were compared at a similar level of IMF. In a limited subset of pigs, we obtained that in GM C20:4 was around 35 times more abundant in phospholipids than in neutral lipids. These results indicate that the ability of C20:4 to get incorporated into membrane phospholipids, and likely to mediate in cell signaling events, could be muscle-specific. Results in mice evidenced that FADS2 deficiency alters the membrane phospholipidomic profiling, affecting the maturation of transcription factor sterol-regulatory- element-binding protein and therefore lipid homeostasis9. In this sense, an interesting piece of research to address the nuances of functionality of C20:4 in pig muscle is to determine how differently the FADS2 genotypes affect phospholipid and neutral lipid fatty acid composition and whether their allocation relates with fat content. IMF is a relevant trait for the pig industry in general, but particularly in Duroc lines used in premium quality meat markets, where pigs are raised to display a high level of IMF. For this reason, rs321384923 cannot be discarded as a candidate marker to increase IMF without altering lean weight.
In line with findings in humans27, our results confirm that the porcine FADS2 is subjected to functional genetic variation while providing evidence that the rs321384923 SNP in its promoter region impacts gene expression. We showed that there is an haplotype tagging SNP in the promoter region of FADS2 that results in a more efficient transformation of C18:2 into C20:4. However, we were not able to observe any consistent implication of this on the traits usually selected for in pig populations. Evidence in humans indicates that fatty acid desaturases affect plasma and tissue lipid profiles and therefore associated disease risk factors. Recent findings in humans suggest that FADS genes have been subjected to strong positive selection in response to C18:2 consumption and that this event is not neutral in relation to plasma cholesterol levels28 and to chronic and inflammatory disorders27. Further studies are needed to identify the molecular mechanisms by which variation in FADS2 modulates gene expression and functional phenotypes. In this regard, the present work confirms that selected pig populations can be an interesting genetic resource.
Electronic supplementary material
Acknowledgements
This research has received funding from the Spanish Ministry of Economy and Competitiveness and the European Union Regional Development Funds (AGL2015–65846-R grant) and was partially supported by the Centre for the Development of Industrial Technology (IDI-20150115 project). SG is recipient of a PhD scholarship from the Spanish Ministry of Science and Innovation (BES-2014-FPU13/04975). We acknowledge the staff at Selección Batallé for their cooperation.
Author Contributions
J.E. and R.N.P. conceived and designed the experiment; S.G., R.N.P. and M.T. performed the experiment; S.G. and J.E. analyzed the data and prepared the tables and figures; J.E., S.G. and R.N.P. wrote the manuscript; M.F.R. contributed to the scientific discussion. All authors read and approved the final manuscript.
Data Availability
All data supporting the findings of this study are available within the article and Supplementary Information, or are available from the corresponding author upon reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32710-w.
References
- 1.Markworth JF, Cameron-Smith D. Arachidonic acid supplementation enhances in vitro skeletal muscle cell growth via a COX-2-dependent pathway. AJP Cell Physiol. 2013;304:C56–C67. doi: 10.1152/ajpcell.00038.2012. [DOI] [PubMed] [Google Scholar]
- 2.Markworth JF, et al. Arachidonic acid supplementation modulates blood and skeletal muscle lipid profile with no effect on basal inflammation in resistance exercise trained men. Prostaglandins Leukot. Essent. Fat. Acids. 2018;128:74–86. doi: 10.1016/j.plefa.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Wood JD, et al. Effects of breed, diet and muscle on fat deposition and eating quality in pigs. Meat Sci. 2004;67:651–667. doi: 10.1016/j.meatsci.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Wood JD, et al. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008;78:343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair, J. & Mantf, N. J. Symposium: Biological Effects of Dietary Arachidonic Acid Short-term Diets Rich in Arachidonic Acid Influence Plasma Phospholipid Polyunsaturated Fatty Acid Levels and Prostacyclin and Thromboxane Production. 5, 0–4 (1996). [DOI] [PubMed]
- 6.Warren HE, et al. Effects of breed and a concentrate or grass silage diet on beef quality in cattle of 3 ages. I: Animal performance, carcass quality and muscle fatty acid composition. Meat Sci. 2008;78:256–269. doi: 10.1016/j.meatsci.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Ntawubizi M, et al. Genetic parameters for intramuscular fatty acid composition and metabolism in pigs. J. Anim. Sci. 2010;88:1286–1294. doi: 10.2527/jas.2009-2355. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura MT, Nara TY. Structure, Function, and Dietary Regulation of Δ6, Δ5, and Δ9 Desaturases. Annu. Rev. Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 9.Stoffel W, et al. Obesity resistance and deregulation of lipogenesis in D 6 -fatty acid desaturase (FADS 2) deficiency. EMBO Rep. 2014;15:110–120. doi: 10.1002/embr.201338041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taniguchi M, et al. Genomic structural analysis of porcine fatty acid desaturase cluster on chromosome 2. Anim. Sci. J. 2015;86:369–377. doi: 10.1111/asj.12308. [DOI] [PubMed] [Google Scholar]
- 11.Park WJ, et al. A novel FADS1 isoform potentiates FADS2 -mediated production of eicosanoid precursor fatty acids. J. Lipid Res. 2012;53:1502–1512. doi: 10.1194/jlr.M025312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, et al. Genome-wide association studies for fatty acid metabolic traits in five divergent pig populations. Sci. Rep. 2016;6:24718. doi: 10.1038/srep24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renaville B, et al. Candidate gene marker associations with fatty acid profiles in heavy pigs. Meat Sci. 2013;93:495–500. doi: 10.1016/j.meatsci.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Estany J, Ros-Freixedes R, Tor M, Pena RN. A functional variant in the stearoyl-CoA desaturase gene promoter enhances fatty acid desaturation in pork. PLoS One. 2014;9:1–11. doi: 10.1371/journal.pone.0086177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ros-Freixedes R, et al. Genome-wide association study singles out SCD and LEPR as the two main loci influencing intramuscular fat content and fatty acid composition in duroc pigs. PLoS One. 2016;11:1–18. doi: 10.1371/journal.pone.0152496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ros-Freixedes R, Reixach J, Tor M, Estany J. Expected genetic response for oleic acid content in pork. J. Anim. Sci. 2012;90:4230–4238. doi: 10.2527/jas.2011-5063. [DOI] [PubMed] [Google Scholar]
- 17.Bosch L, Tor M, Reixach J, Estany J. Estimating intramuscular fat content and fatty acid composition in live and post-mortem samples in pigs. Meat Sci. 2009;82:432–437. doi: 10.1016/j.meatsci.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Yuan JS, Reed A, Chen F, Stewart CN. Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:1–12. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitson AP, Marks KA, Shaw B, Mutch DM, Stark KD. Treatment of ovariectomized rats with 17β-estradiol increases hepatic delta-6 desaturase enzyme expression and docosahexaenoic acid levels in hepatic and plasma phospholipids. Prostaglandins Leukot. Essent. Fat. Acids. 2013;89:81–88. doi: 10.1016/j.plefa.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Dziedzic, B. et al. DHA upregulates FADS2 expression in primary cortical astrocytes exposed to vitamin A. Physiol. Res. athttp://europepmc.org/abstract/MED/29750879 (2018). [DOI] [PubMed]
- 21.Lattka Eva, Illig Thomas, Koletzko Berthold, Heinrich Joachim. Genetic variants of the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Current Opinion in Lipidology. 2010;21(1):64–69. doi: 10.1097/MOL.0b013e3283327ca8. [DOI] [PubMed] [Google Scholar]
- 22.Revilla M, et al. Expression analysis of candidate genes for fatty acid composition in adipose tissue and identification of regulatory regions. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-018-20473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoffel W, et al. Δ6-Desaturase (FADS2) deficiency unveils the role of ω3- and ω6-polyunsaturated fatty acids. EMBO J. 2008;27:2281–2292. doi: 10.1038/emboj.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosch L, Tor M, Reixach J, Estany J. Age-related changes in intramuscular and subcutaneous fat content and fatty acid composition in growing pigs using longitudinal data. Meat Sci. 2012;91:358–363. doi: 10.1016/j.meatsci.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Naughton SS, Mathai ML, Hryciw DH, McAinch AJ. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016;125:90–99. doi: 10.1016/j.prostaglandins.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Belury MA, et al. Erythrocyte linoleic acid, but not oleic acid, is associated with improvements in body composition in men and women. Mol. Nutr. Food Res. 2016;60:1206–1212. doi: 10.1002/mnfr.201500744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merino DM, Ma DW, Mutch DM. Genetic variation in lipid desaturases and its impact on the development of human disease. Lipids Health Dis. 2010;9:63. doi: 10.1186/1476-511X-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley MT, et al. Selection in Europeans on fatty acid desaturases associated with dietary changes. Mol. Biol. Evol. 2017;34:1307–1318. doi: 10.1093/molbev/msx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the article and Supplementary Information, or are available from the corresponding author upon reasonable request.