Human babesiosis is an emerging zoonotic infectious disease caused by intraerythrocytic protozoan parasites of the genus Babesia. Most cases of human babesiosis are caused by Babesia microti and often manifest in individuals over the age of 50 years or in patients with a compromised immune system.
KEYWORDS: antigen, Babesia microti, babesiosis, BmGPI12, diagnosis, transfusion
ABSTRACT
Human babesiosis is an emerging zoonotic infectious disease caused by intraerythrocytic protozoan parasites of the genus Babesia. Most cases of human babesiosis are caused by Babesia microti and often manifest in individuals over the age of 50 years or in patients with a compromised immune system. Patients who develop symptomatic B. microti infections usually experience months of asymptomatic infection after the acute infection has resolved. About one-fifth of B. microti-infected adults never develop symptoms. These asymptomatically infected individuals sometimes donate blood and thus can transmit B. microti through blood transfusion. Current assays for detection of active B. microti infections can be used to screen donor blood prior to transfusion, but they rely primarily on microscopy or PCR methods, which have sensitivity and technical limitations. Here we report the development of an antigen capture enzyme-linked immunosorbent assay (BmGPAC) based on a major secreted immunodominant antigen of B. microti (BmGPI12/BmSA1), and we provide evidence that this assay is superior for detection of active B. microti infections, compared to available microscopy methods and serological assays. The assay has been evaluated using supernatants of B. microti-infected erythrocytes cultured in vitro, sera from B. microti-infected laboratory mice, and sera from wild mice and human patients. Our data suggest that the BmGPAC assay is a reliable assay for detection of active B. microti infections and is superior to real-time PCR and antibody assays for diagnosis of acute B. microti infections, screening of the blood supply, and epidemiological surveys of humans and animal reservoir hosts.
INTRODUCTION
Human babesiosis, which is caused by intraerythrocytic apicomplexan parasites of the genus Babesia, is an emerging infectious disease with global distribution (1). Among the five species of Babesia that infect humans, Babesia microti is responsible for most cases of human babesiosis reported to date. Although this parasite is transmitted primarily by ixodid ticks, blood transfusion is another important route of infection (1). Common babesiosis symptoms include fever, fatigue, chills, sweats, headache, myalgia, arthralgia, and/or anorexia. Laboratory abnormalities include thrombocytopenia, hemolytic anemia, and/or elevated liver enzymes (1). Severe symptoms are often experienced by patients with a compromised immune system due to asplenia, cancer, or HIV infection and by those who acquire the infection through blood transfusion. Death resulting from B. microti infection has been reported in up to one-fifth of such patients (2–5). In states in which babesiosis was reported prior to 2011, a recent assessment by the U.S. Food and Drug Administration (FDA) showed that, in the 5-year period ending in 2015, 3 of 18 fatal transfusion-related infections in the United States were caused by babesiosis (6).
In 2011, babesiosis was added to the CDC list of notifiable diseases (7). The number of babesiosis cases reported to the CDC increased from 1,124 in 2011 to 1,613 in 2016. However, because immunocompetent individuals are often asymptomatic, with submicroscopic levels of parasitemia, the incidence of babesiosis is likely to be significantly underestimated (1). Recently, Stein and colleagues analyzed Wisconsin babesiosis surveillance data for 2001 to 2015, in 3-year periods, and found that the incidence of confirmed babesiosis increased 26-fold (8). This increase in the occurrence of babesiosis was concomitant with the geographic expansion of the vector (8). There have been concomitant increases in both tick-transmitted infections and transfusion-transmitted infections (9).
Various laboratory diagnostic methods, including microscopy, immunofluorescence assay (IFA), and PCR methods, have been developed for the detection of B. microti infections. In 2017, a nucleic acid-based test (Procleix Babesia; Grifols) received approval for use for blood screening under a U.S. FDA investigational new drug application. The assay can detect B. microti, Babesia duncani, Babesia divergens, and Babesia venatorum in human whole blood (10), with the 95% lower limit of detection (LOD) ranging from 7.10 to 13.51 copies/ml. For B. microti (n = 9), the sensitivity of the Grifols assay at 95% LOD ranged from 0.64 to 3.61 parasites/ml. Furthermore, two investigational assays, the Immunetics enzyme immunoassay (EIA) and the Imugen arrayed fluorescence immunoassay (AFIA) for the detection of antibodies to B. microti, have been considered for screening of the blood supply. These assays have been used for seroprevalence analyses in regions in which babesiosis is endemic and regions in which it is not endemic; the reported rates varied from 0.28 to 0.75% and from 0.025 to 0.13%, respectively, with equivalent specificities varying from 99.93 to 99.98% (11–13). While DNA-based techniques can be specific and sensitive, they can also detect residual parasite DNA in the absence of active infection. Similarly, assays that detect antibodies to B. microti can provide positive signals even after the infection has been eliminated.
The completion of genome sequencing and annotation of the B. microti R1 clinical isolate in 2012 (14) and the subsequent DNA and RNA sequencing of other clinical and field strains isolated from patient blood or infected ticks created a unique opportunity to identify new drug targets and novel antigens for diagnosis of active B. microti active infections (15–17). Recently, a multidisciplinary approach combining in silico analysis and immunoproteomics identified BmGPI12 (also known as BmSA1) as a glycosylphosphatidylinositol (GPI)-anchored protein and an immunodominant antigen of B. microti (16, 17). BmGPI12 is a member of the B. microti BMN multigene family (18) and was shown previously to be present in sera from B. microti-infected hamsters (19). Using sera from B. microti-infected mice, it was shown that anti-BmGPI12 IgM and IgG antibodies can be detected as early as 4 days and 8 days postinfection, respectively (16, 17). Antibodies against this protein were also detected in sera of Babesia-infected wild mice and humans (17). Although detection of anti-BmGPI12 IgM and IgG antibodies in sera of humans and mice is specific to B. microti and is highly sensitive, the assay cannot reliably distinguish between past and current infections. Here we show that an antigen capture (BmGPAC) assay for detection of secreted BmGPI12 protein in plasma or serum from humans and animals is a superior method and results correlate with active B. microti infection.
MATERIALS AND METHODS
Mouse and parasite strains.
All experimental protocols involving animals followed Yale University institutional guidelines for the care and use of laboratory animals and were approved by the institutional animal care and use committee at Yale University. Rules for ending experiments in mice were to be followed if animals showed any signs of distress or appeared moribund. Parasitemia was determined by light microscopy, by counting a minimum of 5,000 red blood cells (RBCs) on Giemsa-stained blood smears. The B. microti LabS1 and PRA-99 strains were routinely maintained in SCID mice. SCID (CB17/Icr-Prkdcscid/IcrIcoCrl) mice, Swiss Webster outbred [Crl:CFW(SW)] mice, and BALB/c inbred mice were purchased from Charles River Laboratories. Both Swiss Webster mice and BALB/c mice were used in two independent in vivo studies. Sera from wild-caught white-footed mice (Peromyscus leucopus) caught in the towns of North Branford, Redding, and Storrs, Connecticut, were collected as reported previously (17).
Patient sera.
Blood was drawn from B. microti-infected patients into serum collection tubes and subjected to centrifugation at 1,300 × g. Blood collections were performed according to Yale University human investigation committee guidelines.
Animal injection, plasma and serum collection, DNA extraction, and PCR methods.
Groups of mice consisting of 5 Swiss Webster males, 5 Swiss Webster females, 5 BALB/c males, and 5 BALB/c females were injected intraperitoneally (i.p.) with 106 B. microti-infected RBCs. As a control, another group of 5 BALB/c females were injected with B. microti-infected erythrocytes but also were subjected to combination therapy (atovaquone plus ELQ-334, 10 mg/kg each, daily for 7 days starting at day 4 postinfection), as described previously (20). Smears, serum samples, and plasma samples were collected prior to infection and every 4 days postinfection for 42 days. Genomic DNA extraction and real-time PCR were carried out as reported previously (31).
Short-term in vitro culture.
Blood collected from B. microti (LabS1 strain)-infected mice was used in a short-term in vitro culture as follows. Fifty microliters of infected mouse RBCs (15% parasitemia) was mixed with 250 μl of uninfected mouse RBCs (10% hematocrit), 100 μl of uninfected human RBCs (50% hematocrit), and 1 ml of culture medium supplemented with HB 101 (product no. T151; Irvine Scientific), l-glutamine (product no. B90210; Atlanta Biologicals), 1× antibiotic/antimycotic (product no. 30-004-C1; Corning), and hypoxanthine (200 μM)-thymidine (30 μM) (product no. H0137; Sigma). Parasitemia was monitored over time by counting a minimum of 5,000 RBCs in Giemsa-stained thin blood smears. For immunoblot analyses, 1-ml samples of cultured infected and uninfected human RBCs were collected 6 h and 24 h after initiation of the in vitro culture. These samples were centrifuged at 400 × g for 10 min, and the supernatants were collected. The remaining cell pellets were treated with 1% saponin, incubated on ice for 30 min, and centrifuged at 9,300 × g for 10 min at 4°C. The resulting supernatant (hemolysate) was collected, and the final pellet (parasite fraction) was washed once with cold phosphate-buffered saline (PBS). Supernatant, hemolysate, and parasite fractions were separated by SDS-PAGE and analyzed by immunoblotting using preimmune and anti-BmGPI12 sera.
Expression and purification of recombinant BmGPI12.
An open reading frame encoding amino acids 1 to 302 of BmGPI12 (GenBank accession no. JX112361) was synthesized and cloned into the BamHI and XhoI restriction sites of plasmid pGEX6p-1 (GE Healthcare). The resulting plasmid was introduced into Escherichia coli strain BL21. Expression of the glutathione S-transferase-BmGPI12 fusion was performed in LB culture medium with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), using standard microbiological techniques. Cells were lysed with B-PER (Thermo Fisher Scientific), and the lysate was clarified by centrifugation at 15,000 rpm for 30 min at 4°C. The fusion protein was purified by affinity chromatography on a glutathione chromatography cartridge, as recommended by the manufacturer (Pierce). The glutathione S-transferase moiety was cleaved using PreScission protease (GE Healthcare) and was removed by glutathione affinity chromatography. Recombinant BmGPI12 was further purified by anion-exchange chromatography on a Mono Q column (GE Healthcare). The identity of recombinant BmGPI12 was ascertained by N-terminal sequencing, intact mass spectrometry, and tandem mass spectrometry (MS/MS) protein identification.
Preparation of hyperimmune rabbit antiserum against BmGPI12.
A New Zealand White rabbit (Cocalico Biologicals, Reamstown, PA) was immunized with three i.p. injections of 0.5 mg recombinant antigen. The endpoint dilution titer of the resulting antiserum was greater than 108 when tested against recombinant BmGPI12. This antiserum recognizes a single protein in Babesia-infected RBCs with the molecular weight expected for BmGPI12. IgG was purified from the antiserum by affinity chromatography on a HiTrap protein G column (GE Healthcare), as recommended by the manufacturer. Carboxyl groups of the IgG molecules were biotinylated with an EZ-Link amine-PEG3-biotin kit (Thermo Fisher Scientific), according to the manufacturer's instructions.
Immunodetection of BmGPI12.
Supernatant, hemolysate, and RBC membrane or parasite fractions from control (uninfected RBCs) or B. microti-infected RBCs cultured in vitro were collected 6 and 24 h postinoculation, mixed with an equal volume of 2× SDS-PAGE loading dye, boiled for 5 min, loaded onto 10% SDS-PAGE gels, and transferred to nitrocellulose membranes. Membranes were blocked with 5% milk and incubated overnight at 4°C with anti-BmGPI12 serum (1:250 dilution) or preimmune serum (1:250 dilution). The next day, membranes were washed with TBST (0.02 M Tris base, 0.15 M NaCl, and 0.1% Tween 20) and incubated for 1 h with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (1:10,000 dilution). Following additional washings, the membranes were reacted with enhanced chemiluminescence (ECL) Western blotting detection reagents (GE Healthcare, Amersham) and exposed to X-ray film. To detect BmGPI12 in human plasma, a sample that previously tested positive for B. microti by Giemsa staining and PCR was subjected to Western blotting using the protocol described above. Western blot analyses were performed with animal sera using the same procedure.
BmGPAC assay.
The BmGPAC assay was developed to detect BmGPI12 in biological samples. For this, 96-well white enzyme-linked immunosorbent assay (ELISA) plates (Pierce) were coated overnight at 4°C with 400 ng per well of purified unlabeled anti-BmGPI12 IgG. The following day, unbound anti-BmGPI12 IgG was removed, 200 μl blocking solution (consisting of 5% bovine serum albumin [BSA] in PBS with 0.05% Tween 20 [PBST]) was added, and the plates were incubated for 1 h at room temperature. BmGPI12-containing samples were diluted with PBST to 100 μl, added to wells, and incubated for 2 h at room temperature. Wells were washed four times with PBST, biotinylated anti-BmGPI12 IgG was added at a concentration of 10 μg/ml, and plates were incubated for 1 h at room temperature. After four washes, streptavidin conjugated to alkaline phosphatase (Kirkegaard & Perry Laboratories) was added at a dilution of 1:1,000 in PBST, and plates were incubated for 1 h at room temperature. After four washes, 100 μl developing solution (PhosphaGlo reagent; Kirkegaard & Perry Laboratories) was added and the luminescence was measured with a BioTek FLx800 fluorescence plate reader. Multiple standard curves were generated using recombinant BmGPI12. The lower LOD was typically 20 pg/μl.
RESULTS
BmGPI12 is a secreted antigen of B. microti.
Previous studies using immunofluorescence analysis showed strong expression of BmGPI12 in B. microti-infected erythrocytes (16, 17). The protein was also reported previously to be present in sera from infected hamsters and serum from an infected patient (21). Together, these data suggested that BmGPI12 either is released from B. microti-infected erythrocytes at the end of the intraerythrocytic life cycle or is actively secreted into the host environment by B. microti-infected erythrocytes. To validate the secretion of BmGPI12 by B. microti-infected erythrocytes, we raised rabbit polyclonal antibodies against the protein and used them to detect the presence of the protein in sera or plasma of mice and humans infected with this parasite, as well as in the supernatant of an in vitro culture of B. microti. As shown in Fig. 1, BmGPI12 protein was readily detected by immunoblotting in plasma from mice infected with B. microti strain LabS1 or PRA99. As controls, no protein could be detected in samples from uninfected mice or in plasma samples tested using rabbit preimmune sera (Fig. 1A). We further examined the presence of BmGPI12 in plasma from a patient who underwent exchange transfusion following an infection with B. microti that resulted in high levels of parasitemia. As shown in Fig. 1B, BmGPI12 could be readily detected in as little as 10 μl of the patient's plasma. The BmGPI12 concentration was determined based on the signal detected using recombinant BmGPI12 as a standard. As expected, BmGPI12 was not detected in human plasma samples tested using preimmune rabbit serum (not shown).
FIG 1.
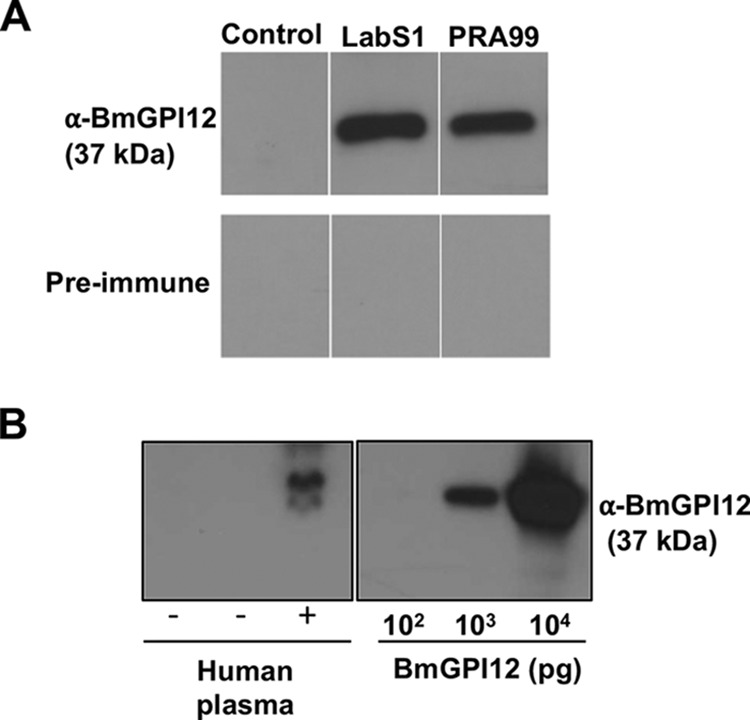
Evidence for secretion of BmGPI12 in mouse and human blood. (A) Immunoblotting detection, using anti-BmGPI12 rabbit serum, of BmGPI12 in plasma from mice infected with either B. microti LabS1 or PRA99. Plasma from uninfected mice and preimmune rabbit serum were used as controls. (B) Immunoblotting detection, using anti-BmGPI12, of BmGPI12 in 10 μl of plasma from a B. microti PCR-positive human patient (parasitemia, ∼10%), compared with plasma from healthy donors. +, individuals confirmed positive for babesiosis; −, healthy donors with no babesiosis. Amounts of BmGPI12 in human plasma were compared with 102, 103, and 104 pg of purified recombinant BmGPI12 protein.
To further demonstrate secretion of this protein from infected erythrocytes, we set up a short-term in vitro culture to maintain infected erythrocytes in serum-free culture medium immediately after collection from mice infected with the B. microti strain LabS1. Infected erythrocytes were washed extensively to remove any residual secreted proteins, and the presence of the protein in the parasite, RBC cytoplasm, and culture supernatant was monitored over a 24-h culture period. As shown in Fig. 2A, during this short-term in vitro culture assay, B. microti developed from an early ring form to a mature ring with long extensions and divided to form 4 daughter parasites (tetrad). Immunoblot analysis showed increased expression of BmGPI12 over time, with the protein being detected not only in the parasite fraction but also in the hemolysate and supernatant of B. microti-infected erythrocytes. These data demonstrate that BmGPI12 is increasingly synthesized during parasite intraerythrocytic development, actively secreted by the parasite into the RBC cytoplasm, and subsequently released outside the infected erythrocyte (Fig. 2B).
FIG 2.
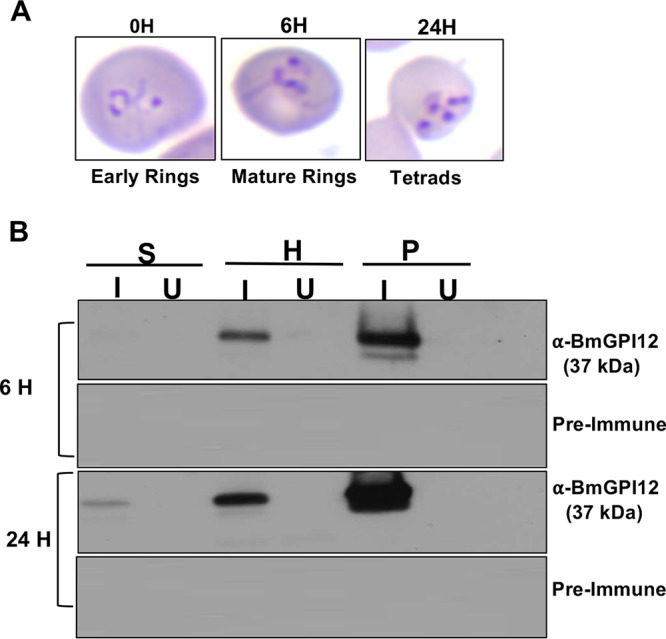
Short-term in vitro cultures of B. microti. (A) Giemsa-stained blood smears, indicating the developmental stages of B. microti 0, 6, and 24 h postinoculation. Percentages of stages were calculated as the number of B. microti-infected cells in at least 5,000 RBCs. (B) Detection of BmGPI12 in in vitro B. microti cultures collected 6 and 24 h following inoculation. At each time point, supernatant (S), hemolysate (H), and pellet (P) fractions from uninfected (U) or B. microti-infected (I) cultures were analyzed by immunoblotting. No signal could be detected with preimmune rabbit serum.
The BmGPI12-based antigen capture assay detects active B. microti infections.
The finding that BmGPI12 can be readily detected as a secreted antigen in sera and plasma of infected animals and humans led us to develop an antigen capture assay using antibodies against BmGPI12, referred to as the BmGPAC assay. The antigen was captured on a solid surface coated with anti-BmGPI12 polyclonal antibodies and was detected using biotin-coupled antibodies (Fig. 3A). The antigen-antibody complex was then detected using alkaline phosphatase-streptavidin, and the signal was measured using a luminescence spectrophotometer. A standard curve generated using serial dilutions of the protein yielded a typical detection limit of 20 pg/μl (Fig. 3B). To evaluate the LOD of the BmGPAC assay in vitro, B. microti-infected RBCs were isolated from an infected mouse, extensively washed in serum-free medium, and serially diluted from 106 B. microti-infected RBCs to 1 infected RBC. The hematocrit level of the cultures was adjusted to 2% and the cultures were gassed and maintained at 37°C using the short-term in vitro culture assay described in Materials and Methods. Supernatant fractions collected from each culture were then analyzed using the BmGPAC assay. As shown in Fig. 3C, BmGPI12 levels above the LOD could be found in 5 μl of a supernatant from a 1-ml culture containing as few as 10 B. microti-infected erythrocytes (Fig. 3C). To evaluate the use of the BmGPAC assay for detection of B. microti infections, the assay was employed to test sera from babesiosis patients. Consistent with the levels of parasitemia in the patients with confirmed babesiosis, the concentrations of BmGPI12 in patients' blood ranged between 20 and 15,000 pg/μl of serum. Human blood samples that were determined to be PCR negative for B. microti were also negative using the BmGPAC assay (Fig. 4). As a control for the specificity of the BmGPAC assay, no signal could be detected using plasma from a Plasmodium vivax-infected patient (parasitemia, 0.1%) or a healthy human donor (Fig. 4). To further evaluate the cross-reactivity of the assay, we tested supernatants from in vitro cultures of B. duncani (parasitemia, 0.1% to 6.8%) and sera from noninfected or Babesia bovis-infected cattle using the BmGPAC assay; no signals could be detected (Fig. 4).
FIG 3.
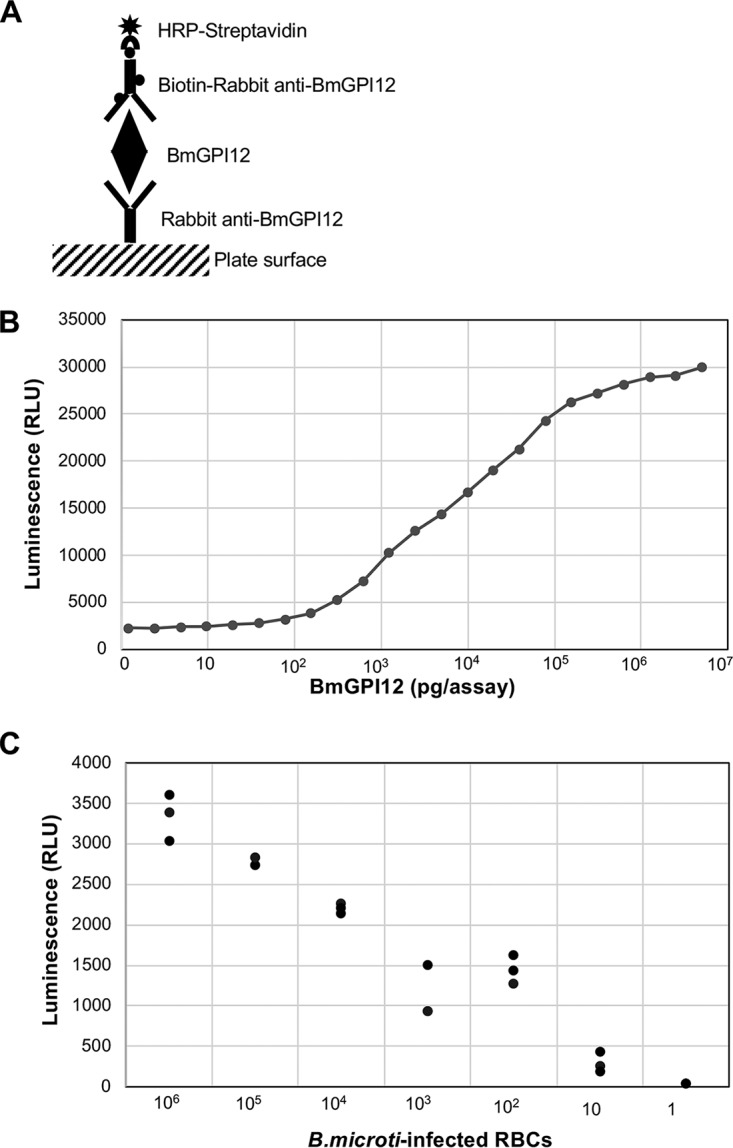
Sandwich ELISA for detection of BmGPI12. (A) Schematic representation of the BmGPAC assay components. (B) Representative standard curve generated using 5 μl recombinant BmGPI12 at different protein concentrations. (C) Detection of BmGPI12 released from infected mouse RBCs. A total of 106 infected mouse RBCs were serially diluted and incubated for 6 h at 37°C, using short-term in vitro culture conditions. Culture supernatants were used in the BmGPAC assay. Three replicates were used for each condition. RLU, relative light units.
FIG 4.
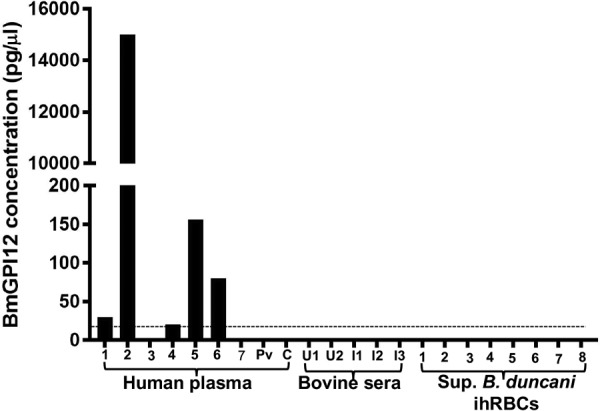
Detection of BmGPI12 in plasma from patients and control noninfected individuals. 1, babesiosis symptoms but PCR negative; smears and IFA were not done; 2, 11.5% parasitemia determined by blood smear; 3, 0.3% parasitemia determined by blood smear; 4, 1.8% parasitemia determined by blood smear and PCR positive; 5, 10% parasitemia determined by blood smear and PCR positive; 6, 5.4% parasitemia determined by blood smear and PCR positive; 7, Lyme disease, with no babesiosis documented. Plasma samples from B. microti PCR-positive patients or PCR-negative donors (1 to 7) were compared with the following samples: plasma from a P. vivax patient (Pv) and a healthy donor (C), noninfected bovine sera (U1 and U2), infected B. bovis sera (I1 to I3), and supernatants (Sup.) from in vitro cultures of B. duncani-infected human RBCs (ihRBCs) (1 to 8), with levels of parasitemia ranging from 0.12 to 6.8%. As expected, negative controls did not show any signal in the BmGPAC assay. The dotted line represents the LOD of BmGPI12 in the assay (20 pg/μl).
Next, we compared the BmGPAC assay to real-time PCR and microscopy for detection of active infections in animals. Immunocompetent outbred Swiss Webster mice (males and females) and inbred BALB/c mice (males and females) were infected with B. microti (strain LabS1), and blood samples were analyzed using the three assays. BmGPI12 antigen above the 100 pg/assay (20 pg/μl) cutoff value could be detected in all infected Swiss Webster animals as early as 6 h postinoculation, and levels increased over the next 11 days, reaching a peak of 225 pg/assay (45 pg/μl) between day 11 and day 14 (Fig. 5A; also see Table S1 in the supplemental material). This increase was concomitant with the increase in parasitemia determined by light microscopy and the decrease in threshold cycle (CT) values below the cutoff value of 35 determined by real-time PCR (Fig. 5B and C; also see Tables S2 and S3). Between day 14 and day 28, BmGPI12 levels decreased gradually to reach 20 pg/μl. Between day 28 and day 35, whereas BmGPI12 levels were below 20 pg/μl and no infected RBCs could be found in thin blood smears, real-time PCR CT values remained above the detection limit. Similar results were obtained using inbred BALB/c mice, although the parasite loads and the rates of clearance of parasitemia varied between the two mouse strains (Fig. 6A and B; also see Tables S1 and S2). To determine whether the differences in the BmGPAC and real-time PCR assay results during the resolution stage of infection could be due to residual B. microti DNA detected by real-time PCR even after parasite clearance, BALB/c mice were treated with atovaquone and the endochin-like quinolone ELQ-334 (10 mg/kg each). This combination was shown previously to result in radical cures in mice (20). Consistent with the rapid killing effect and decline in parasitemia induced by the combination therapy, the levels of BmGPI12 antigen and parasite DNA in treated mice reached their peaks on day 11 and decreased thereafter, falling below detection levels by day 21. The decline, however, was much more precipitous with the BmGPAC assay than with the real-time PCR (Fig. 6A and B). This finding suggests that, after parasite elimination, DNA remains in circulation longer than antigen, suggesting that the BmGPAC assay is a more reliable method for detection of active infections.
FIG 5.
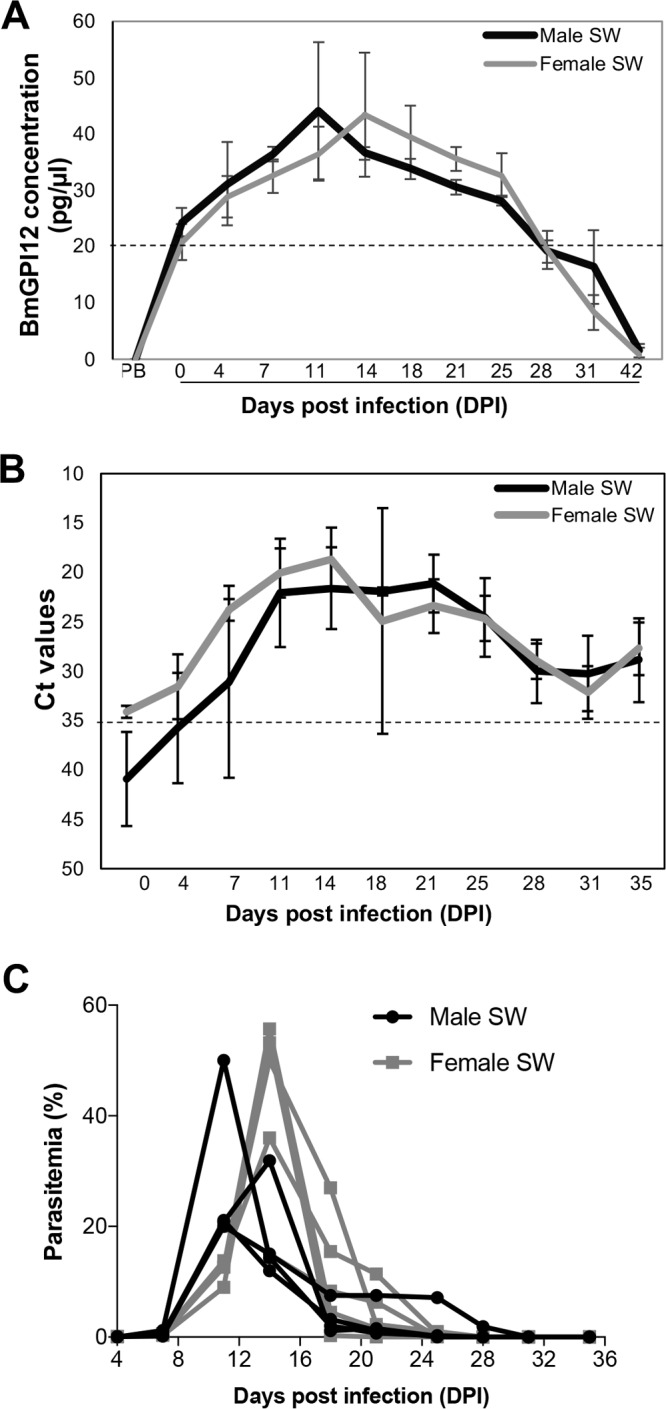
BmGPI12 detection, 18S rDNA expression, and parasitemia in Swiss-Webster (SW) mice inoculated with 106 B. microti-infected RBCs. (A) BmGPI12 detection in plasma from Swiss Webster mice. PB, prebleed (blood collection prior to B. microti infection); DPI, days postinfection. Each point represents an average of 5 mice. The dotted line represents the lower LOD. (B) CT values obtained with real-time PCR using DNA isolated from Swiss Webster plasma. The dotted line represents the CT cutoff value. (C) Parasitemia curves for Swiss Webster mice. Each point represents parasitemia for each male or female Swiss Webster mouse.
FIG 6.
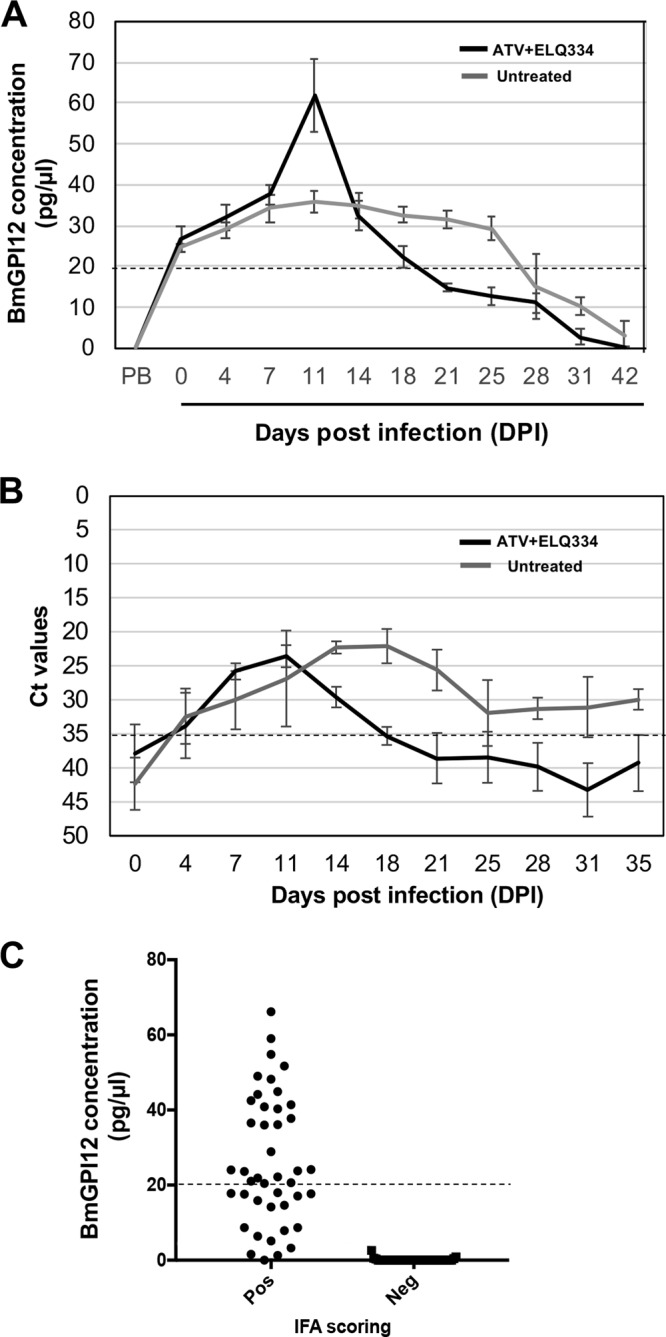
BmGPAC and real-time PCR assays with blood from BALB/c mice infected with 106 B. microti-infected RBCs and detection of BmGPI12 in the plasma of 84 wild-caught Peromyscus leucopus mice. (A) BmGPI12 detection in plasma from untreated BALB/c mice versus combination therapy (atovaquone plus ELQ-334)-treated BALB/c mice. Each point represents the average of 5 mice. PB, prebleed (blood collection prior to B. microti infection). The dotted line represents the LOD. (B) CT values in real-time PCR assays using DNA isolated from plasma from untreated versus treated (atovaquone plus ELQ-334) BALB/c mice. Each point represents the average of 5 mice. The dotted line represents the CT cutoff value. (C) Correlation of IFA results and BmGPI12 levels. Plasma samples from all animals were initially evaluated with an IFA to detect anti-Babesia antibodies and were classified as IFA positive or IFA negative. Five microliters of plasma was assayed using the BmGPAC assay. Dotted lines represent the LODs.
The BmGPAC assay detects B. microti infection in the reservoir host.
Previous studies examining the presence of anti-B. microti IgG antibodies in the mouse reservoir Peromyscus leucopus showed variations in titers for different animals (22). However, detection of the presence of these antibodies is not a reliable method for detection of active infections. More recently, it was shown that IFA-positive sera from wild mice were generally also positive for IgM and IgG antibodies against BmGPI12 (17). To determine whether the BmGPAC assay could detect the presence of BmGPI12 antigen in sera from wild mice, we screened 39 IFA-negative and 45 IFA-positive P. leucopus sera using the assay. All IFA-negative sera had levels below the cutoff value of 20 pg/μl, whereas 28 of the 45 IFA-positive sera had levels above 20 pg/μl and another 17 had levels above the levels detected in the IFA-negative samples (Fig. 6C; also see Table S4).
DISCUSSION
We have developed an antigen capture assay (the BmGPAC assay) that confirms active B. microti infection through detection of secreted BmGPI12 protein. The assay can detect as little as 20 pg of BmGPI12 per μl of blood from infected animals and humans. The assay can be used to confirm acute B. microti infection, to screen blood donations for prevention of transfusion-transmitted babesiosis, and to conduct epidemiological surveys of B. microti infection in humans and reservoir hosts.
Detection of B. microti blood infection during blood donor screening is a major priority to prevent transfusion-transmitted babesiosis in the United States (9, 23). Current strategies for detection of subclinical infections have significant limitations. IFA-based diagnostic assays cannot reliably distinguish between current and past infections. PCR assays may not detect low-level parasitemia because of sampling limitations with small blood volumes (e.g., 1 to 2 ml from 500 ml of donated blood). Such limitations can have major consequences, as reported recently by Glanternik and colleagues regarding a cluster of B. microti cases among three premature infants in one neonatal intensive care unit that were traced to a single blood donor (24).
During its intraerythrocytic development, B. microti secretes several proteins into the RBC cytoplasm. Some of these proteins reside in the cytoplasm or traffic to the RBC membrane, whereas others are secreted into the host environment. Recent annotation of the B. microti proteome estimated that 398 proteins are secreted by the parasite (17). The roles these secreted proteins play in host cell remodeling, nutrient acquisition, or modulation of host immunity remain to be determined. Secreted proteins have been shown to play a critical role in protective immunity following infection with B. divergens, B. bovis, or Babesia rossi (25–27). The ability of some B. microti proteins to trigger strong immune responses in mammals led to the development of various assays aimed at detecting antibodies against these proteins in humans and animals. These antibody detection assays targeted not only anti-BmGPI12 antibodies (19) but also antibodies against the B. microti BMN1-17 family (18), BmP32 (28), and BmP94 (29). The ability to detect a secreted B. microti antigen as a biomarker of infection was first reported by Luo and colleagues (21). Using a double antibody sandwich ELISA, they demonstrated successful detection of BmSA1/BmGPI12 in the blood of B. microti-infected hamsters.
Our data used two different isolates of B. microti (LabS1 and PRA99) and provided in vitro and in vivo evidence that BmGPI12 is secreted by infected RBCs in amounts sufficient to develop a reliable diagnostic assay for detection of active B. microti infection. Using a short-term in vitro growth assay with blood freshly collected from infected animals during the exponential phase of growth and extensively washed to remove any residual secreted BmGPI12, we demonstrated that the overall levels of BmGPI12 increased with the time in culture, concomitant with the development of the parasite from an early ring form to a mature ring and then to a tetrad. Immediately after inoculation, BmGPI12 could be detected primarily in the parasite and RBC cytoplasm fractions. Over the course of the 24-hour intraerythrocytic development, the overall amount of the protein synthesized by the parasite was more than double the amount detected at 6 h. Interestingly, significant amounts of the protein could also be detected in the supernatant of the culture and accumulated over time to levels in the micromolar range. Consistent with the active secretion of BmGPI12 by B. microti-infected RBCs, our data showed that the protein could be readily detected in sera and plasma from B. microti-infected mice and plasma from an infected patient.
Our finding that the BmGPAC assay could detect the presence of the protein in amounts as low as 100 pg per assay is an improvement over the findings of the previous study by Luo and colleagues, who used a double antibody sandwich ELISA with a quantitation limit of 250 pg per assay for detection of BmSA1/BmGPI12 (21). Importantly, there was no cross-reactivity when the BmGPAC assay was used with sera from P. vivax-infected patients or culture supernatants of other Babesia species, including B. duncani and B. bovis. Our limiting dilution in vitro assays showed that secreted BmGPI12 levels could be detected reliably in the culture supernatants of 10 B. microti-infected erythrocytes.
Using outbred Swiss Webster mice, we found that the results of the three assays (BmGPAC assay, smears, and real-time PCR assay) showed positive correlation during the acute phase of infection, with peak levels being detected between day 11 and day 14 by all assays. During the resolution phase, however, BmGPI12 levels above 20 pg/μl could be detected up to 28 days postinfection but DNA detection by real-time PCR remained above the CT cutoff value until day 35. Using the drug combination of atovaquone and ELQ-334, which results in rapid killing of parasites and clearance of parasitemia, the BmGPAC and real-time PCR assays showed major increases in antigen and DNA levels at day 11, consistent with parasite killing and release of protein and DNA contents into the host environment. This was followed by rapid decreases in these levels, with both assays revealing the presence of the antigen and DNA at detection levels by day 18 and below those levels by day 21. The decline was more precipitous with the BmGPAC assay than with real-time PCR, however, suggesting that, following parasite clearance, the antigen is rapidly cleared but DNA can persist in the circulation. Persistence of parasite DNA after successful therapy has been widely documented but was systematically investigated in malaria patients only recently. Homann and colleagues showed, using blood from 31 successfully treated malaria patients, that DNA could be detected by msp2- and species-specific PCR assays up to 31 and 42 days after treatment, respectively, whereas parasites could be detected by microscopy up to 2 days after treatment (30).
We showed that the BmGPAC assay also could be used to detect infection in wild mice and thus could be useful for epidemiological studies. Using the BmGPAC assay, we found that sera from animals that had been found previously to be IFA negative had no detectable amounts of BmGPI12, whereas 63% of IFA-positive sera contained levels of BmGPI12 above 20 pg/μl and 91% had antigen levels above 4 pg/μl. Mice with levels of BmGPI12 between 1.4 and 4 pg/μl represent animals that either have cleared the infection or maintain very low levels of parasitemia.
Together, these data demonstrate that the BmGPAC assay is a reliable assay for detection of active B. microti infections and could be a suitable assay for large-scale screening of the blood supply. Future efforts will aim to optimize the BmGPAC assay, to compare it to blood smears and real-time PCR assays for B. microti-infected and noninfected human blood, and to use it to screen randomly collected human blood samples from healthy adults from areas in which the parasite is endemic and areas in which it is not endemic.
Supplementary Material
ACKNOWLEDGMENTS
C.B.M. and members of his laboratory were supported by grants from the National Institutes of Health (grants AI097218, GM110506, AI123321, and R43AI136118) and the Bill and Melinda Gates Foundation (grant OPP1021571). M.L. and S.M. were supported by NIH grant R43AI136118.
We thank David Allred for providing B. bovis samples, Michael Riscoe for providing ELQ drugs, and Chloe Baudy for her help with microscopic analyses.
J.T., S.M., L.L., B.A.P., and M.G. performed the experiments; J.T., S.M., M.L., and C.B.M. analyzed the data; M.L. and C.B.M. designed the study; J.T., S.M., L.L., M.L., and C.B.M. wrote the manuscript; S.W. provided sera from wild mice and edited the manuscript; J.S.D. provided the ELQ334; and P.J.K. provided the human blood sample and contributed to the writing of the manuscript.
We declare we have no conflicts of interest regarding the contents of this article.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00067-18.
REFERENCES
- 1.Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. 2015. Babesiosis. Infect Dis Clin North Am 29:357–370. doi: 10.1016/j.idc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Genda J, Negron EA, Lotfipour M, Balabhadra S, Desai DS, Craft DW, Katzman M. 2016. Severe Babesia microti infection in an immunocompetent host in Pennsylvania. J Investig Med High Impact Case Rep 4:2324709616663774. doi: 10.1177/2324709616663774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause PJ, Gewurz BE, Hill D, Marty FM, Vannier E, Foppa IM, Furman RR, Neuhaus E, Skowron G, Gupta S, McCalla C, Pesanti EL, Young M, Heiman D, Hsue G, Gelfand JA, Wormser GP, Dickason J, Bia FJ, Hartman B, Telford SR III, Christianson D, Dardick K, Coleman M, Girotto JE, Spielman A. 2008. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 46:370–376. doi: 10.1086/525852. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez E, Vannier E, Wormser GP, Hu LT. 2016. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA 315:1767–1777. doi: 10.1001/jama.2016.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vannier E, Krause PJ. 2012. Human babesiosis. N Engl J Med 366:2397–2407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. 2015. Fatalities reported to FDA following blood collection and transfusion: annual summary for fiscal year 2014. Food and Drug Administration, Silver Spring, MD: http://www.notifylibrary.org/sites/default/files/FDA%20Fatality%20Report-2014.pdf. [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2011. Summary of notifiable diseases, United States 2011. MMWR Morb Mortal Wkly Rep 60:1–117. [PubMed] [Google Scholar]
- 8.Stein E, Elbadawi LI, Kazmierczak J, Davis JP. 2017. Babesiosis surveillance: Wisconsin, 2001–2015. MMWR Morb Mortal Wkly Rep 66:687–691. doi: 10.15585/mmwr.mm6626a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. 2011. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 155:509–519. doi: 10.7326/0003-4819-155-8-201110180-00362. [DOI] [PubMed] [Google Scholar]
- 10.Bres V, Proctor MC, Self D, Portugal M, Gurrola A, Tonnetti L, Bakkour S, Lobo C, Busch MP, Stramer SL, Linnen JM. 2017. Screening donated blood from babesia endemic regions of the United States using a transcription-mediated amplification assay on a fully automated system. Abstr AABB Annu Meet, abstr C57-A03J: https://aabb.confex.com/aabb/2017/meetingapp.cgi/Paper/1935. [Google Scholar]
- 11.Levin AE, Williamson PC, Bloch EM, Clifford J, Cyrus S, Shaz BH, Kessler D, Gorlin J, Erwin JL, Krueger NX, Williams GV, Penezina O, Telford SR IV, Branda JA, Krause PJ, Wormser GP, Schotthoefer AM, Fritsche TR, Busch MP. 2016. Serologic screening of United States blood donors for Babesia microti using an investigational enzyme immunoassay. Transfusion 56:1866–1874. doi: 10.1111/trf.13618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moritz ED, Winton CS, Johnson ST, Krysztof DE, Townsend RL, Foster GA, Devine P, Molloy P, Brissette E, Berardi VP, Stramer SL. 2014. Investigational screening for Babesia microti in a large repository of blood donor samples from nonendemic and endemic areas of the United States. Transfusion 54:2226–2236. doi: 10.1111/trf.12693. [DOI] [PubMed] [Google Scholar]
- 13.Moritz ED, Winton CS, Tonnetti L, Townsend RL, Berardi VP, Hewins ME, Weeks KE, Dodd RY, Stramer SL. 2016. Screening for Babesia microti in the U.S. blood supply. N Engl J Med 375:2236–2245. doi: 10.1056/NEJMoa1600897. [DOI] [PubMed] [Google Scholar]
- 14.Cornillot E, Hadj-Kaddour K, Dassouli A, Noel B, Ranwez V, Vacherie B, Augagneur Y, Brès V, Duclos A, Randazzo S, Carcy B, Debierre-Grockiego F, Delbecq S, Moubri-Ménage K, Shams-Eldin H, Usmani-Brown S, Bringaud F, Wincker P, Vivarès CP, Schwarz RT, Schetters TP, Krause PJ, Gorenflot A, Berry V, Barbe V, Ben Mamoun C. 2012. Sequencing of the smallest Apicomplexan genome from the human pathogen Babesia microti. Nucleic Acids Res 40:9102–9114. doi: 10.1093/nar/gks700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpi G, Walter KS, Mamoun CB, Krause PJ, Kitchen A, Lepore TJ, Dwivedi A, Cornillot E, Caccone A, Diuk-Wasser MA. 2016. Babesia microti from humans and ticks hold a genomic signature of strong population structure in the United States. BMC Genomics 17:888. doi: 10.1186/s12864-016-3225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornillot E, Dassouli A, Pachikara N, Lawres L, Renard I, Francois C, Randazzo S, Brès V, Garg A, Brancato J, Pazzi JE, Pablo J, Hung C, Teng A, Shandling AD, Huynh VT, Krause PJ, Lepore T, Delbecq S, Hermanson G, Liang X, Williams S, Molina DM, Ben Mamoun C. 2016. A targeted immunomic approach identifies diagnostic antigens in the human pathogen Babesia microti. Transfusion 56:2085–2099. doi: 10.1111/trf.13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva JC, Cornillot E, McCracken C, Usmani-Brown S, Dwivedi A, Ifeonu OO, Crabtree J, Gotia HT, Virji AZ, Reynes C, Colinge J, Kumar V, Lawres L, Pazzi JE, Pablo JV, Hung C, Brancato J, Kumari P, Orvis J, Tretina K, Chibucos M, Ott S, Sadzewicz L, Sengamalay N, Shetty AC, Su Q, Tallon L, Fraser CM, Frutos R, Molina DM, Krause PJ, Ben Mamoun C. 2016. Genome-wide diversity and gene expression profiling of Babesia microti isolates identify polymorphic genes that mediate host-pathogen interactions. Sci Rep 6:35284. doi: 10.1038/srep35284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lodes MJ, Houghton RL, Bruinsma ES, Mohamath R, Reynolds LD, Benson DR, Krause PJ, Reed SG, Persing DH. 2000. Serological expression cloning of novel immunoreactive antigens of Babesia microti. Infect Immun 68:2783–2790. doi: 10.1128/IAI.68.5.2783-2790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Y, Jia H, Terkawi MA, Goo Y-K, Kawano S, Ooka H, Li Y, Yu L, Cao S, Yamagishi J, Fujisaki K, Nishikawa Y, Saito-Ito A, Igarashi I, Xuan X. 2011. Identification and characterization of a novel secreted antigen 1 of Babesia microti and evaluation of its potential use in enzyme-linked immunosorbent assay and immunochromatographic test. Parasitol Int 60:119–125. doi: 10.1016/j.parint.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Lawres LA, Garg A, Kumar V, Bruzual I, Forquer IP, Renard I, Virji AZ, Boulard P, Rodriguez EX, Allen AJ, Pou S, Wegmann KW, Winter RW, Nilsen A, Mao J, Preston DA, Belperron AA, Bockenstedt LK, Hinrichs DJ, Riscoe MK, Doggett JS, Ben Mamoun C. 2016. Radical cure of experimental babesiosis in immunodeficient mice using a combination of an endochin-like quinolone and atovaquone. J Exp Med 213:1307–1318. doi: 10.1084/jem.20151519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo Y, Terkawi MA, Jia H, Aboge GO, Goo Y-K, Cao S, Li Y, Yu L, Ooka H, Kamyingkird K, Masatani T, Zhang S, Nishikawa Y, Igarashi I, Xuan X. 2012. A double antibody sandwich enzyme-linked immunosorbent assay for detection of secreted antigen 1 of Babesia microti using hamster model. Exp Parasitol 130:178–182. doi: 10.1016/j.exppara.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Magnarelli LA, Williams SC, Norris SJ, Fikrig E. 2013. Serum antibodies to Borrelia burgdorferi, Anaplasma phagocytophilum, and Babesia microti in recaptured white-footed mice. J Wildl Dis 49:294–302. doi: 10.7589/2012-06-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang DC, McCullough J. 2016. Transfusion-transmitted Babesia microti. Transfus Med Rev 30:132–138. doi: 10.1016/j.tmrv.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Glanternik JR, Baine IL, Tormey CA, Rychalsky MR, Baltimore RS. 2018. A cluster of cases of Babesia microti among neonates traced to a single unit of donor blood. Pediatr Infect Dis J 37:269–271. doi: 10.1097/INF.0000000000001803. [DOI] [PubMed] [Google Scholar]
- 25.Patarroyo JH, Prates AA, Tavares CAP, Mafra CL, Vargas MI. 1995. Exoantigens of an attenuated strain of Babesia bovis used as a vaccine against bovine babesiosis. Vet Parasitol 59:189–199. doi: 10.1016/0304-4017(94)00756-3. [DOI] [PubMed] [Google Scholar]
- 26.Schetters TPM, Strydom T, Crafford D, Kleuskens JAGM, van de Crommert J, Vermeulen AN. 2007. Immunity against Babesia rossi infection in dogs vaccinated with antigens from culture supernatants. Vet Parasitol 144:10–19. doi: 10.1016/j.vetpar.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Valentin A, Precigout E, L'Hostis M, Carcy B, Gorenflot A, Schrevel J. 1993. Cellular and humoral immune responses induced in cattle by vaccination with Babesia divergens culture-derived exoantigens correlate with protection. Infect Immun 61:734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ooka H, Terkawi MA, Cao S, Aboge G, Goo Y-K, Luo Y, Li Y, Nishikawa Y, Igarashi I, Xuan X. 2012. Molecular and immunological characterization of a novel 32-kDa secreted protein of Babesia microti. J Parasitol 98:1045–1048. doi: 10.1645/GE-2999.1. [DOI] [PubMed] [Google Scholar]
- 29.Ooka H, Terkawi MA, Goo Y-K, Luo Y, Li Y, Yamagishi J, Nishikawa Y, Igarashi I, Xuan X. 2011. Babesia microti: molecular and antigenic characterizations of a novel 94-kDa protein (BmP94). Exp Parasitol 127:287–293. doi: 10.1016/j.exppara.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Homann MV, Emami SN, Yman V, Stenstrom C, Sonden K, Ramstrom H, Karlsson M, Asghar M, Farnert A. 2017. Detection of malaria parasites after treatment in travelers: a 12-months longitudinal study and statistical modelling analysis. EBioMedicine 25:66–72. doi: 10.1016/j.ebiom.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G, Wormser GP, Zhuge J, Villafuerte P, Ip D, Zeren C, Fallon JT. 2015. Utilization of a real-time PCR assay for diagnosis of Babesia microti infection in clinical practice. Ticks Tick-Borne Dis 6(3):376–382. doi: 10.1016/j.ttbdis.2015.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


