Abstract
MicroRNA‐1 (miR‐1) stands out as the most prominent microRNA (miRNA) in regulating cardiac function and has been perceived as a new potential therapeutic target. Lycium barbarum polysaccharides (LBPs) are major active constituents of the traditional Chinese medicine based on L. barbarum. The purpose of this study was to exploit the cardioprotective effect and molecular mechanism of LBPs underlying heart failure. We found that LBPs significantly reduced the expression of myocardial miR‐1. LBPs improved the abnormal ECG and indexes of cardiac functions in P‐V loop detection in transgenic (Tg) mice with miR‐1 overexpression. LBPs recovered morphological changes in sarcomeric assembly, intercalated disc and gap junction. LBPs reversed the reductions of CaM and cMLCK, the proteins targeted by miR‐1. Similar trends were also obtained in their downstream effectors including the phosphorylation of MLC2v and both total level and phosphorylation of CaMKII and cMyBP‐C. Collectively, LBPs restored adverse structural remodelling and improved cardiac contractile dysfunction induced by overexpression of miR‐1. One of the plausible mechanisms was that LBPs down‐regulated miR‐1 expression and consequently reversed miR‐1‐induced repression of target proteins relevant to myocardial contractibility. LBPs could serve as a new, at least a very useful adjunctive, candidate for prevention and therapy of heart failure.
Keywords: calmodulin, cardiac function, cardiac myosin light chain kinase, Lycium barbarum polysaccharides, microRNA‐1, structural remodelling
1. INTRODUCTION
Heart failure (HF) is a common, disabling and potentially deadly condition and remains the only cardiovascular disease with an increasing hospitalization burden and an ongoing drain on health care expenditures, despite several therapeutic approaches have reduced cardiovascular morbidity and mortality.1, 2 Therefore, to explore the pathogenesis of such disease and look for novel effective drugs with low side‐effects would have great impacts on its prevention and clinical management of HF. Recently, constituents from natural herbs have attracted attention with regard to pharmaceutical development. MicroRNA (miRNA), a small non‐coding RNA, is emerged as a critical node in post‐transcriptional regulation and a newly discovered class of gene regulatory factors at the core of human physiology and disease.3, 4 Increasing lines of evidence have been rapidly evolving for the crucial roles of miRNAs in regulating diverse aspects of cardiac function and progression of HF.4, 5 And notably microRNA‐1 (miR‐1), a cardiac‐enriched miRNA, is in most close relation to heart conditions, and the changes in its expression have been discovered in a variety of heart diseases.6, 7, 8, 9, 10 Our previous report revealed that outcomes of miR‐1 overexpression induced adverse structural remodelling, which impaired cardiac contractile and diastolic function and even caused HF.11 MiR‐1 could result in an epigenetic defect as cardiac hypertrophy by microinjecting fragments of miR‐1.12 The functional significance of the findings uncovered that miR‐1 might be a trigger and sustainer for the structural remodelling and dysfunction of HF. Down‐regulation of miR‐1 may produce cardioprotective effects7, 8, 13 and miR‐1 has been perceived as a new therapeutic target for heart diseases. Therefore, it is valuable to develop new candidates regulating miR‐1 by which to treat relevant heart diseases.
Lycium barbarum polysaccharides (LBPs) are important active constituents extracted from the traditional Chinese herb L. barbarum. There are scientific proofs of its pharmacological and biological functions including anti‐oxidative properties,14 immunomodulation,15 antitumor activity,16 anti‐ageing effect,17 neuroprotection,18 hypoglycaemic and hypolipidemic effects19 and male fertility‐facilitating,20 indicating extensive application prospects on relevant diseases. To the best of our knowledge, there are only a few studies concerning the effect of LBPs on cardiovascular system. Electrocardiographic and biochemical evidence were found that LBPs elicit a typical cardioprotective effect against Doxorubicin‐related oxidative stress in rats and dogs.21, 22 LBPs reduced myocardial apoptosis and injury in ischemia/reperfusion process of rat heart and could prevent the development of cardiovascular diseases.23, 24 However, until now, no available information has addressed the effects of LBPs on cardiac structure and function in HF. Given its biological property, it is conceivable that LBPs may produce beneficial actions in preventing the development of HF and act as a potential therapy option. This study was aimed to investigate the effects of LBPs on impaired cardiac function and structural remodelling induced by overexpression of miR‐1 and to unravel the underlying molecular mechanism for exploiting therapeutic potential on HF.
2. MATERIALS AND METHODS
2.1. Preparation of Lycium barbarum polysaccharides
Lycium barbarum polysaccharides were prepared using the extraction procedure optimized by Jingcheng Tang.25 Briefly, the dry fruits of L. barbarum (0.5 kg, purchased from Xi'an Tianyuan Biologics Plant, China) were immersed in deionized water for 12 hours, boiled for 1.5 hours and extracted twice. The combined aqueous extracts were concentrated in vacuum and precipitated by 95% ethanol for 48 hours at room temperature. The precipitate was filtered at reduced pressure and dried by lyophilization. We got a yield of 37.8% LBPs powder. The crude LBPs have been deproteinated by the Sevag method, and intensively dialysed for 2 days against distilled water (cut‐off Mw 3500 Da). The solution of LBPs samples were scanned under UV‐VIS spectrometer in the range from 190 to 400 nm, and there was no absorbance peak at 280 and 260 nm, implying that the protein and nucleic acid were absent in this polysaccharide. LBPs were diluted by ddH2O in our study.
2.2. Generation of miR‐1 transgenic (Tg) mice
All experimental procedures and protocols used in this investigation received approval by the Institutional Animal Care and Use Committee of Harbin Medical University, which conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85‐23, revised 1985). We generated Tg mouse line for cardiac‐specific overexpression of miR‐1 driven by the α‐myosin heavy chain (α‐MHC) promoter as previously reported.11 Briefly, sexually immature female C57BL/6 mice (4‐5 weeks of age) were applied to obtain sufficient quantity of eggs (>250) for microinjection. The mice used in this study were the fifth generation or later.
2.3. Administration of LBPs to mice
Animals were divided into three groups: C57BL/6 male wild‐type (WT) littermate mice, miR‐1 Tg mice, miR‐1 Tg mice received LBPs. LBPs were delivered into miR‐1 Tg mice at 3 months of age through intragastric administration at a dosage of 200 mg/kg/d22, 26 for 1 month. The age‐matched WT mice and miR‐1 Tg mice were received 0.9% NaCl (0.2 mL/d) as the vehicle. In the next 2 months, LBPs was mixed in water and administered to the mice by drink at the same dose. Monitoring of drinking quantity manifested no change in all mice. Measurements were made when the mice were at an age of 6 months.
2.4. Culture of neonatal rat ventricular cardiomyocytes (NRVCs)
Hearts from 1‐ to 3‐day‐old Wistar rats were excised, and the ventricular myocardium was minced in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) and cells were dissociated with 0.25% trypsin‐EDTA solution (Beyotime, China). After centrifugation, the collected isolated cells were plated onto 25 cm2 cell culture flask (Corning Incorporated, USA) for 100 minutes to separate ventricular myocytes from the faster attaching non‐myocytes. The re‐collected cells were then seeded in a six‐well plate (2 × 105/well) in DMEM containing 10% foetal bovine serum (FBS) and 0.1 mmol/L bromodeoxyuridine (sigma). Cells were used for experiments 48‐72 hours after isolation when demonstrating rhythmic contractions.
2.5. Administration of LBPs to NRVCs
NRVCs (2 × 105/well) were incubated with 2 mL fresh FBS‐free medium in six‐well plates and administered incremental dose of LBPs (100, 400 and 800 μg/mL).27, 28 For miR‐1 overexpression treated groups, NRVCs were transfected with 2.5 μg miR‐1 or negative control (NC) siRNA with X‐treme GENE siRNA transfection reagent (Cat.#04476093001, Roche). LBPs were administered with miR‐1 at the same time. At 48 hours post‐transfection, cells were harvested for total RNA or protein extraction. MiR‐1 and NC were synthesized by Shanghai GenePharma Co., Ltd. The sequence of rno‐miR‐1 is 5′‐UGGAAUGUAAAGAAGUGUGUAU‐3′. The sequence of NC is 5′‐UUCUCCGAACGUGUCACGUTT‐3′.
2.6. Detection of heart function
Mice were anaesthetized with sodium pentobarbital (60 mg/kg, intraperitoneal). After recording ECG for 10 minutes, pressure‐volume (PV) loops (Scisense, Ontario, Canada) measurements were performed with a 1.2F mouse pressure‐volume catheter (Pressure‐Volume Control Unit FV896B) which was retrogradely inserted into the left ventricle cavity through the right carotid artery to measure baseline arterial pressure haemodynamics in the closed chest. All data were analysed with iWork Labscribe2 Data Recording and Analysis software. Baseline haemodynamic values were obtained by averaging 300 beats recorded during steady‐state periods. The main measured parameters included ejection fraction (EF), cardiac output (CO), end‐systolic pressure (ESP), end‐diastolic pressure (EDP), end‐systolic volume (ESV), end‐diastolic volume (EDV), maximum derivative of change in systolic pressure over time (dP/dtmax) and maximum derivative of change in diastolic pressure over time (dP/dtmin).
2.7. Evaluation of morphological remodelling
For transmission electron microscopy (TEM) detection, heart tissues from left ventricles were removed and immersed in stationary liquid (pH 7.3) containing 3% glutaraldehyde, and then fixed in 2% Osmic acid (OsO4). After gradient dehydration tissues were embedded in epon with propylene oxide as an intermediary solvent. Ultrathin sections were processed, mounted onto formvar‐coated slot grids, and stained with uranyl acetate and lead citrate. Images were captured by a Hitachi H‐7650 electron microscope (Hitachi, H‐7650, Tokyo, Japan). The quantitative analysis was to measure the lengths of damaged and total intercalated disc by Image J. The result was represented as the percentage of damaged length to the total. Haematoxylin and eosin (H&E) staining was performed by routine method. Hearts were taken and fixed in zinc formalin for 24‐48 hours then processed using a Sakura Tissue Tek VIP5 processor. Samples were embedded in paraffin and sectioned longitudinally from the identical plane of the initial portion of the ascending aorta at 4 μm using a microtome. Sections were stained with haematoxylin and eosin for identification. Heart‐to‐bodyweight ratio (HW/BW) was calculated for each group. The length of sarcomeres was evaluated by Image‐Pro Plus 6.0 (Media Cybernetics, Bethesda, MA).
2.8. Quantification of miR‐1 levels in Tg mice or NRVCs
The total RNA samples were isolated using Trizol and phenol/chloroform extraction procedures. MiR‐1 level was quantified by the TaqMan® MicroRNA Reverse Transcription Kit (Cat.#4366596, Applied Biosystems) and the TaqMan® MicroRNA Assay (for mice: target sequences: UGGAAUGUAAAGAAGUAUGUAU, Cat.#002222, Applied Biosystems; for rats: target sequences: UGGAAUGUAAAGAAGUGUGUAU, Cat.#002064, Applied Biosystems). U6 (Cat.#001973, Applied Biosystems) was used as an internal control. The quantitative Real‐time PCR (qRT‐PCR) was performed on 7500 FAST Real‐Time PCR System (Applied Biosystems) for 40 cycles.
2.9. Western blot analysis
The left ventricles of mice or cultured NRVCs were homogenized in lysis buffer (RIPA buffer 60%, SDS 40% and protease inhibitor cocktail 1%) on ice and then centrifuged at 18 000 g at 4°C for 30 minutes to remove the insoluble pellet. Protein concentration in the supernatant was determined by the BCA Protein Assay Kit (Bio‐Rad, Hercules, CA, USA). Equal amounts of protein (60 or 80 μg) were loaded on a 10% or 15% SDS‐PAGE gel. The lysates were resolved by electrophoresis (70 V for 30 minutes and 100 V for 1.5 hours) and transferred onto nitrocellulose membranes. After being blocked in 5% nonfat milk for 2 hours at room temperature, the membranes were treated with anti‐CaM (1:1000, ab45689, Abcam, MA, USA), anti‐MLC2v (1:1000, ab79935, Abcam, MA, USA), anti‐MLC2v (phospho‐S20) (1:1000, ab2480, Abcam, MA, USA), anti‐cMLCK (ARM‐Mylk3 (150‐164), 1:50, generated in our lab),11 anti‐cMyBP‐C3 (M‐190) (1:200, sc‐67353, Santa Cruz Bio., Inc., CA, USA), anti‐cMyBP‐C‐Ser 282 (1:1000, ALX‐215‐057, Enzo Life Sci., USA), anti‐CaMKIIδ (T287) (1:200, sc‐5392, Santa Cruz Bio., Inc., CA, USA), anti‐CaMKIIδ‐p (pT287) (1:200, sc‐5392p, Santa Cruz Bio., Inc., CA, USA) and anti‐Connexin43 (1:200, sc‐13558, Santa cruz Bio., Inc., CA, USA) at 4°C overnight. The membranes were then washed and incubated with secondary antibody (1:10 000) for 1 hour at room temperature. Protein loading was confirmed using GAPDH (1:5000, G8795, Sigma, Saint Louis, MO, USA) as an internal control. Blots were detected with the Odyssey infrared imaging system (Licor, USA). Western blot bands were quantified using Quantity One software to measure band intensity (area × OD) and normalized to GAPDH band intensity. The final results were expressed as fold changes compared with the control values.
2.10. Data analysis
Data were calculated as mean ± SEM (standard error of the means) except the length of sarcomeres as mean ± SD (standard deviation). The ANOVA test was performed for statistical comparisons among multiple groups. Differences were considered statistically significant at P < .05. SPSS13.0 was used for all statistical analyses.
3. RESULTS
3.1. LBPs reduced the expression of myocardial miR‐1 in vitro and in vivo
We quantified the miR‐1 levels in neonatal rat ventricular cardiomyocytes (NRVCs) with incremental doses of LBPs. Excitingly, our data from qRT‐PCR showed that LBPs significantly reduced the expression of endogenic miR‐1 (Figure 1A). Similar results were observed in the overexpression model of miR‐1 by transfecting miRNA mimics in NRVCs (Figure 1B). Consistently, in vivo study with miR‐1 Tg mice also confirmed the down‐regulated expression of miR‐1 in the application of LBPs (1.96 ± 0.11 vs 2.66 ± 0.18, P < .05) even not to WT level (1.96 ± 0.11 vs 1.0 ± 0.09, P < .05) (Figure 1C).
Figure 1.
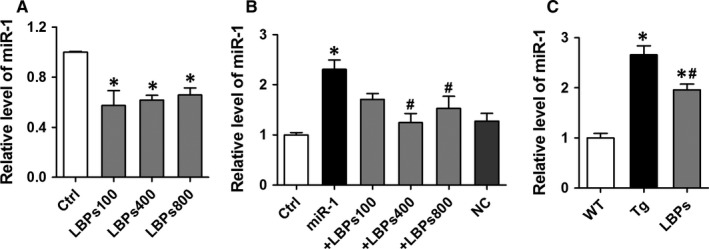
Lycium barbarum polysaccharides (LBPs) down‐regulated the expression level of miR‐1 in vitro and in vivo. A, The expression levels of endogenic miR‐1 in the treatment of LBPs in neonatal rat ventricular cardiomyocytes (NRVCs). NRVCs, neonatal rat ventricular cardiomyocytes; Ctrl, control. *P < .05 vs Ctrl, mean ± SEM, n = 3 independent RNA samples for each group. B, The effect of LBPs on the expression levels of miR‐1 in NRVCs overexpression model of miR‐1. *P < .05 vs Ctrl or NC, # P < .05 vs miR‐1; mean ± SEM, n = 8 for Ctrl, +LBPs100, +LBPs400, NC, 7 for miR‐1, and 6 for +LBPs800. C, The effect of LBPs on the expression levels of miR‐1 in miR‐1 Tg mice. Tg, transgenic; WT, wild type. *P < .05 vs WT, # P < .05 vs Tg; mean ± SEM, n = 6 for WT, 8 for Tg, and 5 for LBPs
3.2. LBPs restored cardiac dysfunction induced by overexpression of miR‐1
ECG analysis illustrated significantly widened QRS complex (21.03 ± 1.94 vs 15.17 ± 0.38, P < .01) and prolonged P‐R interval (70.78 ± 3.07 vs 46.36 ± 1.48, P < .01) in Tg mice, indicating slowing down of cardiac conduction. Representative ECG tracing also recorded arrhythmias in Tg mice. Treatment with LBPs significantly improved the abnormal QRS complex and P‐R interval (16.88 ± 0.47 vs 21.03 ± 1.94 and 59.37 ± 3.01 vs 70.78 ± 3.07, respectively, P < .05) with no arrhythmias (Figure 2A,B). TEM examination found that intercalated discs were dissolved markedly with vacuolar degeneration of gap junctions and decreased density of the macula adherents in the hearts of Tg mice but not in those treated with LBPs (Figure 2C,D). Figure 2D presented the quantitative analysis of percentage of damaged length in intercalated disc organization.
Figure 2.
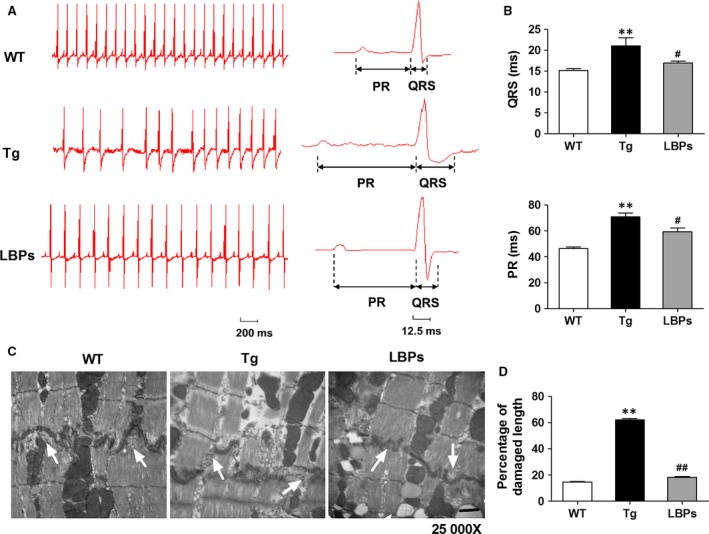
The protective effect of Lycium barbarum polysaccharides (LBPs) on impaired cardiac conduction function induced by miR‐1 overexpression. A, Representative surface ECG recordings showing QRS complex and P‐R interval and arrhythmias in three groups. B, Quantitative comparison of QRS and PR interval in all groups. *P < .05, **P < .01 vs WT, # P < .05 vs Tg; mean ± SEM, n = 15 for WT, 9 for Tg, and 7 for LBPs. C, Representative intercalated disc organization showing gap junction and macula adherents detected by TEM. Magnification ×25 000, scale bar: 1 μm. D, Quantitative analysis of percentage of damaged length in intercalated disc. **P < .01 vs WT, ## P < .01 vs Tg, data presented as mean ± SEM, n = 3 independent TEM images for each group
P‐V loop detection revealed the changes of indexes of cardiac function. Compared to WT mice, ejection fraction (EF), the most important parameter representing contractile function, was significantly reduced by 42.99% in Tg mice (42.08 ± 2.00 vs 73.81 ± 3.25, P < .01). However, in LBPs‐treated mice EF showed significant mitigation (66.65 ± 2.41 vs 42.08 ± 2.00, P < .01) which almost moved back to normal level (Figure 3A). In addition, the impaired cardiac contractile and diastolic functions of Tg mice were indicated by decreased CO, ESP, dP/dtmax and dP/dtmin, as well as increased EDP, ESV and EDV (Figure 3B‐H). Interestingly, in the treatment of LBPs, the indexes of cardiac contractile function including CO, ESP, ESV and dp/dtmax were seen significant restorations. However, except EDV, the indexes of cardiac diastolic function including EDP and dP/dtmin were not improved significantly in mice treated with LBPs (Figure 3B‐H). These implied the beneficial effect of LBPs on contractile function and at least partial recovery on diastolic function.
Figure 3.
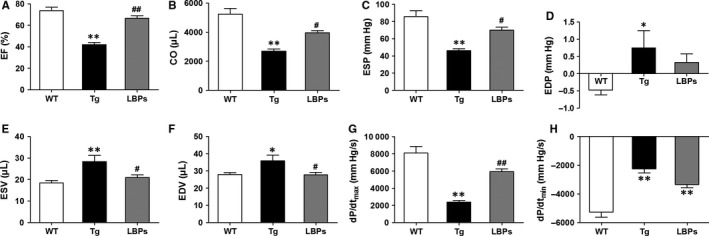
The mitigating effect of Lycium barbarum polysaccharides (LBPs) on damaged cardiac contractile and diastolic functions by miR‐1 overexpression. A‐H, Changes of parameters of cardiac systolic and diastolic functions in three groups. *P < .05, **P < .01 vs WT, # P < .05, ## P < .01 vs Tg; mean ± SEM, n = 8 for WT, 6 for Tg, and 6 for LBPs. CO, cardiac output; dP/dtmax, maximum derivative of change in systolic pressure over time; dP/dtmin, maximum derivative of change in diastolic pressure over time; EDP, end‐diastolic pressure; EDV, end‐diastolic volume; EF, ejection fraction; ESP, end‐systolic pressure; ESV, end‐systolic volume
3.3. LBPs recovered adverse cardiac structural remodelling in miR‐1 Tg mice
Decreases in bodyweight and increases in heart‐to‐bodyweight ratios (HW/BW) were obtained from Tg mice as compared to WT ones. In the treatment of LBPs, significant reversion was observed in bodyweight but not in HW/BW (Figure 4A,B). H&E staining exhibited that the hearts of Tg mice were enlarged in the diastolic state and LBPs‐treated ones were not recovered to normal (Figure 4C), which was consistent with the result of HW/BW. TEM examination displayed shortened and uneven sarcomeric length (asynchronous contraction) as well as the loss of clear zone and H‐zone in Tg mouse hearts, and even severe myofibrillar fragmentation and dissolution of cardiomyocytes (Figure 4D,E), suggesting that remodelled sarcomeric assembly participated in the detrimental alterations of heart function in Tg mice. But remarkably, these phenomena were not found in mice treated with LBPs, indicating the protective effect of LBPs on adverse structural remodelling and consequent heart dysfunction (Figure 4D,E). Figure 4E presents a summary of length of sarcomeres with error bar (standard deviation) expressing the variation of sarcomeres.
Figure 4.
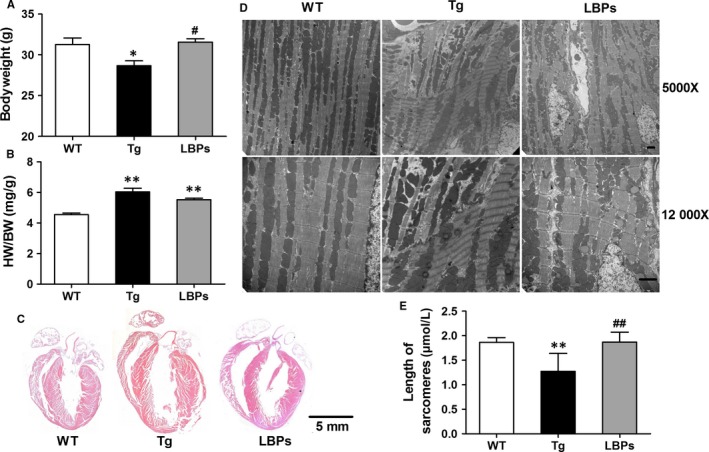
The reversing effect of Lycium barbarum polysaccharides (LBPs) on cardiac structural remodelling produced by miR‐1 overexpression. A, The change of bodyweight in three groups. *P < .05 vs WT, # P < .05 vs Tg; mean ± SEM, n = 13 for WT, 12 for Tg, and 7 for LBPs. B, The change of ratio of heart to bodyweight in three groups. **P < .01 vs WT; mean ± SEM, n = 13 for WT, 12 for Tg, and 7 for LBPs. C, Longitudinal sections of hearts stained by H&E from mice of three groups, scale bar: 5 mm. D, TEM examination of cardiac myofilament showing sarcomeric length, clear zone and H‐zone, and myofibrillar fragmentation and dissolution (in Tg mice). Up panels: magnification ×5000, scale bar: 2 μm and down panels: magnification ×12 000, scale bar: 2 μm. E, Comparison of length and variation of sarcomeres. **P < .01 vs WT, ## P < .01 vs Tg, data presented as mean ± SD (for variation), n = 80 from three hearts for each group
3.4. LBPs reversed the reductions of target proteins of miR‐1 and key contractile proteins
In the present study, the repressive effects of miR‐1 on the target proteins calmodulin (CaM) and cardiac myosin light chain kinase (cMLCK) are shown in Figure 5A,F as observed by a weaker signal vs WT band. Administration of LBPs displayed more intense band indicating increased expression of target proteins. Similarly, both the total and phosphorylated protein levels of Ca2+/calmodulin‐dependent protein kinase II (CaMKII) were decreased in Tg hearts as compared to those from WT, and consistently, the reversion were observed in LBPs treated ones (Figure 5B,C). Similar trends were also obtained in cardiac myosin binding protein C (cMyBP‐C) (Figure 5D,E). The reduction in phosphorylation status of myosin light chain 2v (MLC2v) in hearts from Tg mice was significantly recovered in those from LBPs‐treated mice (Figure 5G), although the expression of the total protein level of MLC2v was in the same range in three groups (Figure 5H). Furthermore, MYLK3 (encoding cMLCK) mRNA level was also decreased in Tg mice and reversed by LBPs treatment, but mRNA levels of CALM1 and CALM2, both encoding CaM, were not changed in three groups (Figure 5I). These results indicated that the observed decrease in CaM protein expression may be ascribed at least partially to the post‐transcriptional regulation.
Figure 5.
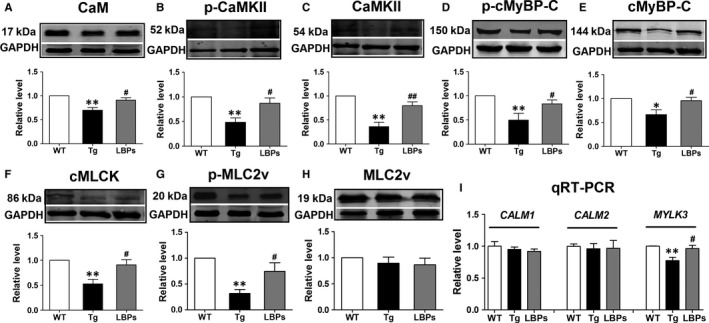
The restoration of Lycium barbarum polysaccharides (LBPs) on reduced expression of key cardiac contractile regulatory proteins in miR‐1 Tg mice. A‐H, Representative examples of Western blot bands of each protein and statistical bar graph indicating the result of densitometric analysis of the bands as normalized to the quantity of GADPH protein. *P < .05, **P < .01 vs WT, # P < .05, ## P < .01 vs Tg; mean ± SEM, n = 5, 6, 6, 4, 5, 5, 7, 6 independent protein samples for each group in sequence from A to H. CaM, calmodulin; CaMKII, Ca2+/calmodulin‐dependent protein kinase II; cMyBP‐C, cardiac myosin binding protein C; cMLCK, cardiac myosin light chain kinase; MLC2v, myosin light chain 2v. I, Comparison of mRNA levels of CALM1,CALM2 and MYLK3 by qRT‐PCR in all groups. **P < .01 vs WT, # P < .05 vs Tg; mean ± SEM, n = 5 independent RNA samples for each group
4. DISCUSSION
MiRNA‐based therapy has been recognized to be a promising novel therapeutic strategy for the treatment of cardiovascular diseases.29, 30 The principal finding of this study is the first to identify that LBPs protect the hearts from adverse structural remodelling and impaired cardiac function in miR‐1 transgenic (Tg) mouse line. The mechanism of cardioprotective effects of LBPs is due, at least partially, to down‐regulating the expression of miR‐1 to reverse miR‐1‐induced repression of target proteins relevant to myocardial contractibility. LBPs could serve as a new candidate for the treatment of HF.
At present, the conventional treatments for HF are mainly angiotensin converting enzyme inhibitors, β‐adrenoceptor blockers and diuretics. The important effectiveness, especially in short‐term use, cannot be denied, but long‐term use does not prolong the life‐span of those patients with many adverse side‐effects such as producing poor compliance, promoting myocardial injury and remodelling, deteriorating cardiac function and even increasing the rate of sudden death caused by cardiac arrhythmia.1, 31 Treatment of HF remains unsatisfactory as available treatments often fail to control the symptoms.32 Therefore, the current major goal for HF is still to develop rational approaches to improve the quality of life and prolong life‐span. The use of natural compounds to improve human health has long been recognized in the history and increased in popularity in the modern society. The traditional Chinese herb, compared to the Western medicine, has a prominent advantage because of a stable curative effect with significantly less toxicity. With the progression of modern technology, pharmaceutics of herbal medicine products is undergoing rapid development in China, and more and more herbal compound extracts are being authenticated, standardized and administered successfully in clinical practice.33, 34 Traditional Chinese medicine Tongxinluo (TXL) mediates endothelial preservation by mitigating atherogenesis35 and improves cardiac functions in response to ischaemia–reperfusion injury.36 Qili qiangxin capsule, a traditional Chinese medicine, has been approved in China to be used in combination therapy for chronic HF.34 Of note, several other traditional Chinese medicines such as Huangqi injection, Berberine, Shenfu decoction, Shengmai, Hawthorn, Curcumin, Resveratrol and Cannabinoids may have potential therapeutic use in HF as adjunctive treatments in clinical practice or animal models of human disease.31, 37, 38, 39, 40 All these findings manifest that herbal medicine products play more and more important role in modern medical therapy.
Lycium barbarum (also known as Goji berry or wolfberry), a famous Chinese medicinal herb and also a functional food, has a long history of use in a broad spectrum of diseases to nourish liver, kidneys and eyes and becomes increasingly popular in Europe and North America.19, 41, 42 Polysaccharides are the most important functional ingredient that is approximately 40% of dry mass in L. barbarum fruits.18 Till recent several years LBPs are given increasing attention in the field and most researches focus on its anti‐oxidative property as a free radical scavenger for immunomodulation and anticancer.14, 15, 16 The studies of LBPs on cardiovascular aspect are only limited to the protective phenomena (no discussion of mechanism) on doxorubicin‐induced cardiotoxicity and ischaemia/reperfusion injury of rat heart.21, 22, 23, 24 In the present study, we used the model of miR‐1 Tg mice and demonstrated that LBPs restored the cardiac function impaired by overexpression of miR‐1, as indicated by increase in EF, CO, ESP, and dp/dtmax, as well as decrease in ESV and EDV. Notably, although the indexes of cardiac diastolic function EDP and dP/dtmin were not improved significantly, TEM results of administration of LBPs did not show morphological damages on sarcomeric assembly, such as shortened and uneven sarcomeric length, loss of clear zone and H‐zone and even severe myofibrillar fragmentation and dissolution as displayed in Tg mouse hearts. Additionally, the treatment with LBPs displayed normal intercalated disc and gap junction in TEM and reversed conduction abnormalities induced by miR‐1 in ECG, strongly implying the protective effect of LBPs on cardiac conduction function. Therefore, our study has uncovered for the first time that LBPs possess the protective action against the structural and functional abnormalities of the heart, the essential aetiology of HF.
HF with complicated pathogenesis is associated with loss of cardiac contractility, abnormalities in Ca2+ handling and altered phosphorylation states of cardiac contractile regulatory protein.43, 44 Our previous study has elucidated that overexpression of miR‐1 repressed potential target proteins CaM and cMLCK, which attenuated the phosphorylation of CaMKII, cMyBP‐C and MLC2v, leading to impaired sarcomeric assembly and consequent heart dysfunction.11 CaM, a known transducer of Ca2+ signal, activates CaMKII45 to directly phosphorylate cMyBP‐C, which is a thick filament protein with physiological significance for normal myocardial contractility and stability and serves as a convergent node for signalling processes in the cardiomyocyte.46, 47 Activation of cMLCK, also regulated by CaM, appears to be pivotal to maintain the phosphorylation of MLC2v, which functions as an essential component of thick myofilament assembly and plays a critical role in maintaining normal myocardial contractility and function.48, 49 The dephosphorylation of both MLC2v and cMyBP‐C is associated with a declined cardiac function in failing human hearts and animal models of HF.46, 48 The present study exhibited that LBPs restored the reductions in phosphorylation of MLC2v and both total level and phosphorylation of CaMKII and cMyBP‐C in Tg mice, as well as their upstream activators cMLCK and CaM, target proteins affected by miR‐1. Undoubtedly all these data supported that the structural and functional protection of LBPs on Tg mouse hearts was due largely to the down‐regulation of miR‐1 level and subsequent restoration of target proteins essential for cardiac contractile function. Other studies found that tanshinone IIA, an active component of a traditional Chinese medicine based on Salvia Miltiorrhiza, protected against arrhythmogenesis after myocardial infarction and cardiac sudden death induced by lethal arrhythmias via repression of miR‐1,8, 13 and Propranolol exerted ischaemic cardioprotection related to down‐regulation of miR‐1,7 providing powerful supports for our cardioprotective result of LBPs on HF by down‐regulation of miR‐1. These findings not only help us understand the mechanisms underlying the beneficial effects of LBPs on HF, but also conceptually advance our view regarding miRNAs to serve as potential therapeutic drug targets. Notably, although the suppression did not completely back to normal levels as indicated in WT mice, the remarkable mitigation of LBPs on adverse structural remodelling, cardiac contractile function and key contractile regulatory proteins, revealed that, besides down‐regulation of miR‐1 expression, LBPs might perform cardioprotective effects by directly affecting key contractile proteins or other signalling pathway which remains to be illuminated in future study (Figure 6).
Figure 6.
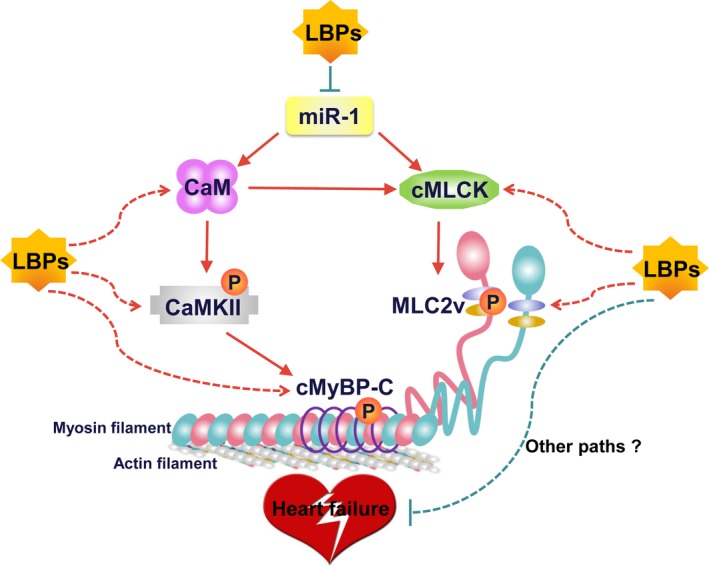
Schematic illustration explaining the possible targeting and signalling mechanisms by which Lycium barbarum polysaccharides (LBPs) restore the impairment of cardiac contractility induced by miR‐1 overexpression. LBPs restored the reductions of CaM and cMLCK, target proteins affected by miR‐1, and their corresponding downstream proteins for myocardial contraction including CaMKII, cMyBP‐C and MLC2v. LBPs might perform cardioprotective effects by affecting key contractile proteins directly or other signalling pathway. CaM: calmodulin; cMLCK, cardiac specific myosin light chain kinase; CaMKII, calmodulin‐dependent protein kinase II; cMyBP‐C, myosin binding protein‐C; MLC2v, myosin regulatory light chain 2
It is undeniable that our animal model cannot represent totally as a conventional HF model. Usually in laboratory research, there are two main HF models including transaortic constriction (TAC) and permanent ligation of the left anterior descending coronary artery (LAD) for 8 weeks.50, 51 A limitation of the study is the use of miR‐1 Tg mice. In our study, in view of the important role of miR‐1, we focus on the effect of miR‐1 on HF by the animal model of miR‐1 Tg mice and try to develop new candidates regulating miR‐1 by which to treat HF. We will verify our results in conventional HF models in future. The other limitation is that we only observed the changes of structural and functional alterations in ventricles but not in atria, which merit future studies to exploit these possibilities and better interpret the mechanisms of action of miR‐1 on heart diseases.
Systematic characterization of functional compounds in medicinal herbs and their mechanisms of action are important for providing the rationale for their efficacy and developing modern evidence‐based medicine. While L. barbarum fruit is widely used in China, there is still a lack of in‐depth study on the pharmacological effects of its active ingredients, especially those that exert cardioprotection. Our research addressed the protection of LBPs on cardiac contraction and conduction dysfunction and adverse structural remodelling induced by overexpression of miR‐1 via direct and indirect improvement of key proteins essential for cardiac contractile function, and provided new insights into the significant role of LBPs as a new, at least a very useful adjunctive, candidate for prevention and therapy of HF. Herein, we have accumulated scientific evidence for LBPs’ cardioprotective role and hope that these novel findings will develop its potential as an evidence‐based medicine and expand the application of LBPs on heart disease.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (81470523, 81271207, 81471115 and 81671052). J.A. designed the research study; R.Z. analysed the data and wrote the manuscript; H.N. and Y.X. performed the research; T.T. helped writing the manuscript; T.B. and L.Z. contributed essential tools. All authors read and gave their approval for the final version of the manuscript.
Zhang R, Xu Y, Niu H, et al. Lycium barbarum polysaccharides restore adverse structural remodelling and cardiac contractile dysfunction induced by overexpression of microRNA‐1. J Cell Mol Med. 2018;22:4830–4839. 10.1111/jcmm.13740
Contributor Information
Rong Zhang, Email: rongzhang77@163.com.
Jing Ai, Email: azhrbmu@126.com.
REFERENCES
- 1. Bozkurt B, Mann DL. The treatment of heart failure in the 21st century: is the glass half empty or half full? Methodist Debakey Cardiovasc J. 2013;9:3‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McNally EM, Barefield DY, Puckelwartz MJ. The genetic landscape of cardiomyopathy and its role in heart failure. Cell Metab. 2015;21:174‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Plasterk RH. Micro RNAs in animal development. Cell. 2006;124:877‐881. [DOI] [PubMed] [Google Scholar]
- 4. Sucharov CC, Kao DP, Port JD, et al. Myocardial microRNAs associated with reverse remodeling in human heart failure. JCI Insight. 2017;2:e89169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piubelli C, Meraviglia V, Pompilio G, et al. microRNAs and cardiac cell fate. Cells. 2014;3:802‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang B, Lin H, Xiao J, et al. The muscle‐specific microRNA miR‐1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486‐491. [DOI] [PubMed] [Google Scholar]
- 7. Lu Y, Zhang Y, Shan H, et al. MicroRNA‐1 downregulation by propranolol in a rat model of myocardial infarction: a new mechanism for ischaemic cardioprotection. Cardiovasc Res. 2009;84:434‐441. [DOI] [PubMed] [Google Scholar]
- 8. Shan H, Li X, Pan Z, et al. Tanshinone IIA protects against sudden cardiac death induced by lethal arrhythmias via repression of microRNA‐1. Br J Pharmacol. 2009;158:1227‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ai J, Zhang R, Li Y, et al. Circulating microRNA‐1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun. 2010;391:73‐77. [DOI] [PubMed] [Google Scholar]
- 10. Izarra A, Moscoso I, Canon S, et al. miRNA‐1 and miRNA‐133a are involved in early commitment of pluripotent stem cells and demonstrate antagonistic roles in the regulation of cardiac differentiation. J Tissue Eng Regen Med. 2017;11:787‐799. [DOI] [PubMed] [Google Scholar]
- 11. Ai J, Zhang R, Gao X, et al. Overexpression of microRNA‐1 impairs cardiac contractile function by damaging sarcomere assembly. Cardiovasc Res. 2012;95:385‐393. [DOI] [PubMed] [Google Scholar]
- 12. Wagner KD, Wagner N, Ghanbarian H, et al. RNA induction and inheritance of epigenetic cardiac hypertrophy in the mouse. Dev Cell. 2008;14:962‐969. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Zhang L, Chu W, et al. Tanshinone IIA inhibits miR‐1 expression through p38 MAPK signal pathway in post‐infarction rat cardiomyocytes. Cell Physiol Biochem. 2010;26:991‐998. [DOI] [PubMed] [Google Scholar]
- 14. Varoni MV, Pasciu V, Gadau SD, et al. Possible antioxidant effect of Lycium barbarum polysaccharides on hepatic cadmium‐induced oxidative stress in rats. Environ Sci Pollut Res Int. 2017;24:2946‐2955. [DOI] [PubMed] [Google Scholar]
- 15. Bo R, Zheng S, Xing J, et al. The immunological activity of Lycium barbarum polysaccharides liposome in vitro and adjuvanticity against PCV2 in vivo. Int J Biol Macromol. 2016;85:294‐301. [DOI] [PubMed] [Google Scholar]
- 16. Zhang XJ, Yu HY, Cai YJ, Ke M. Lycium barbarum polysaccharides inhibit proliferation and migration of bladder cancer cell lines BIU87 by suppressing Pi3K/AKT pathway. Oncotarget. 2017;8:5936‐5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yi R, Liu XM, Dong Q. A study of Lycium barbarum polysaccharides (LBP) extraction technology and its anti‐aging effect. Afr J Tradit Complement Altern Med. 2013;10:171‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li SY, Yang D, Yeung CM, et al. Lycium barbarum polysaccharides reduce neuronal damage, blood‐retinal barrier disruption and oxidative stress in retinal ischemia/reperfusion injury. PLoS ONE. 2011;6:e16380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao J, Xing F, Huo J, et al. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non‐alcoholic steatohepatitis rats and cellular steatosis model. Sci Rep. 2014;4:5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qian L, Yu S. Protective effect of polysaccharides from Lycium barbarum on spermatogenesis of mice with impaired reproduction system induced by cyclophosphamide. Am J Reprod Immunol. 2016;76:383‐385. [DOI] [PubMed] [Google Scholar]
- 21. Xin Y, Zhang S, Gu L, et al. Electrocardiographic and biochemical evidence for the cardioprotective effect of antioxidants in acute doxorubicin‐induced cardiotoxicity in the beagle dogs. Biol Pharm Bull. 2011;34:1523‐1526. [DOI] [PubMed] [Google Scholar]
- 22. Xin YF, Wan LL, Peng JL, Guo C. Alleviation of the acute doxorubicin‐induced cardiotoxicity by Lycium barbarum polysaccharides through the suppression of oxidative stress. Food Chem Toxicol. 2011;49:259‐264. [DOI] [PubMed] [Google Scholar]
- 23. Lu SP, Zhao PT. Chemical characterization of Lycium barbarum polysaccharides and their reducing myocardial injury in ischemia/reperfusion of rat heart. Int J Biol Macromol. 2010;47:681‐684. [DOI] [PubMed] [Google Scholar]
- 24. Hou YM, Wang J, Zhang XZ. Lycium barbarum polysaccharide exhibits cardioprotection in an experimental model of ischemia‐reperfusion damage. Mol Med Rep. 2017;15:2653‐2658. [DOI] [PubMed] [Google Scholar]
- 25. Tang JC, Zhang JN, Wu YT, Li ZX. Effect of the water extract and ethanol extract from traditional Chinese medicines Angelica sinensis (Oliv.) Diels, Ligusticum chuanxiong Hort. and Rheum palmatum L. on rat liver cytochrome P450 activity. Phytother Res. 2006;20:1046‐1051. [DOI] [PubMed] [Google Scholar]
- 26. Xin YF, You ZQ, Gao HY, et al. Protective effect of Lycium barbarum polysaccharides against doxorubicin‐induced testicular toxicity in rats. Phytother Res. 2012;26:716‐721. [DOI] [PubMed] [Google Scholar]
- 27. Mao F, Xiao B, Jiang Z, Zhao J, Huang X, Guo J. Anticancer effect of Lycium barbarum polysaccharides on colon cancer cells involves G0/G1 phase arrest. Med Oncol. 2011;28:121‐126. [DOI] [PubMed] [Google Scholar]
- 28. Luo Q, Li Z, Yan J, Zhu F, Xu RJ, Cai YZ. Lycium barbarum polysaccharides induce apoptosis in human prostate cancer cells and inhibits prostate cancer growth in a xenograft mouse model of human prostate cancer. J Med Food. 2009;12:695‐703. [DOI] [PubMed] [Google Scholar]
- 29. Mishra PK, Tyagi N, Kumar M, Tyagi SC. MicroRNAs as a therapeutic target for cardiovascular diseases. J Cell Mol Med. 2009;13:778‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu X, Wang H, Liu F, et al. Identification of micro‐RNA networks in end‐stage heart failure because of dilated cardiomyopathy. J Cell Mol Med. 2013;17:1173‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang K, Wu J, Duan X, et al. Huangqi injection in the treatment of chronic heart failure: a systematic review and meta‐analysis. Medicine (Baltimore). 2017;96:e8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aronson D, Krum H. Novel therapies in acute and chronic heart failure. Pharmacol Ther. 2012;135:1‐17. [DOI] [PubMed] [Google Scholar]
- 33. Zhang L, Liu Y, Lu XT, et al. Traditional Chinese medication Tongxinluo dose‐dependently enhances stability of vulnerable plaques: a comparison with a high‐dose simvastatin therapy. Am J Physiol Heart Circ Physiol. 2009;297:H2004‐H2014. [DOI] [PubMed] [Google Scholar]
- 34. Li X, Zhang J, Huang J, et al. A multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled study of the effects of qili qiangxin capsules in patients with chronic heart failure. J Am Coll Cardiol. 2013;62:1065‐1072. [DOI] [PubMed] [Google Scholar]
- 35. Ma L, Ni M, Hao P, et al. Tongxinluo mitigates atherogenesis by regulating angiogenic factors and inhibiting vasa vasorum neovascularization in apolipoprotein E‐deficient mice. Oncotarget. 2016;7:16194‐16204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Q, Cui HH, Yang YJ, et al. Quantitative proteomics analysis of ischemia/reperfusion injury‐modulated proteins in cardiac microvascular endothelial cells and the protective role of Tongxinluo. Cell Physiol Biochem. 2017;41:1503‐1518. [DOI] [PubMed] [Google Scholar]
- 37. Xia LM, Luo MH. Study progress of berberine for treating cardiovascular disease. Chronic Dis Transl Med. 2015;1:231‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo N, Yang D, Wang X, Dai J, Wang M, Lei Y. Metabonomic study of chronic heart failure and effects of Chinese herbal decoction in rats. J Chromatogr A. 2014;1362:89‐101. [DOI] [PubMed] [Google Scholar]
- 39. Zhou Q, Qin WZ, Liu SB, Kwong JS, Zhou J, Chen J. Shengmai (a traditional Chinese herbal medicine) for heart failure. Cochrane Database Syst Rev. 2014:CD005052. [DOI] [PubMed] [Google Scholar]
- 40. Fu S, Zhang J, Menniti‐Ippolito F, et al. Huangqi injection (a traditional Chinese patent medicine) for chronic heart failure: a systematic review. PLoS ONE. 2011;6:e19604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang D, So KF, Lo AC. Lycium barbarum polysaccharide extracts preserve retinal function and attenuate inner retinal neuronal damage in a mouse model of transient retinal ischaemia. Clin Exp Ophthalmol. 2017;45:717‐729. [DOI] [PubMed] [Google Scholar]
- 42. Potterat O. Goji (Lycium barbarum and L. chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010;76:7‐19. [DOI] [PubMed] [Google Scholar]
- 43. Kampourakis T, Sun YB, Irving M. Myosin light chain phosphorylation enhances contraction of heart muscle via structural changes in both thick and thin filaments. Proc Natl Acad Sci USA. 2016;113:E3039‐E3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fischer TH, Eiringhaus J, Dybkova N, et al. Ca(2+)/calmodulin‐dependent protein kinase II equally induces sarcoplasmic reticulum Ca(2+) leak in human ischaemic and dilated cardiomyopathy. Eur J Heart Fail. 2014;16:1292‐1300. [DOI] [PubMed] [Google Scholar]
- 45. Luczak ED, Anderson ME. CaMKII oxidative activation and the pathogenesis of cardiac disease. J Mol Cell Cardiol. 2014;73:112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosas PC, Liu Y, Abdalla MI, et al. Phosphorylation of cardiac Myosin‐binding protein‐C is a critical mediator of diastolic function. Circ Heart Fail. 2015;8:582‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guilbert A, Lim HJ, Cheng J, Wang Y. CaMKII‐dependent myofilament Ca2+ desensitization contributes to the frequency‐dependent acceleration of relaxation. Cell Calcium. 2015;58:489‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Massengill MT, Ashraf HM, Chowdhury RR, et al. Acute heart failure with cardiomyocyte atrophy induced in adult mice by ablation of cardiac myosin light chain kinase. Cardiovasc Res. 2016;111:34‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kampourakis T, Irving M. Phosphorylation of myosin regulatory light chain controls myosin head conformation in cardiac muscle. J Mol Cell Cardiol. 2015;85:199‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang Y, Wang Y, Park KM, et al. MicroRNA‐150 protects the mouse heart from ischaemic injury by regulating cell death. Cardiovasc Res. 2015;106:387‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wahlquist C, Jeong D, Rojas‐Munoz A, et al. Inhibition of miR‐25 improves cardiac contractility in the failing heart. Nature. 2014;508:531‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]


