Yellow fever (YF) is the prototypical hemorrhagic fever and results from infection with yellow fever virus (YFV), which is endemic to regions of Africa and South America. Despite the availability of an effective vaccine, YFV continues to cause disease throughout regions where it is endemic, including intermittent large outbreaks among undervaccinated populations.
KEYWORDS: yellow fever virus, diagnostics, molecular methods, serology
ABSTRACT
Yellow fever (YF) is the prototypical hemorrhagic fever and results from infection with yellow fever virus (YFV), which is endemic to regions of Africa and South America. Despite the availability of an effective vaccine, YFV continues to cause disease throughout regions where it is endemic, including intermittent large outbreaks among undervaccinated populations. A number of diagnostic methods and assays have been described for the detection of YFV infection, including viral culture, molecular testing, serology, and antigen detection. Commercial diagnostics are not widely available, and testing is generally performed at a small number of reference laboratories. The goal of this article, therefore, is to review available clinical diagnostics for YFV, which may not be familiar to many practitioners outside areas where it is endemic. Additionally, we identify gaps in our current knowledge about YF that pertain to diagnosis and describe interventions that may improve YFV detection.
INTRODUCTION
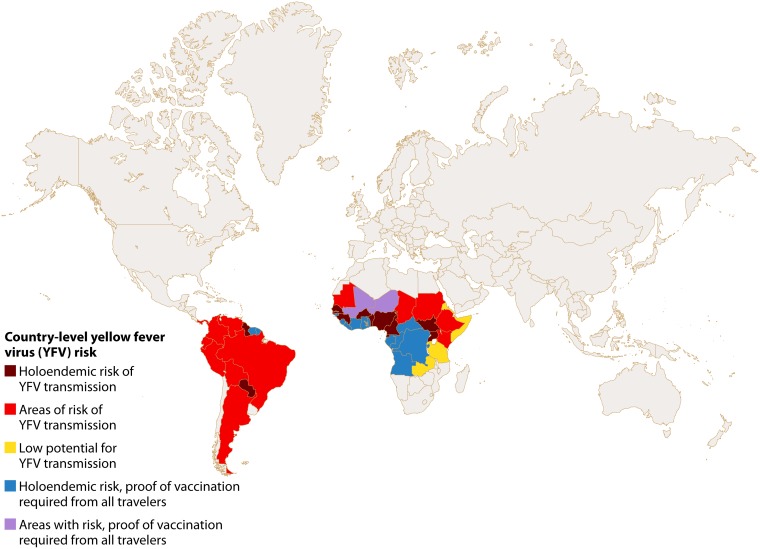
Yellow fever (YF) is a potentially fatal arboviral disease resulting from human infection with yellow fever virus (YFV), which is endemic to regions of Africa and South America (Fig. 1) (1–3). YFV is the prototype virus of the family Flaviviridae (genus Flavivirus) and the virus for which the family was named (flavus being Latin for yellow). The flaviviruses are enveloped, positive-sense, single-stranded RNA viruses, and these are predominantly transmitted by the bite of infected arthropod vectors (arboviruses) such as mosquitoes and ticks (1). Over 100 species comprise the genus Flavivirus, which includes other notable human pathogens, such as dengue virus (DENV), Japanese encephalitis virus (JEV), Zika virus (ZIKV), West Nile virus (WNV), Kyasanur Forest disease virus, and tick-borne encephalitis virus. The YFV genome is approximately 11 kb in length, and untranslated regions at the 5′ and 3′ ends flank the genes for 10 proteins, namely, 3 structural proteins (capsid, premembrane, and envelope) and 7 nonstructural proteins (termed NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) (1).
FIG 1.
Map of countries with areas of YFV endemicity and countries with requirements for proof of vaccination for all incoming travelers (updated 5 July 2018).
Severe YF occurs in ∼12% of infected individuals (95% confidence interval, 5 to 26%) and may manifest with jaundice, hemorrhage, and multisystem organ failure. Although the case fatality rate for severe YF is ∼47%, the case fatality rate for all YFV infections is estimated to be 5% (4). This occurs because a majority of human YFV infections are asymptomatic or result in a mild, undifferentiated systemic febrile illness that may not present to medical attention. These mild and asymptomatic cases create significant challenges in the diagnosis of YF, and, similarly to related flavivirus infections, contribute to underestimates of disease incidence in areas where YF is endemic (2, 5, 6).
Recent and ongoing YFV outbreaks have affected areas of YF endemicity in West Africa and extended into a region of southeastern Brazil that was previously thought to be at low risk for transmission (7, 8). A number of severe YF cases, including fatalities, have been reported among unvaccinated travelers to these regions (9–11). Repeated shortages of global vaccine stockpiles occurred during these outbreaks, and it is estimated that 45 to 52% of individuals in areas of YF endemicity require vaccination (12). YF outbreaks are therefore likely to continue, complicated by the movement of infected travelers to areas where YF is not endemic. Our objectives here are to review the available diagnostics for YFV, which may be unfamiliar to practitioners outside regions of endemicity, and highlight areas where additional information or new methods are needed.
HISTORICAL PERSPECTIVE
In the United States, YF outbreaks that resulted from imported cases occurred with some regularity in the early 20th century. Initially, the disease was thought to result from poor sanitary conditions and to be potentially transmissible from person to person (13). The understanding of YFV epidemiology and virology advanced significantly during studies performed by Walter Reed and the United States Army Yellow Fever Commission, which began in 1900 in Cuba. The commission proved a theory proposed by Carlos Finlay and others that YFV was transmitted by the bite of infected female mosquitoes, and this research led directly to interventions aimed at decreasing YF incidence through vector control (1). The last outbreak of YF in the United States occurred in 1905 in New Orleans.
YFV was first isolated in 1927 through the inoculation of macaques, using blood from symptomatic human cases in Ghana (14). As new laboratory methods became available for the detection of viral infections, they were developed for YFV. Hemagglutination inhibition testing was first reported for YFV in 1953, and the development of an indirect immunofluorescence (IIF) assay was reported in 1981 to simplify serological testing and shorten turnaround time (15, 16). YFV was the first arbovirus to be extensively characterized using whole-genome sequencing, with the publication of the Asibi and 17D strains in 1987 (17, 18). Finally, reverse transcription-PCRs (RT-PCRs) have been in use for YFV detection since the early 1990s (19, 20). Despite the application of new technologies for the detection of YFV, early successes in YF prevention (through vector control and vaccination), combined with difficulties in the timely detection of YF cases, have limited the opportunities for use of these methods on a larger clinical scale. As a result, the characterization of YFV infections using current diagnostic techniques has not kept pace with other arboviral infections, such as those caused by DENV or Chikungunya virus.
TRANSMISSION AND RISK IN AREAS OF YF ENDEMICITY
YFV is maintained in nature through transmission between nonhuman primates (NHPs) and the sylvatic mosquitoes that feed upon them, predominantly Aedes species in Africa and Haemagogus species in the Americas. Humans may become infected with YFV when bitten by an infected sylvatic mosquito, and sustained transmission in a human population may also occur with A. aegypti as the vector of domestic transmission (1, 2). In South America, YFV is primarily transmitted by sylvatic species, with rare outbreaks related to transmission by A. aegypti. In contrast, transmission in Africa is often cause by a mix of Aedes species, including sylvatic and domestic vectors. This leads to a force of infection that is at least 10-fold higher than South America (2, 3). Autochthonous transmission of YFV (the local acquisition of YFV infection) has never been confirmed in Southeast Asia, despite the presence of potential mosquito vectors and susceptible hosts. The cause of this absence of YFV remains controversial, but preexisting flavivirus immunity, and in particular DENV immunity, is one possible explanation (21, 22).
World Health Organization (WHO) updates from YFV outbreaks dating back to 2000 are readily available (http://www.who.int/csr/don/archive/disease/yellow_fever/en/), but reported YF cases likely represent a dramatic underestimation of YFV infections, with estimates ranging from 10 to 250 YFV infections for each reported case (1, 4, 23). This results from many factors, including limitations to available diagnostics, frequent occurrence of mild cases that may not present to medical attention, and cases that occur in remote areas without convenient access to care (2, 5). A modeling study using data from Africa estimated that 130,000 YF cases with fever and jaundice occurred each year on the continent, with 78,000 deaths (5). Such data are not available for South America. For unvaccinated travelers, risk estimates have been published. During a 2-week visit to areas of YF endemicity in West Africa in an interepidemic period, the risk of YF and death from YF are estimated to be 50 and 10 per 100,000, respectively. Estimates for YF and death from YF are 10-fold lower for areas of YF endemicity in South America (3). Risk estimates for travel during an outbreak period are expected to be higher.
YF outbreaks occur among unvaccinated or undervaccinated populations, which may result from lapses in vaccination programs, or through the emergence of YFV into new geographical regions (7, 8). The prediction of outbreaks is hampered by difficulties in YFV surveillance. Monitoring epizootics by identifying unusual monkey deaths is possible in the Americas, as many New World monkey species are susceptible to YFV, but this strategy may not be applicable in Africa, where many Old-World monkeys remain asymptomatic (2, 7). Early detection through the collection and testing of sentinel mosquito pools is inefficient (7). Therefore, surveillance recommendations from the WHO, which are codified in the International Health Regulations (IHR), focus on the importance of early detection and reporting of human cases, with a single confirmed human case defining a YF outbreak (24, 25).
CLINICAL PRESENTATION AND ROUTINE LABORATORY TESTING
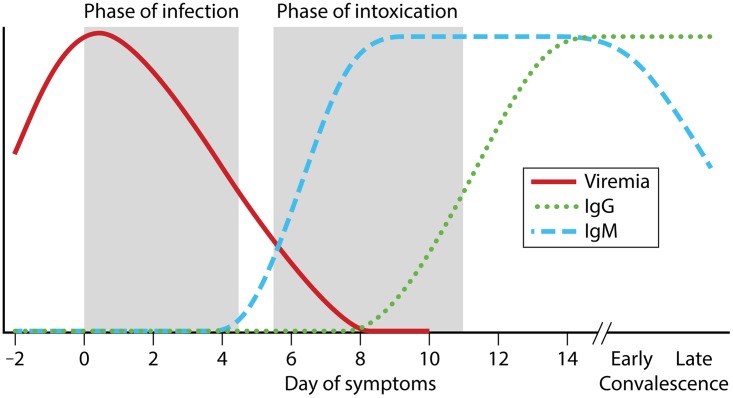
Humans develop symptoms at a median of 4 days (middle 95%, 2 to 9 days) after YFV transmission through the bite of an infected mosquito (26). The clinical course of YF has been classically described in three phases, namely, infection, remission, and intoxication (Fig. 2) (1, 2, 27). Symptom onset occurs during the phase of infection and is typically abrupt, with the development of fever, headache, myalgia, malaise, and nausea and vomiting. Fever may be high, with measured temperatures up to 41°C in adults, and patients may have a slow pulse relative to the degree of fever (Faget's sign) (2, 11). YFV may be detectable in serum/plasma by viral culture or molecular methods during this phase of illness. Following the infection phase, there may be a short phase of remission (2 to 48 h), with resolution of fever and improvement in clinical signs and symptoms. Many patients will recover during this stage of illness and not progress to more severe disease.
FIG 2.
Model time course for YF. Shaded areas represent periods of infection and intoxication. For many patients, YF will resolve during the phase of remission (unshaded region between the aforementioned phases), and it is estimated that 12% of patients will enter the period of intoxication. YFV RNA may be detectable in urine into early convalescence, although sensitivity of detection is unknown. Duration of viremia has been variable and may extend past the identified time period in severe cases.
Using data from 11 YF outbreaks, it is estimated that 12% of YFV-infected patients will progress to the phase of intoxication (4). This final phase of illness generally begins 3 to 6 days after the onset of initial symptoms, and it is marked by the return of fever, along with abdominal pain, nausea and vomiting, oliguria, and jaundice. Hepatic dysfunction results from apoptosis with limited inflammation and manifests with elevated transaminases and bilirubin, as well as decreased synthesis of clotting factors synthesized by the liver (2, 28). Renal failure is likely multifactorial, with elements of hepatorenal syndrome, prerenal azotemia, and possibly direct viral injury. Patients develop oliguria, and, in fatal cases, complete anuria may occur. Hemorrhagic manifestations occur during the phase of intoxication and can involve bleeding from mucosal sites, oozing from needle puncture sites and lines, petechiae, and ecchymoses. Encephalopathy can occur at later stages of disease. This is thought to result from metabolic changes, as YFV encephalitis is rare (1). Viremia ends during this phase, and anti-YFV antibodies become detectable (29).
The results of routine laboratory tests, such as the hemogram and standard chemistries, are not specific for YFV early in the disease course. Patients may have leukopenia with relative neutropenia, but laboratory abnormalities that are consistent with YF do not develop until later stages, when the disease carries a poor prognosis (1, 2, 10, 11, 30). Transaminases begin to rise 2 to 3 days after symptom onset. In YF, the rise in aspartate aminotransferase/serum glutamic-oxaloacetic transaminase (AST/SGOT) may exceed that for alanine aminotransferase/serum glutamic-pyruvic transaminase (ALT/SGPT), which differs from other forms of viral hepatitis and may be related to skeletal muscle or cardiac injury (10, 11, 31). Direct bilirubin levels may rise to 5 to 10 mg/dl. Levels of transaminases and bilirubin have been correlated with disease outcome, with higher levels occurring in more severe cases (32). Additional laboratory findings include thrombocytopenia and increased prothrombin time, and patients may develop evidence of diffuse intravascular coagulation (DIC). With renal dysfunction, high levels of proteinuria can develop, along with azotemia and elevated serum creatinine.
The differential diagnosis for YF is broad, particularly during the phase of intoxication, when the disease manifests as an undifferentiated acute febrile illness. Acute viral hepatitis caused by any of the viral hepatidities can present in a manner similar to YF, and hepatitis E in particular can present with severe disease in pregnancy (33, 34). In one study from the Democratic Republic of Congo, 218/498 (43.7%) suspected YF cases that tested negative for anti-YFV IgM had one or more detectable hepatitis viruses (most commonly Hepatitis B virus) (34). Dengue fever classically presents with fever, headache, and myalgia. Patients may develop hemorrhagic manifestations, and liver involvement can occur, although is less common than in YF (35). Malaria is on the differential diagnosis, as fever and jaundice occur in the setting of this infection. Importantly, coinfections of patients with malaria and YFV have been documented (36). Hemorrhagic fever viruses, including filoviruses, arenaviruses, and certain bunyaviruses, can mimic YF. Although these pathogens typically do not present with significant liver damage, Rift Valley fever virus, a bunyavirus (genus Phlebovirus), can present with hemorrhage and hepatic injury (37). Additionally, Crimean-Congo hemorrhagic fever (CCHF), caused by a tick-borne bunyavirus (genus Nairovirus), may present with severe liver disease, hemorrhage, renal failure, and altered mental status (38). CCHF may be distinguished from YF by epidemiologic exposure, and the diagnosis is made by RT-PCR and/or serologic testing.
YFV DIAGNOSTICS
WHO surveillance definitions for YF define a suspected case as any patient with an acute febrile illness who develops jaundice within 14 days of symptom onset (http://www.who.int/csr/disease/yellowfev/case-definition/en/). The results of laboratory testing for YFV are then used to categorize cases as probable or confirmed (described in detail below). While these definitions provide a useful framework for interpreting the results of diagnostic testing, the definition of a suspected case is too restrictive for clinical use, as it focuses on patients in the phase of intoxication and may not be met at the time of presentation.
For patients who present soon after symptom onset, the diagnosis of YF requires a high degree of clinical suspicion and the availability of specific laboratory tests. A number of methods and assays may be utilized in the clinical laboratory for the confirmation of YFV infections, including viral culture, serology, molecular testing, and antigen detection (references 24, 29; see also http://www.who.int/csr/disease/yellowfev/case-definition/en/). These methods will be discussed here in the context of clinical diagnostic testing. Classic findings on liver pathology have been described, such as the formation of Councilman bodies, and YFV antigens can be detected in tissues by immunohistochemistry (1, 2). However, antemortem tissue biopsy samples should not be taken due to the risk of hemorrhage in acute YF. Tissue pathology, therefore, should only be used to confirm the diagnosis postmortem.
YFV is a biosafety level 3 (BSL3) pathogen, and laboratorians who perform YFV testing should be vaccinated prior to working with the virus. Enhanced biosafety precautions should be considered if handling specimens from patients with hemorrhagic fever who may be infected with pathogens that require BSL4 facilities (e.g., filoviruses or arenaviruses) (29). Testing is generally performed at national or regional reference laboratories and this, combined with the frequent occurrence of YF cases in rural or remote areas, limits the detection of YFV infections in both epidemic and endemic settings.
VIRAL ISOLATION
Viral isolation is not routinely performed as a clinical diagnostic test for YF. These techniques are labor-intensive, require weeks for confirmation, and may necessitate additional biosafety precautions compared to other methods (29, 39). Additionally, viral isolation is considered to be less sensitive than YFV detection by molecular methods. Although rigorous comparisons are not available, reports document YFV RNA detection by reverse transcriptase PCR (RT-PCR) or real-time RT-PCR (rRT-PCR) in samples that were culture negative (40, 41). Despite these limitations, viral isolation remains vitally important for the characterization and banking of YFV strains. Also, infectious particles can only be demonstrated and quantified by viral isolation (39, 42). If resources are available, viral culture is recommended for these reasons (2).
Viremia develops rapidly following human infection with YFV (Fig. 2). Virus may be detectable in serum or plasma as soon as 1 to 2 days after infection and viral load reportedly peaks around day 3, near the time of symptom onset (2, 26, 43). YFV is most commonly cultured in Vero cells or C6/36 (Aedes albopictus) cells (29). Additionally, YFV can be isolated by mosquito inoculation or intracerebral injection into suckling mice, but these latter techniques are unlikely to be applicable to the clinical laboratory setting (2, 44). Blood samples collected in the first few days following symptom onset (phase of infection) are more likely to yield viral isolates, but a case has been described where virus was isolated from serum out to day 12 (mouse injection) (44). YFV can also been cultured from tissue specimens collected at autopsy (45).
ANTI-YFV ANTIBODY DETECTION
YFV exists as a single antigenic type or serotype (46). Due to the antigenic similarity between strains, antibody responses to the YFV-17D vaccine strain cannot be readily differentiated from responses to wild-type virus. The accurate interpretation of serologic testing, therefore, requires knowledge of a patient's vaccination history. According to WHO case definitions, a probable YF case is defined by the detection of anti-YFV IgM in a suspected YF case in the absence of YFV vaccination within the 30 days preceding illness onset (http://www.who.int/csr/disease/yellowfev/case-definition/en/). A case is considered confirmed by serology if there is (i) a ≥4-fold rise in anti-YFV IgM or IgG titers between acute-phase and convalescent-phase samples and/or (ii) detection of specific anti-YFV IgM or neutralizing antibodies (by plaque reduction neutralization testing) (reference 29 and WHO case definition, above). In this definition, “specific detection” requires negative results from concurrent testing for IgM or neutralizing antibodies to other circulating flaviviruses. Although viral diversity has not been expected to impact the performance of serological tests, strain-specific differences in antibody detection were observed in an external quality assessment (47). Therefore, this warrants consideration when new assays are being evaluated.
The most common techniques for the detection of anti-YFV IgM and IgG are IIF and enzyme-linked immunosorbent assay (ELISA) (47). Antigen preparations are typically derived from YFV-infected whole-cell lysates (48, 49). Commercial serological reagents are not widely available and are only produced by a limited number of suppliers, including EUROIMMUN (IgG) and the Instituto Nacional de Salud de Perú (IgM). Assays for anti-YFV IgM antibodies are most often used for the diagnosis of acute YF in areas of endemicity. Although a single commercial IIF kit for anti-YFV IgM has been evaluated (50), this performed poorly in an international quality assessment study (47). Validated, laboratory-developed tests are often utilized, and an IgM antibody capture ELISA (MAC-ELISA) kit has been developed and evaluated by the U.S. Centers for Disease Control and Prevention (CDC) (48).
A proposed time course for the development of anti-YFV antibodies is shown in Fig. 2. During the phase of infection, antibodies are not detectable. As the patient transitions through the phase of remission and possibly into the phase of intoxication, anti-YFV IgM becomes detectable as virus becomes undetectable (by isolation and/or RT-PCR). Anti-YFV IgG levels rise a few days after those of IgM, and this is detected in almost all patients by day 14 after symptom onset. However, the timing of antibody detection in natural YFV infections has not been well documented using current IIF or ELISA techniques, and the best data have been generated from the study of responses to the live-attenuated YFV vaccine (51–53). Additionally, the clinical sensitivity of IgM detection for the diagnosis of natural YFV infection remains unclear, but there is evidence from vaccination trials that anti-YFV IgM levels may be lower among patients with previous flavivirus infections (50, 54). Although IgM assays are relied upon for the diagnosis of YFV, a number of cases are likely missed even when testing is performed at the appropriate time.
Anti-YFV IgM may persist following vaccination, which obscures the use of such assays for diagnosis in vaccinated patients. In a study of flavivirus-naive YFV vaccine recipients (YF-Vax), 29/40 participants (73%) had detectable anti-YFV IgM 3 to 4 years after vaccine administration (53). Anti-YFV IgM may decay faster among residents in regions where YF is endemic, as evidenced by a study of 17DD vaccine recipients in Recife, Brazil, in which 13/30 individuals (43.3%) remained positive at the last time point tested (100 to 349 days after vaccination) (54). The differences in results likely have multiple explanations, including the patient populations (86.6% of the Brazilian cohort was DENV experienced), vaccine preparations, and IgM assays. Nonetheless, these data indicate that anti-YFV IgM is detectable in vaccine recipients well past the 30-day cutoff used in surveillance guidelines, and this should be considered when interpreting anti-YFV IgM results.
Testing for anti-YFV IgG is less often performed for the diagnosis of YF, as these tests require the use of acute-phase and convalescent-phase samples and appear to be less specific than other serological methods (reference 29; see also http://www.who.int/csr/disease/yellowfev/case-definition/en/). Serum neutralization testing is considered to be the most specific serological test for YFV (29). This technique is labor intensive and has a prolonged turnaround time, and, as a result, neutralization testing is typically only performed at reference laboratories for diagnostic confirmation or research purposes. Titers in IIF and ELISAs may not correlate with neutralizing titers, and individuals may have neutralizing antibodies but test negative by other serological methods (50, 54).
Finally, serological cross-reactions between the flaviviruses have been well described (48, 55–58). Sera from patients with YF may produce positive results in assays for other flaviviruses, most commonly DENV, which may result in underdiagnosis of YF cases. Additionally, sera from patients with other flaviviruses, including DENV, WNV, and ZIKV, may yield positive results in YFV serological assays. False-positive cross-reactions may be more common using assays for anti-YFV IgG, but this effect is also seen for anti-YFV IgM and even neutralization testing (55–57). The cross-reactive nature of whole-virus YFV antigen with heterotypic flavivirus antibodies limits the diagnostic accuracy of these assays. The development and application of antigens with reduced cross-reactivity is, therefore, needed to improve both flavivirus infection serodiagnosis and estimates of disease burden (59).
RNA DETECTION
A number of molecular tests for YFV have been described in the literature, including conventional RT-PCR (41, 60–62), real-time RT-PCR (rRT-PCR) with hydrolysis probes or SYBR green (39, 41, 42, 63–67), multiplex rRT-PCR (68–70), and isothermal methods (64, 71–73). Representative real-time and isothermal assays are shown in Table 1. As real-time platforms become more accessible, conventional RT-PCR is being utilized less frequently for YFV detection. However, conventional assays continue to be used for the generation of larger amplicons for sequencing and phylogenetic analysis (62). In all published clinical evaluations of YFV molecular assays, RNA extraction has been performed using a commercial kit. Results following minimal specimen processing or direct specimen testing have not been reported.
TABLE 1.
Real-time and isothermal molecular tests for YFV
| Assay type and author (year) | Detection method(s)a | Genome target(s)b | Clinical samples testedc | Molecular comparatord | Notes | Reference |
|---|---|---|---|---|---|---|
| rRT-PCR | ||||||
| Drosten et al. (2003) | Hydrolysis probe | 5′ UTR | Yes | No | 65 | |
| Bae et al. (2003) | Hydrolysis probe | NS3 and 3′ UTR | Yes | No | Two separate assays developed | 39 |
| Mantel et al. (2008)e | Hydrolysis probe | NS5 | No | No | Designed from only YF-17D-204 sequence | 42 |
| Weidmann et al. (2010)f | LNA probe | 5′ UTR | No | No | 66 | |
| Nunes et al. (2011) | SYBR green | NS5 | Yes | Yes | 41 | |
| Dash et al. (2012) | SYBR green | 5′ UTR–capsid | No | No | 63 | |
| Domingo et al. (2012)f | Hydrolysis probe | 5′ UTR | Yes | Yes | 64 | |
| Hughes et al. (2018)f | LNA probe | NS5–3′ UTR | Yes | Yes | Specifically detects YF-17D strain only | 67 |
| Multiplex rRT-PCR | ||||||
| Chao et al. (2007) | Hydrolysis probe | NS5 | No | No | Developed for mosquito samples | 68 |
| Fischer et al. (2017)g | Hydrolysis probe | NS1 | Yes | Yes | Lineage-specific duplex assay | 69 |
| Rojas et al. (2018)f | Hydrolysis probe | 5′ UTR | Yes | Yes | Includes pan-DENV detection and internal control | 70 |
| Isothermal methods | ||||||
| Kwallah et al. (2013) | RT-LAMP, turbidimeter | NS1 | No | Yes | 60 min reaction, designed from strain 17D sequences | 72 |
| Nunes et al. (2015) | RT-LAMP, SYBR green | NS1 | No | Yes | 60 min reaction, SYBR added postamplification | 73 |
| Domingo et al. (2012)f | HDA | 5′ UTR | Yes | Yes | 2 h with lateral flow detection | 64 |
| Escadafal et al. (2018)f | RPA | 5′ UTR | No | No | TwistDx kit for real-time performance | 71 |
HDA, helicase-dependent amplification; LNA, locked nucleic acid; and RPA, recombinase polymerase amplification; RT-LAMP, reverse transcription–loop-mediated isothermal amplification.
NS, nonstructural; and UTR, untranslated region.
Testing of any human sample reported in original assay validation.
Comparison with a separate published molecular assay described in assay validation.
Assay designed specifically to detect YF-17D strain.
Published evaluation included at least one strain each from both South America and Africa (excluding the 17D strain).
Lineage-specific detection of YFV vaccine and South American genotype strains.
Seven major genotypes of YFV have been identified, including five African (West Africa I and II, East Africa, East/Central Africa, and Angola) and two South American genotypes (South America I and II) (46). With few exceptions, molecular tests have been designed with the goal of detecting all genotypes or strains (67, 69). However, the majority of published molecular tests have only been evaluated using isolates from one or a few genotypes. In such instances, suboptimal assay performance may occur with the other genotypes but remain undetected. Indeed, in an international external quality assessment study, 13/32 (41%) laboratories produced nonoptimal results due to failure to detect one or more YFV strains (47). Associations between specific strains and disease severity have been observed, but these are not reliable enough to identify “high-risk” subtypes that could be targeted in a prognostic test (74).
Viral kinetics of YFV during human infection are poorly understood. The time course of viremia shown in Fig. 2 is consistent with published guidelines and expert opinion (2, 26, 29, 43). Viremia data in the literature come from case reports or small case series, which predominantly focus on severe cases in returning travelers and which utilize a variety of assays with different reported units of measurement (10, 31, 39, 65, 69, 75–77). Detectable viremia may occur as early as 2 to 3 days postinfection (26, 43), and it is expected that patients develop relatively high viral loads in serum/plasma, which are necessary to infect Aedes mosquitoes and maintain human-mosquito-human transmission. Indeed, serum/plasma viral loads of >8.0 log10 genome equivalents/ml have been reported (39, 69). It also appears that serum/plasma viral loads may decay slowly in severe YF cases (10, 31, 69, 75), at least compared to dengue cases (78), and in a single case, YFV viremia was detected until day 20 (77). Current recommendations from the Pan American Health Organization (PAHO) support molecular testing during the first 10 days of illness (29). Disease severity may be associated with viremia (79), but quantitative data are not available for human case series. Additionally, further studies are needed to determine if viral kinetic parameters differ between severe and mild (or asymptomatic) cases and based on prior flavivirus infections.
Molecular testing performed on other specimens may extend the window of YFV RNA detection. In urine samples, YFV RNA has been detected as early as 1 day after vaccination and up to 21 to 27 days following vaccination or the onset of symptoms in naturally occurring infection (30, 77, 80, 81). A single individual has been described who had a positive urine YFV rRT-PCR 198 days after vaccination (81). The clinical sensitivity of YFV detection in urine remains unknown, but testing of this specimen is warranted in suspected cases. A single report of a YF case documented viral detection in semen at 21 days (30). RNA from ZIKV and WNV have been detected in whole blood for prolonged periods (82, 83), but no published data are currently available for YFV. Finally, the dilution of primary serum/plasma samples has facilitated YFV detection by molecular methods in early severe YF cases that initially tested negative (31, 77). Although dilution clearly decreases the eluate RNA concentration, the dilution of the sample and/or the extracted nucleic acids may result in a dilution of PCR inhibitors, thereby allowing for YFV detection (84). The specific cause of RT-PCR inhibition in these cases of YF was not reported.
YFV detection may be improved in areas of endemicity if testing is performed in multiplex with related pathogens that cause an acute febrile illness. However, few multiplex rRT-PCRs that specifically detect YFV have been developed (68–70). Of those, only the assay by Rojas et al. detects another related human pathogen (DENV) and has been developed for and evaluated with clinical samples (70). Different isothermal methods for YFV detection have been developed and evaluated (Table 1) (64, 71–73). These assays hold promise for addressing some of the limitations to available YF diagnostics, as these might provide accurate assays that require less laboratory infrastructure than real-time RT-PCR. However, more data are needed regarding the real-world use of these assays for YFV detection.
NS1
Although frequently used for the diagnosis of DENV in countries where it is endemic, few reports have described the specific detection of NS1 antigen as a diagnostic test for YFV (previously identified in YFV studies as gp48). Ricciardi-Jorge et al. described the development of a quantitative YFV NS1-capture ELISA with 80% sensitivity (12/15 cases) and 100% specificity compared to those of RT-PCR (85). NS1 concentrations ranged from 177.6 to 4,597.6 ng/ml of serum and the 3 negative samples came from patients on days 4 (n = 2) and 7. Yen et al. included DENV and YFV NS1 detection in a proof-of-concept report of a multiplexed silver nanoparticle-based assay. This test had a limit of detection of 150 ng/ml but was not evaluated with clinical samples (86).
MANAGEMENT AND OUTCOME
Currently, there are no specific antiviral treatments for YF, and patients are managed symptomatically. The majority of YF cases will resolve spontaneously, although severe cases often require admission to intensive care. Treatment may consist of fluid replacement, gastric acid suppression, transfusion (when indicated), vasopressors, and renal replacement therapy (2, 27). Unfortunately, even advanced intensive care does not appear to significantly impact YF outcomes. The case fatality rate for patients that enter the phase of intoxication ranges from 20 to 50% in different outbreaks and case series (1, 2, 23, 32, 87). In general, reported mortality in outbreaks that occur in Africa is lower than that in outbreaks in the Americas. This may be related to a number of factors, including differences in the human hosts and YFV strain-related differences in virulence (88). Factors that have been associated with poor outcomes include increasing patient age and higher levels of AST, ALT, and bilirubin (32, 89).
A number of antiviral compounds are under investigation for use in YF, including sofosbuvir, which is an FDA-approved drug for hepatitis C and which has documented efficacy against ZIKV (90). This direct-acting antiviral is currently being evaluated for the treatment of YF in a compassionate-use protocol in Brazil (Thiago Moreno L. Souza, personal communication, and reference 91). There are no data regarding the impact of early diagnostic confirmation on clinical outcomes in YF cases. If efficacious medications are identified, early administration may improve outcome, but this needs to be evaluated in clinical studies. Beyond individual therapy, early case detection may also have a positive impact on outbreak control.
YELLOW FEVER PREVENTION
Live-attenuated YFV vaccines have been widely available since the 1940s (43). All current vaccines are derived from the 17D strain, which was isolated following attenuation of the 1927 Asibi strain through serial passage. YFV vaccines generally produce robust and long-lived humoral and cellular immune responses in virtually all recipients (51, 52, 54). Anti-YFV neutralizing antibodies have been detected for as long as 35 years postvaccination (92), and the current recommendation is for a single life-time dose of YFV vaccine for travelers to areas with risk of transmission (Fig. 1). A caveat is that repeat administration can be considered for individuals traveling to outbreak areas who last received the YFV vaccine more than 10 years prior (3). There is evidence that patients with preexisting DENV immunity may have decreased antibody responses to YF vaccination, but the impact of this effect on the durability of anti-YFV immune responses is unclear (93).
In general, YFV vaccines are very safe. Although severe adverse events have been reported following vaccination, including yellow fever vaccine-associated viscerotropic disease (YEL-AVD) and acute neurotropic disease (YEL-AND), the incidence of these severe events remains very low, at <1 case per 100,000 doses of vaccine (2, 94). The risk of YEL-AVD and YEL-AND are increased among individuals at the extremes of age (<9 months and >70 years old) and those who are immune suppressed or have thymus dysfunction. YEL-AVD typically develops within the first week following vaccination and clinically presents as YF, with detectable viremia, organ failure, and a high mortality rate (43, 94). Differentiating YEL-AVD from YF in an outbreak setting motivated the development of lineage specific rRT-PCRs (67, 69). The duplex assay by Fisher et al. was designed for use in the Americas and requires further evaluation for use with African strains (69).
CONCLUSIONS
YFV remains an important tropical infectious disease, and recent large outbreaks and vaccine supply issues have again brought this to the fore. Due to the existence of natural reservoir hosts (monkeys) and widespread vector populations, YFV elimination will not be possible. Therefore, effort must be focused on prevention and control, which in turn require accurate diagnostic tests and screening protocols for the identification of cases. In order to improve YFV detection, human data regarding viral kinetics is needed, both generally and in relation to host immune responses and disease severity. Decisions regarding test selection and utilization would benefit from rigorous method comparisons using well-characterized specimens, and in an age of increasingly rapid global travel, assays should be evaluated for all known genotypes. Finally, improved detection and characterization of mild or asymptomatic cases are needed to better understand host responses, risk factors for severe disease among YFV infections, and transmission dynamics. YF has proven that it will not go quietly, and an important aspect of disease monitoring and outbreak control continues to be early detection through the availability and appropriate use of laboratory diagnostics.
REFERENCES
- 1.Gardner CL, Ryman KD. 2010. Yellow fever: a reemerging threat. Clin Lab Med 30:237–260. doi: 10.1016/j.cll.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monath TP, Vasconcelos PF. 2015. Yellow fever. J Clin Virol 64:160–173. doi: 10.1016/j.jcv.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Gershman MD, Staples JE. 2018. Yellow fever. In Burnette GD. (ed), CDC yellow book 2018: health information for international travel. Oxford University Press, New York, NY. [Google Scholar]
- 4.Johansson MA, Vasconcelos PF, Staples JE. 2014. The whole iceberg: estimating the incidence of yellow fever virus infection from the number of severe cases. Trans R Soc Trop Med Hyg 108:482–487. doi: 10.1093/trstmh/tru092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garske T, Van Kerkhove MD, Yactayo S, Ronveaux O, Lewis RF, Staples JE, Perea W, Ferguson NM, Yellow Fever Expert C. 2014. Yellow fever in Africa: estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med 11:e1001638. doi: 10.1371/journal.pmed.1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson SE, Hull BP, Tomori O, Bele O, LeDuc JW, Esteves K. 1996. Yellow fever: a decade of reemergence. JAMA 276:1157–1162. doi: 10.1001/jama.1996.03540140045025. [DOI] [PubMed] [Google Scholar]
- 7.Dexheimer Paploski IA, Souza RL, Tauro LB, Cardoso CW, Mugabe VA, Pereira Simoes Alves AB, de Jesus Gomes J, Kikuti M, Campos GS, Sardi S, Weaver SC, Reis MG, Kitron U, Ribeiro GS. 2018. Epizootic outbreak of yellow fever virus and risk for human disease in Salvador, Brazil. Ann Intern Med 168:301–302. doi: 10.7326/M17-1949. [DOI] [PubMed] [Google Scholar]
- 8.Zhao S, Stone L, Gao D, He D. 2018. Modelling the large-scale yellow fever outbreak in Luanda, Angola, and the impact of vaccination. PLoS Negl Trop Dis 12:e0006158. doi: 10.1371/journal.pntd.0006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamer DH, Angelo K, Caumes E, van Genderen PJJ, Florescu SA, Popescu CP, Perret C, McBride A, Checkley A, Ryan J, Cetron M, Schlagenhauf P. 2018. Fatal yellow fever in travelers to Brazil, 2018. MMWR Morb Mortal Wkly Rep 67:340–341. doi: 10.15585/mmwr.mm6711e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Liu L, Lv Y, Zhang W, Li J, Zhang Y, Di T, Zhang S, Liu J, Li J, Qu J, Hua W, Li C, Wang P, Zhang Q, Xu Y, Jiang R, Wang Q, Chen L, Wang S, Pang X, Liang M, Ma X, Li X, Wang Q, Zhang F, Li D. 2016. A fatal yellow fever virus infection in China: description and lessons. Emerg Microbes Infect 5:e69. doi: 10.1038/emi.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wouthuyzen-Bakker M, Knoester M, van den Berg AP, GeurtsvanKessel CH, Koopmans MP, Van Leer-Buter C, Oude Velthuis B, Pas SD, Ruijs WL, Schmidt-Chanasit J, Vreden SG, van der Werf TS, Reusken CB, Bierman WF. 2017. Yellow fever in a traveller returning from Suriname to the Netherlands, March 2017. Euro Surveill 22(11):pii=30488. doi: 10.2807/1560-7917.ES.2017.22.11.30488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shearer FM, Moyes CL, Pigott DM, Brady OJ, Marinho F, Deshpande A, Longbottom J, Browne AJ, Kraemer MUG, O'Reilly KM, Hombach J, Yactayo S, de Araujo VEM, da Nobrega AA, Mosser JF, Stanaway JD, Lim SS, Hay SI, Golding N, Reiner RC Jr. 2017. Global yellow fever vaccination coverage from 1970 to 2016: an adjusted retrospective analysis. Lancet Infect Dis 17:1209–1217. doi: 10.1016/S1473-3099(17)30419-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphreys M. 1992. Yellow fever and the South. Johns Hopkins University Press, Baltimore, MD. [Google Scholar]
- 14.Stokes A, Bauer JH, Hudson NP. 1928. Experimental transmission of yellow fever to laboratory animals. Am J Trop Med Hyg S1-8:103–164. doi: 10.4269/ajtmh.1928.s1-8.103. [DOI] [Google Scholar]
- 15.Casals J, Brown LV. 1953. Hemagglutination with certain arthropod-borne viruses. Proc Soc Exp Biol Med 83:170–173. doi: 10.3181/00379727-83-20299. [DOI] [PubMed] [Google Scholar]
- 16.Monath TP, Cropp CB, Muth DJ, Calisher CH. 1981. Indirect fluorescent antibody test for the diagnosis of yellow fever. Trans R Soc Trop Med Hyg 75:282–286. doi: 10.1016/0035-9203(81)90335-7. [DOI] [PubMed] [Google Scholar]
- 17.Hahn CS, Dalrymple JM, Strauss JH, Rice CM. 1987. Comparison of the virulent Asibi strain of yellow fever virus with the 17D vaccine strain derived from it. Proc Natl Acad Sci U S A 84:2019–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang E, Ryman KD, Jennings AD, Wood DJ, Taffs F, Minor PD, Sanders PG, Barrett AD. 1995. Comparison of the genomes of the wild-type French viscerotropic strain of yellow fever virus with its vaccine derivative French neurotropic vaccine. J Gen Virol 76(Part 11):2749–2755. doi: 10.1099/0022-1317-76-11-2749. [DOI] [PubMed] [Google Scholar]
- 19.Brown TM, Chang GJ, Cropp CB, Robbins KE, Tsai TF. 1994. Detection of yellow fever virus by polymerase chain reaction. Clin Diagn Virol 2:41–51. doi: 10.1016/0928-0197(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 20.Eldadah ZA, Asher DM, Godec MS, Pomeroy KL, Goldfarb LG, Feinstone SM, Levitan H, Gibbs CJ Jr, Gajdusek DC. 1991. Detection of flaviviruses by reverse-transcriptase polymerase chain reaction. J Med Virol 33:260–267. doi: 10.1002/jmv.1890330410. [DOI] [PubMed] [Google Scholar]
- 21.Xiao SY, Guzman H, da Rosa AP, Zhu HB, Tesh RB. 2003. Alteration of clinical outcome and histopathology of yellow fever virus infection in a hamster model by previous infection with heterologous flaviviruses. Am J Trop Med Hyg 68:695–703. [PubMed] [Google Scholar]
- 22.Izurieta RO, Macaluso M, Watts DM, Tesh RB, Guerra B, Cruz LM, Galwankar S, Vermund SH. 2009. Anamnestic immune response to dengue and decreased severity of yellow Fever. J Glob Infect Dis 1:111–116. doi: 10.4103/0974-777X.56257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monath TP, Craven RB, Adjukiewicz A, Germain M, Francy DB, Ferrara L, Samba EM, N′Jie H, Cham K, Fitzgerald SA, Crippen PH, Simpson DI, Bowen ET, Fabiyi A, Salaun JJ. 1980. Yellow fever in the Gambia, 1978–1979: epidemiologic aspects with observations on the occurrence of orungo virus infections. Am J Trop Med Hyg 29:912–928. doi: 10.4269/ajtmh.1980.29.912. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. 2008. WHO—recommended standards for surveillance of selected vaccine-preventable diseases. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 25.World Health Organization. 2014. International Health Regulations (2005), 3rd ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 26.Johansson MA, Arana-Vizcarrondo N, Biggerstaff BJ, Staples JE. 2010. Incubation periods of yellow fever virus. Am J Trop Med Hyg 83:183–188. doi: 10.4269/ajtmh.2010.09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministério da Saúde. 2017. Febre amarela: guia para profissionais de saúde. Ministério da Saúde, Brasília, Brazil. [Google Scholar]
- 28.Quaresma JA, Barros VL, Pagliari C, Fernandes ER, Andrade HF Jr, Vasconcelos PF, Duarte MI. 2007. Hepatocyte lesions and cellular immune response in yellow fever infection. Trans R Soc Trop Med Hyg 101:161–168. doi: 10.1016/j.trstmh.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Pan American Health Organization. 2018. Laboratory diagnosis of yellow fever virus infection. Pan American Health Organization, Washington, DC. [Google Scholar]
- 30.Barbosa CM, Di Paola N, Cunha MP, Rodrigues-Jesus MJ, Araujo DB, Silveira VB, Leal FB, Mesquita FS, Botosso VF, Zanotto PMA, Durigon EL, Silva MV, Oliveira DBL. 2018. Yellow fever virus RNA in urine and semen of convalescent patient, Brazil. Emerg Infect Dis 24. doi: 10.3201/eid2401.171310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colebunders R, Mariage JL, Coche JC, Pirenne B, Kempinaire S, Hantson P, Van Gompel A, Niedrig M, Van Esbroeck M, Bailey R, Drosten C, Schmitz H. 2002. A Belgian traveler who acquired yellow fever in the Gambia. Clin Infect Dis 35:e113–e116. doi: 10.1086/344180. [DOI] [PubMed] [Google Scholar]
- 32.Tuboi SH, Costa ZG, da Costa Vasconcelos PF, Hatch D. 2007. Clinical and epidemiological characteristics of yellow fever in Brazil: analysis of reported cases 1998–2002. Trans R Soc Trop Med Hyg 101:169–175. doi: 10.1016/j.trstmh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Lachish T, Erez O, Daudi N, Shouval D, Schwartz E. 2015. Acute hepatitis E virus in pregnant women in Israel and in other industrialized countries. J Clin Virol 73:20–24. doi: 10.1016/j.jcv.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Makiala-Mandanda S, Le Gal F, Ngwaka-Matsung N, Ahuka-Mundeke S, Onanga R, Bivigou-Mboumba B, Pukuta-Simbu E, Gerber A, Abbate JL, Mwamba D, Berthet N, Leroy EM, Muyembe-Tamfum JJ, Becquart P. 2017. High Prevalence and Diversity of Hepatitis Viruses in Suspected Cases of Yellow Fever in the Democratic Republic of Congo. J Clin Microbiol 55:1299–1312. doi: 10.1128/JCM.01847-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. 2009. Dengue: guidelines for diagnosis, treatment, prevention and control. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 36.Sow A, Loucoubar C, Diallo D, Faye O, Ndiaye Y, Senghor CS, Dia AT, Faye O, Weaver SC, Diallo M, Malvy D, Sall AA. 2016. Concurrent malaria and arbovirus infections in Kedougou, southeastern Senegal. Malar J 15:47. doi: 10.1186/s12936-016-1100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikegami T, Makino S. 2011. The pathogenesis of Rift Valley fever. Viruses 3:493–519. doi: 10.3390/v3050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akinci E, Bodur H, Sunbul M, Leblebicioglu H. 2016. Prognostic factors, pathophysiology and novel biomarkers in Crimean-Congo hemorrhagic fever. Antiviral Res 132:233–243. doi: 10.1016/j.antiviral.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Bae HG, Nitsche A, Teichmann A, Biel SS, Niedrig M. 2003. Detection of yellow fever virus: a comparison of quantitative real-time PCR and plaque assay. J Virol Methods 110:185–191. doi: 10.1016/S0166-0934(03)00129-0. [DOI] [PubMed] [Google Scholar]
- 40.Munera GI, Mendez JA, Rey GJ. 2010. [Serological, molecular and virological analyses associated with yellow fever surveillance in Colombia]. Biomedica 30:345–352. (In Spanish.) doi: 10.7705/biomedica.v30i3.268. [DOI] [PubMed] [Google Scholar]
- 41.Nunes MR, Palacios G, Nunes KN, Casseb SM, Martins LC, Quaresma JA, Savji N, Lipkin WI, Vasconcelos PF. 2011. Evaluation of two molecular methods for the detection of yellow fever virus genome. J Virol Methods 174:29–34. doi: 10.1016/j.jviromet.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantel N, Aguirre M, Gulia S, Girerd-Chambaz Y, Colombani S, Moste C, Barban V. 2008. Standardized quantitative RT-PCR assays for quantitation of yellow fever and chimeric yellow fever-dengue vaccines. J Virol Methods 151:40–46. doi: 10.1016/j.jviromet.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 43.Barnett ED. 2007. Yellow fever: epidemiology and prevention. Clin Infect Dis 44:850–856. doi: 10.1086/511869. [DOI] [PubMed] [Google Scholar]
- 44.Downs WG, Anderson CR, Spence L. 1955. Isolation of yellow fever virus from a human patient on the twelfth day of illness. Trans R Soc Trop Med Hyg 49:577–579. doi: 10.1016/0035-9203(55)90030-4. [DOI] [PubMed] [Google Scholar]
- 45.Anderson CR, Wattley GH. 1955. The isolation of yellow fever virus from human liver obtained at autopsy. Trans R Soc Trop Med Hyg 49:580–581. doi: 10.1016/0035-9203(55)90031-6. [DOI] [PubMed] [Google Scholar]
- 46.Beasley DW, McAuley AJ, Bente DA. 2015. Yellow fever virus: genetic and phenotypic diversity and implications for detection, prevention and therapy. Antiviral Res 115:48–70. doi: 10.1016/j.antiviral.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Domingo C, Escadafal C, Rumer L, Mendez JA, Garcia P, Sall AA, Teichmann A, Donoso-Mantke O, Niedrig M. 2012. First international external quality assessment study on molecular and serological methods for yellow fever diagnosis. PLoS One 7:e36291. doi: 10.1371/journal.pone.0036291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basile AJ, Goodman C, Horiuchi K, Laven J, Panella AJ, Kosoy O, Lanciotti RS, Johnson BW. 2015. Development and validation of an ELISA kit (YF MAC-HD) to detect IgM to yellow fever virus. J Virol Methods 225:41–48. doi: 10.1016/j.jviromet.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol 38:1823–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niedrig M, Kursteiner O, Herzog C, Sonnenberg K. 2008. Evaluation of an indirect immunofluorescence assay for detection of immunoglobulin M (IgM) and IgG antibodies against yellow fever virus. Clin Vaccine Immunol 15:177–181. doi: 10.1128/CVI.00078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akondy RS, Johnson PL, Nakaya HI, Edupuganti S, Mulligan MJ, Lawson B, Miller JD, Pulendran B, Antia R, Ahmed R. 2015. Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. Proc Natl Acad Sci U S A 112:3050–3055. doi: 10.1073/pnas.1500475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edupuganti S, Eidex RB, Keyserling H, Akondy RS, Lanciotti R, Orenstein W, del Rio C, Pan Y, Querec T, Lipman H, Barrett A, Ahmed R, Teuwen D, Cetron M, Mulligan MJ, Team YF-IS. 2013. A randomized, double-blind, controlled trial of the 17D yellow fever virus vaccine given in combination with immune globulin or placebo: comparative viremia and immunogenicity. Am J Trop Med Hyg 88:172–177. doi: 10.4269/ajtmh.2012.12-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibney KB, Edupuganti S, Panella AJ, Kosoy OI, Delorey MJ, Lanciotti RS, Mulligan MJ, Fischer M, Staples JE. 2012. Detection of anti-yellow fever virus immunoglobulin M antibodies at 3–4 years following yellow fever vaccination. Am J Trop Med Hyg 87:1112–1115. doi: 10.4269/ajtmh.2012.12-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Melo AB, da Silva Mda P, Magalhaes MC, Gonzales Gil LH, Freese de Carvalho EM, Braga-Neto UM, Bertani GR, Marques ET Jr, Cordeiro MT. 2011. Description of a prospective 17DD yellow fever vaccine cohort in Recife, Brazil. Am J Trop Med Hyg 85:739–747. doi: 10.4269/ajtmh.2011.10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allwinn R, Doerr HW, Emmerich P, Schmitz H, Preiser W. 2002. Cross-reactivity in flavivirus serology: new implications of an old finding? Med Microbiol Immunol 190:199–202. doi: 10.1007/s00430-001-0107-9. [DOI] [PubMed] [Google Scholar]
- 56.Houghton-Trivino N, Montana D, Castellanos J. 2008. Dengue-yellow fever sera cross-reactivity; challenges for diagnosis. Rev Salud Publica (Bogota) 10:299–307. doi: 10.1590/S0124-00642008000200010. [DOI] [PubMed] [Google Scholar]
- 57.Koraka P, Zeller H, Niedrig M, Osterhaus AD, Groen J. 2002. Reactivity of serum samples from patients with a flavivirus infection measured by immunofluorescence assay and ELISA. Microbes Infect 4:1209–1215. doi: 10.1016/S1286-4579(02)01647-7. [DOI] [PubMed] [Google Scholar]
- 58.Vazquez S, Valdes O, Pupo M, Delgado I, Alvarez M, Pelegrino JL, Guzman MG. 2003. MAC-ELISA and ELISA inhibition methods for detection of antibodies after yellow fever vaccination. J Virol Methods 110:179–184. doi: 10.1016/S0166-0934(03)00128-9. [DOI] [PubMed] [Google Scholar]
- 59.Chiou SS, Crill WD, Chen LK, Chang GJ. 2008. Enzyme-linked immunosorbent assays using novel Japanese encephalitis virus antigen improve the accuracy of clinical diagnosis of flavivirus infections. Clin Vaccine Immunol 15:825–835. doi: 10.1128/CVI.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mendez MC, Domingo C, Tenorio A, Pardo LC, Rey GJ, Mendez JA. 2013. Development of a reverse transcription polymerase chain reaction method for yellow fever virus detection. Biomedica 33(Suppl 1):S190–S196. [PubMed] [Google Scholar]
- 61.Sanchez-Seco MP, Rosario D, Hernandez L, Domingo C, Valdes K, Guzman MG, Tenorio A. 2006. Detection and subtyping of dengue 1–4 and yellow fever viruses by means of a multiplex RT-nested-PCR using degenerated primers. Trop Med Int Health 11:1432–1441. doi: 10.1111/j.1365-3156.2006.01696.x. [DOI] [PubMed] [Google Scholar]
- 62.Vasconcelos PF, Bryant JE, da Rosa TP, Tesh RB, Rodrigues SG, Barrett AD. 2004. Genetic divergence and dispersal of yellow fever virus, Brazil. Emerg Infect Dis 10:1578–1584. doi: 10.3201/eid1009.040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dash PK, Boutonnier A, Prina E, Sharma S, Reiter P. 2012. Development of a SYBR green I based RT-PCR assay for yellow fever virus: application in assessment of YFV infection in Aedes aegypti. Virol J 9:27. doi: 10.1186/1743-422X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Domingo C, Patel P, Yillah J, Weidmann M, Mendez JA, Nakoune ER, Niedrig M. 2012. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J Clin Microbiol 50:4054–4060. doi: 10.1128/JCM.01799-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drosten C, Kummerer BM, Schmitz H, Gunther S. 2003. Molecular diagnostics of viral hemorrhagic fevers. Antiviral Res 57:61–87. doi: 10.1016/S0166-3542(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 66.Weidmann M, Faye O, Faye O, Kranaster R, Marx A, Nunes MR, Vasconcelos PF, Hufert FT, Sall AA. 2010. Improved LNA probe-based assay for the detection of African and South American yellow fever virus strains. J Clin Virol 48:187–192. doi: 10.1016/j.jcv.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 67.Hughes HR, Russell BJ, Mossel EC, Kayiwa J, Lutwama J, Lambert AJ. 2018. Development of a real-time RT-PCR assay for the global differentiation of yellow fever virus vaccine adverse events from natural infections. J Clin Microbiol 56:e00323-. doi: 10.1128/JCM.00323-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chao DY, Davis BS, Chang GJ. 2007. Development of multiplex real-time reverse transcriptase PCR assays for detecting eight medically important flaviviruses in mosquitoes. J Clin Microbiol 45:584–589. doi: 10.1128/JCM.00842-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fischer C, Torres MC, Patel P, Moreira-Soto A, Gould EA, Charrel RN, de Lamballerie X, Nogueira RMR, Sequeira PC, Rodrigues CDS, Kummerer BM, Drosten C, Landt O, Bispo de Filippis AM, Drexler JF. 2017. Lineage-specific real-time RT-PCR for yellow fever virus outbreak surveillance, Brazil. Emerg Infect Dis 23. doi: 10.3201/eid2311.171131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rojas A, Diagne CT, Stittleburg VD, Mohamed-Hadley A, de Guillen YA, Balmaseda A, Faye O, Faye O, Sall AA, Harris E, Pinsky BA, Waggoner JJ. 2018. Internally controlled, multiplex real-time reverse transcription PCR for dengue virus and yellow fever virus detection. Am J Trop Med Hyg 98:1833–1836. doi: 10.4269/ajtmh.18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Escadafal C, Faye O, Sall AA, Faye O, Weidmann M, Strohmeier O, von Stetten F, Drexler J, Eberhard M, Niedrig M, Patel P. 2014. Rapid molecular assays for the detection of yellow fever virus in low-resource settings. PLoS Negl Trop Dis 8:e2730. doi: 10.1371/journal.pntd.0002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwallah A, Inoue S, Muigai AW, Kubo T, Sang R, Morita K, Mwau M. 2013. A real-time reverse transcription loop-mediated isothermal amplification assay for the rapid detection of yellow fever virus. J Virol Methods 193:23–27. doi: 10.1016/j.jviromet.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 73.Nunes MR, Vianez JL Jr, Nunes KN, da Silva SP, Lima CP, Guzman H, Martins LC, Carvalho VL, Tesh RB, Vasconcelos PF. 2015. Analysis of a reverse transcription loop-mediated isothermal amplification (RT-LAMP) for yellow fever diagnostic. J Virol Methods 226:40–51. doi: 10.1016/j.jviromet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Tesh RB, Guzman H, da Rosa AP, Vasconcelos PF, Dias LB, Bunnell JE, Zhang H, Xiao SY. 2001. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J Infect Dis 183:1431–1436. doi: 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- 75.Belsher JL, Gay P, Brinton M, DellaValla J, Ridenour R, Lanciotti R, Perelygin A, Zaki S, Paddock C, Querec T, Zhu T, Pulendran B, Eidex RB, Hayes E. 2007. Fatal multiorgan failure due to yellow fever vaccine-associated viscerotropic disease. Vaccine 25:8480–8485. doi: 10.1016/j.vaccine.2007.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munoz J, Vilella A, Domingo C, Nicolas JM, de Ory F, Corachan M, Tenorio A, Gascon J. 2008. Yellow fever-associated viscerotropic disease in Barcelona, Spain. J Travel Med 15:202–205. doi: 10.1111/j.1708-8305.2008.00209.x. [DOI] [PubMed] [Google Scholar]
- 77.Reusken C, Knoester M, GeurtsvanKessel C, Koopmans M, Knapen DG, Bierman WFW, Pas S. 2017. Urine as sample type for molecular diagnosis of natural yellow fever virus infections. J Clin Microbiol 55:3294–3296. doi: 10.1128/JCM.01113-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tricou V, Minh NN, Farrar J, Tran HT, Simmons CP. 2011. Kinetics of viremia and NS1 antigenemia are shaped by immune status and virus serotype in adults with dengue. PLoS Negl Trop Dis 5:e1309. doi: 10.1371/journal.pntd.0001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.ter Meulen J, Sakho M, Koulemou K, Magassouba N, Bah A, Preiser W, Daffis S, Klewitz C, Bae HG, Niedrig M, Zeller H, Heinzel-Gutenbrunner M, Koivogui L, Kaufmann A. 2004. Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J Infect Dis 190:1821–1827. doi: 10.1086/425016. [DOI] [PubMed] [Google Scholar]
- 80.Domingo C, Yactayo S, Agbenu E, Demanou M, Schulz AR, Daskalow K, Niedrig M. 2011. Detection of yellow fever 17D genome in urine. J Clin Microbiol 49:760–762. doi: 10.1128/JCM.01775-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez MJ, Vilella A, Pumarola T, Roldan M, Sequera VG, Vera I, Hayes EB. 2011. Persistence of yellow fever vaccine RNA in urine. Vaccine 29:3374–3376. doi: 10.1016/j.vaccine.2011.02.075. [DOI] [PubMed] [Google Scholar]
- 82.Rios M, Daniel S, Chancey C, Hewlett IK, Stramer SL. 2007. West Nile virus adheres to human red blood cells in whole blood. Clin Infect Dis 45:181–186. doi: 10.1086/518850. [DOI] [PubMed] [Google Scholar]
- 83.Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, Garcia MN, Correa A, Patel SM, Aagaard K, Mulligan MJ. 2017. Prolonged detection of zika virus in vaginal secretions and whole blood. Emerg Infect Dis 23:99–101. doi: 10.3201/eid2301.161394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scipioni A, Bourgot I, Mauroy A, Ziant D, Saegerman C, Daube G, Thiry E. 2008. Detection and quantification of human and bovine noroviruses by a TaqMan RT-PCR assay with a control for inhibition. Mol Cell Probes 22:215–222. doi: 10.1016/j.mcp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 85.Ricciardi-Jorge T, Bordignon J, Koishi A, Zanluca C, Mosimann AL, Duarte Dos Santos CN. 2017. Development of a quantitative NS1-capture enzyme-linked immunosorbent assay for early detection of yellow fever virus infection. Sci Rep 7:16229. doi: 10.1038/s41598-017-16231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yen CW, de Puig H, Tam JO, Gomez-Marquez J, Bosch I, Hamad-Schifferli K, Gehrke L. 2015. Multicolored silver nanoparticles for multiplexed disease diagnostics: distinguishing dengue, yellow fever, and Ebola viruses. Lab Chip 15:1638–1641. doi: 10.1039/C5LC00055F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vasconcelos PF, Costa ZG, Travassos Da Rosa ES, Luna E, Rodrigues SG, Barros VL, Dias JP, Monteiro HA, Oliva OF, Vasconcelos HB, Oliveira RC, Sousa MR, Barbosa Da Silva J, Cruz AC, Martins EC, Travassos Da Rosa JF. 2001. Epidemic of jungle yellow fever in Brazil, 2000: implications of climatic alterations in disease spread. J Med Virol 65:598–604. doi: 10.1002/jmv.2078. [DOI] [PubMed] [Google Scholar]
- 88.Blake LE, Garcia-Blanco MA. 2014. Human genetic variation and yellow fever mortality during 19th century U.S. epidemics. mBio 5:e01253–e01214. doi: 10.1128/mBio.01253-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ribeiro M, Antunes CM. 2009. [Yellow fever: study of an outbreak]. Rev Soc Bras Med Trop 42:523–531. (In Portuguese.) doi: 10.1590/S0037-86822009000500009. [DOI] [PubMed] [Google Scholar]
- 90.Ferreira AC, Zaverucha-do-Valle C, Reis PA, Barbosa-Lima G, Vieira YR, Mattos M, Silva PP, Sacramento C, de Castro Faria Neto HC, Campanati L, Tanuri A, Bruning K, Bozza FA, Bozza PT, Souza TML. 2017. Sofosbuvir protects Zika virus-infected mice from mortality, preventing short- and long-term sequelae. Sci Rep 7:9409. doi: 10.1038/s41598-017-09797-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Freitas CS, Higa LM, Sacramento C, Ferreira AC, Reis PA, Delvecchio R, Monteiro FL, Barbosa-Lima G, Vieira YR, Mattos M, Boas Hoelz LV, Paes Leme RP, Bastos MM, Bozza FA, Bozza PT, Boechat N, Tanuri A, Souza TML. 2018. Yellow fever virus is susceptible to sofosbuvir both in vitro and in vivo. bioRxiv doi: 10.1101/266361. [DOI] [PMC free article] [PubMed]
- 92.Poland JD, Calisher CH, Monath TP, Downs WG, Murphy K. 1981. Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bull World Health Organ 59:895–900. [PMC free article] [PubMed] [Google Scholar]
- 93.Monath TP, Cetron MS. 2002. Prevention of yellow fever in persons traveling to the tropics. Clin Infect Dis 34:1369–1378. doi: 10.1086/340104. [DOI] [PubMed] [Google Scholar]
- 94.Romano AP, Costa ZG, Ramos DG, Andrade MA, Jayme Vde S, Almeida MA, Vettorello KC, Mascheretti M, Flannery B. 2014. Yellow Fever outbreaks in unvaccinated populations, Brazil, 2008–2009. PLoS Negl Trop Dis 8:e2740. doi: 10.1371/journal.pntd.0002740. [DOI] [PMC free article] [PubMed] [Google Scholar]