The recognition of the role of Kingella kingae as one of the main etiologic agents of skeletal system infections in young children and the recent discovery of the novel Kingella negevensis species have resulted in an increasing interest in these two emerging pediatric pathogens. Both bacteria colonize the oropharynx and are not detected in nasopharyngeal specimens, and the colonized mucosal surface is their portal of entry to the bloodstream.
KEYWORDS: Kingella kingae, Kingella negevensis, carriage, culture, method, oropharynx
ABSTRACT
The recognition of the role of Kingella kingae as one of the main etiologic agents of skeletal system infections in young children and the recent discovery of the novel Kingella negevensis species have resulted in an increasing interest in these two emerging pediatric pathogens. Both bacteria colonize the oropharynx and are not detected in nasopharyngeal specimens, and the colonized mucosal surface is their portal of entry to the bloodstream. Although species-specific nucleic acid amplification assays have significantly improved the detection of kingellae and facilitated patients' management, the increasing use of this diagnostic approach has the potential drawback of neglecting culture recovery of these organisms. The isolation of Kingella species enables the thorough genotyping of strains for epidemiological purposes, the study of the dynamics of asymptomatic colonization and person-to-person transmission, the investigation of the pathogenesis of invasive infections, and the determination of antibiotic susceptibility patterns. The culture isolation of pharyngeal strains and their comparison with isolates derived from normally sterile body sites may also aid in identifying virulence factors involved in the transition from colonization to invasive disease which could represent potential targets for a future protective vaccine. The two species are notoriously fastidious, and their isolation from upper respiratory tract specimens requires a short transport time, plating on selective vancomycin-containing blood-agar medium, and incubation under capnophilic and aerobic conditions. The identification of K. kingae and K. negevensis can be performed by a combination of the typical Gram stain and biochemical tests and confirmed and differentiated by molecular assays that target the groEL and mdh genes.
INTRODUCTION
Since Kingella kingae is a common invasive pediatric pathogen and the most frequent etiology of osteoarticular infections in children aged 6 to 48 months, there is a growing interest in the investigation of the epidemiology of the organism as well as in understanding the virulence factors involved in mucosal colonization and invasive disease (1). The bacterium is notoriously fastidious, and its isolation in blood cultures, exudates from bone and joint infections, and the oropharynx of colonized individuals is frequently unsuccessful (1). Kingella kingae is inhibited by purulent body fluids, and its presence in upper respiratory tract specimens is obscured by the overgrowth of other members of the residing flora. To overcome these obstacles, the isolation of the organism often requires the inoculation of synovial fluid aspirates and bone exudates in blood culture vials (BCVs) and the inoculation of oropharyngeal samples on selective culture medium (BAV agar) (1). In recent years, the use of BAV medium for processing pediatric oropharyngeal specimens has resulted in the unexpected isolation of a novel and difficult-to-cultivate Kingella species, named Kingella negevensis, which was originally misidentified as K. kingae (1, 2).
In recent years, molecular detection methods have made a significant impact in the diagnosis of infectious diseases in general and of those caused by fastidious bacteria in particular. Amplification and sequencing of the 16S rRNA have enhanced by 2-fold the detection of K. kingae organisms from skeletal system infections compared to that of cultures (1). Species-specific nucleic acid amplification tests (NAATs) targeting either the rtx operon or the groEL gene (also known as cpn60) have recently been developed, and their use has further improved the detection of elusive K. kingae organisms in bone and joint exudates by a 4-fold factor, decreasing the time required for making a bacteriological diagnosis from several days to a few hours, even in patients on antibiotic therapy (1, 3–6).
Despite the obvious advantages of NAATs for improving the sensitivity of detection and diagnosis, the culture approach remains critical to obtain isolates in order to expand our understanding of K. kingae and K. negevensis, because it enables in-depth research of the organism and its biological features. The increasing use of the diagnostic molecular approach has the potential drawback of neglecting the culture isolation of the organism. This is especially regrettable for K. negevensis, because the clinical significance of the species is still unclear and the number of strains available for research worldwide is still very small. A loss of the capability to isolate kingellae could hamper in the future our understanding of the immune response to particular components of these organisms, thus compromising the possibility of developing protective vaccines.
The present review describes in detail the culture and identification procedures of K. kingae and K. negevensis followed at the Clinical Microbiology Laboratory of the Soroka University Medical Center (CML-SUMC), Beer-Sheva, Israel, with the aim of familiarizing other laboratories with the detection of these species in upper respiratory tract specimens. Obviously, events of colonization might have been systematically missed by the CML-SUMC customary culture protocol. However, the carriage rate of K. kingae detected in young Israeli children by our cultivation method (1) was similar to that found by a sensitive molecular assay in a Swiss population of comparable age (7), suggesting adequate recovery of the organism by culture.
RESPIRATORY CARRIAGE OF K. KINGAE AND K. NEGEVENSIS
It was long suspected that, similar to other members of the Neisseriaceae family, K. kingae was carried in the respiratory tract. This assumption was ascertained in a pioneer study conducted among young attendees to a day care center facility (8). Additional research demonstrated that K. kingae colonizes the oropharyngeal epithelium and does not colonize the nasopharynx (8, 9), and the carriage rate closely parallels the age-related epidemic curve of invasive infections caused by the organism (1). The colonized oropharyngeal surface is the portal of entry of K. kingae to the bloodstream, frequently facilitated by a previous or concomitant viral infection, from which it disseminates to the skeletal system or the endocardium for which the organism shows a particular affinity (1, 10). Kingella kingae colonization, as well as the occurrence of clinical disease, is exceptional in the first 6 months after birth, suggesting the combined protective effects of maternally derived immunity and limited social contacts in early infancy (1). The carriage rate and prevalence of infection rise rapidly in children older than 6 months, indicating a vanishing immunity coupled with increasing exposure (1). The prevalence of pharyngeal colonization reaches a zenith (10% to 12%) in the second year of life and gradually declines in older children and adults (1).
Studies employing the cultivation of the bacterium have shown that organisms carried by siblings and playmates tend to be genotypically identical, implying that intimate contact facilitates person-to-person spread (8, 9, 11). Transmission is enhanced in young children attending day care centers, among which the organism was detected, on average, in 23% in an Israeli study (1, 8, 11). Genotyping of pharyngeal isolates has revealed that K. kingae colonization is a dynamic process and colonizing organisms are replaced by new strains after weeks or months of asymptomatic carriage (1, 11).
The respiratory colonization of K. negevensis was recently assessed by reexamining data from the original study in which the organism was still erroneously considered to be a small colony variant of K. kingae (1, 2, 9). The age-related acquisition and carriage of the novel species closely followed that of K. kingae, starting after the sixth month of life, reaching a plateau in the second year, and decreasing thereafter, but the overall carriage rate was approximately one-tenth of that noted in K. kingae (9).
TYPING K. KINGAE ISOLATES: INVASIVE STRAINS AND MUCOSAL COLONIZERS
Although the number of healthy carriers is much larger than that of diseased individuals, strain collections of human pathogens are usually heavily biased toward isolates that are particularly virulent, often neglecting less invasive organisms and underestimating the extent of the genetic diversity of the species. The isolation of K. kingae strains colonizing the oropharynx is important for whole-genomic analysis to ascertain genomic relatedness and identify core genes and those associated with colonization fitness, invasiveness, and tissue tropism.
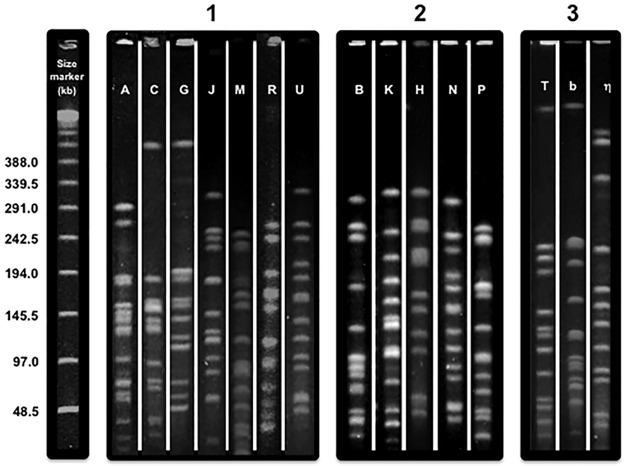
Genotyping of K. kingae isolates has shown that the species exhibits remarkable genomic heterogeneity. More than 60 different multilocus sequence typing (MLST) combinations of alleles and 70 pulsed-field gel electrophoresis (PFGE) clones, named by arbitrarily chosen letters, have been described to date (1, 12, 13). Colonizing organisms differ in their potential virulence, and not all strains found in the oropharynx are capable of causing clinical infections (12, 13). For instance, PFGE clones A, C, G, J, M, R, and U are commonly detected among healthy children but are seldom if ever isolated from patients with clinical K. kingae disease (12, 13) (Fig. 1). The majority of invasive K. kingae infections are caused by a small number of distinct PFGE clones (1, 12). In a countrywide Israeli study, 32 different PFGE clones were found among invasive isolates recovered from 181 patients. Five distinct clones, namely, B, K, H, N, and P, were overrepresented in the sample and collectively comprised 128 (70.7%) of all the strains (13). Genomically similar strains have been also detected in patients with invasive infections in Europe (12), indicating that these organisms combine enhanced virulence and transmission fitness and have spread widely. Remarkably, a few PFGE clones exhibited specific tissue tropism and were significantly associated with skeletal system infections (clone N), bacteremia (clone K), or endocarditis (clone P) (13) (Fig. 1).
FIG 1.
Composite picture showing the pulsed-field electrophoretic profiles of Kingella kingae and K. negevensis obtained with the EagI restriction enzyme. The figure was created by joining pictures taken from multiple gels. 1, K. kingae clones usually associated with asymptomatic carriage; 2, K. kingae clones isolated from patients with invasive disease; 3, the 3 most common K. negevensis clones.
Research conducted using a large assortment of international isolates recovered from asymptomatic respiratory carriers and patients with a variety of clinical infections has revealed that K. kingae elaborates a polysaccharide capsule and an exopolysaccharide that confer invasiveness in juvenile animal models (14–16). Two capsule types (namely, a and b) appear to have enhanced pathogenic properties and are detected in 95% of all isolates from patients with invasive diseases, irrespective of their geographical origin (17, 18). Interestingly, capsules c and d are significantly more abundant in isolates from healthy pharyngeal carriers (17).
PHARYNGEAL CULTURES AND NAATs IN THE INVESTIGATION OF K. KINGAE OUTBREAKS
Over the last 2 decades, clusters of invasive K. kingae infections have been detected among young attendees to day care centers in France, Israel, and the United States (1, 19). These events have been characterized by the dissemination of highly virulent strains in the facility causing bacteremia, a variety of skeletal system infections, tenosynovitis, and endocarditis (1). Investigation of the outbreaks has repeatedly included sampling of the oropharynges of asymptomatic day care center attendees and their caretakers to detect carriers of the causative strain deemed to be at increased risk for infection and candidates to receive prophylactic antibiotics (19). In the course of the epidemiological investigation of a cluster of invasive K. kingae disease among attendees to a day care facility in Paris, the molecular method detected twice as many colonized individuals than the respiratory culture on the Columbia-based selective medium (12 versus 6 carriers, respectively) (20). It should be pointed out, however, that the primer employed in the study fails to distinguish between K. kingae and Simonsiella muelleri, an organism that may also colonize the human upper respiratory tract; therefore, the apparent superior performance of the molecular assay may, in fact, represent a false-positive result (20). Although no rigorous comparisons of the performance of culture methods and molecular assays for the detection of K. kingae colonization of the pharynx have been performed, it is plausible that the sensitivity of cultures is inferior to that of NAATs. It should be pointed out, however, that the recovery of K. kingae by culture enables typing of the isolates to determine the dissemination of the outbreak strain within the day care population (1, 19) as well as the performance of antibiotic susceptibility testing. The typing capability is lost when diagnostic molecular methods are used, because in order to achieve exquisite sensitivity, NAATs amplify highly conserved housekeeping genes that evolve at a slow pace and therefore lack discriminatory power (3–6). Recently, a modified multilocus sequence typing (MLST) protocol was able to differentiate K. kingae strains into distinct sequence types directly from DNA extracted from oropharyngeal swabs without requiring the isolation of the bacterium (21). This level of discrimination is, however, insufficient for the epidemiological investigation of clusters of K. kingae disease in day care centers in which multiple closely related strains belonging to the same sequence type but of lesser virulence may be cocirculating (1, 19).
WHY ISOLATE K. KINGAE AND K. NEGEVENSIS FROM THE OROPHARYNX
Because the colonized upper respiratory epithelium is the source from which K. kingae invades the bloodstream and disseminates to bones, joints, and intervertebral discs, the detection of the organism in the pharynx has been employed to determine the presumptive etiology of a variety of pediatric infections affecting the skeletal system. This approach has been particularly successful for establishing the bacteriological diagnosis of osteomyelitis and septic arthritis involving small bones and joints, tenosynovitis, and spondylodiscitis in young children, since specimens of the infected sites are not easily accessible and cultures of blood and relevant skeletal system exudates and tissues are frequently negative (1, 22–24). It should be noted, however, that K. kingae is carried by approximately 10% of healthy children aged 6 months to 4 years, and the colonization rate is even higher among those attending day care facilities (1, 8). Therefore, the detection of the organism in the pharynx in the absence of its isolation or a positive molecular assay from a normally sterile body site cannot be considered irrefutable proof of the etiology of the infection (1). On the other hand, the negative predictive value of the NAATs is high, and the failure to detect K. kingae-specific DNA sequences in the oropharynx probably excludes the bacterium as the etiology of the disease (1).
The CML-SUMC has been a pioneer in optimizing the culture recovery of K. kingae, demonstrating that the oropharyngeal epithelium is the initial colonization site of this elusive pathogen in the human body (8), and over the years, has been involved in multiple studies of the epidemiology of the carriage and dissemination of the organism in the pediatric population (1, 8, 9). Employing the same culture protocol, we recently recovered a novel Kingella species colonizing the pharynges of healthy young children living in the Negev Desert area, subsequently named K. negevensis sp. nov. (2). The organism has also been detected in France, Switzerland, and the United States (25, 26), indicating a wide geographical distribution, although it has not been isolated yet from patients with invasive infection. Kingella negevensis exhibits genomic heterogeneity, and a few clones, defined by distinct PFGE and sequencing profiles of the 16S rRNA and groEL genes, predominated in a healthy carrier population (2). Although the clinical significance of this species is still being investigated, preliminary data suggest that, similar to K. kingae, the bacterium is associated with osteoarticular infections in young children (26). The performance of additional research on the subject and the culture isolation of multiple K. negevensis strains are of paramount importance to understand the epidemiology and pathogenesis of colonization and disease.
CULTURE PROCEDURE
Sampling site.
Unlike the observations made with other pathogens of respiratory origin, the niche of K. kingae and K. negevensis appears to be restricted to the oropharyngeal epithelium and tonsillar crypts (8, 9). In a cohort study in which 716 children were enrolled and separate cultures from the oropharynges and nasopharynges were sequentially performed between the ages of 2 and 30 months, 376 of 4,472 (8.4%) oropharyngeal cultures, but only a single nasopharyngeal culture, grew K. kingae (P < 0.001 by the chi-square test) (9). In the same study, K. negevensis was isolated from 26 of 4,472 (0.25%) oropharyngeal cultures, and none of the 4,472 nasopharyngeal specimens were positive for the organism (9). Thus, the oropharynx is the optimal sampling site for both species.
Swab material and transport.
A number of commercial swabs and transport systems have been used for the collection of upper respiratory tract specimens in prevalence studies. The materials from which the swabs fibers are made comprise calcium alginate, rayon, polyethylene terephthalate (Dacron), and nylon-flocked tips. Ideally, oropharyngeal swabs should (i) be safe for use in young noncooperative children, (ii) be efficient at extracting the target organism from the mucosal surface onto the swab, (iii) enable easy elution of the target bacteria from the swab, (iv) have no detrimental effect on the viability of the organism, and (v) be compatible with all intended laboratory procedures, including cultures and NAATs (27). Because very few colonization studies using culture-based methods have been carried out outside Israel, there are no published data on the performance of different swab and transport systems other than those employed at the CML-SUMC for the isolation of kingellae. Over the years, we have employed the Amies agar gel medium transport with rayon swabs manufactured by Copan Diagnostics transported at room temperature, resulting in the isolation of hundreds of K. kingae strains from respiratory carriers as well as >50 K. negevensis strains. Although this transport medium was designed to maintain the viability of microorganisms such as Neisseria gonorrhoeae for up to 48 h, its capability to maintain the viability of fastidious kingellae has not been systematically assessed, and due to the highly fastidious nature of Kingella species, as a measure of caution, we prefer to plate the inoculated swabs within <6 h of collection. We have shared our strains with researchers in France, Switzerland, and the United States and shipped them overseas at room temperature in Amies agar gel medium tubes. The transport time has been, on average, 4 to 5 days, and the organisms, if plated immediately upon arrival, can usually be retrieved with minimal losses. Obviously, a prospective and systematic comparison of swabs and transport media should be conducted before definitive recommendations can be made.
Culture media and incubation conditions.
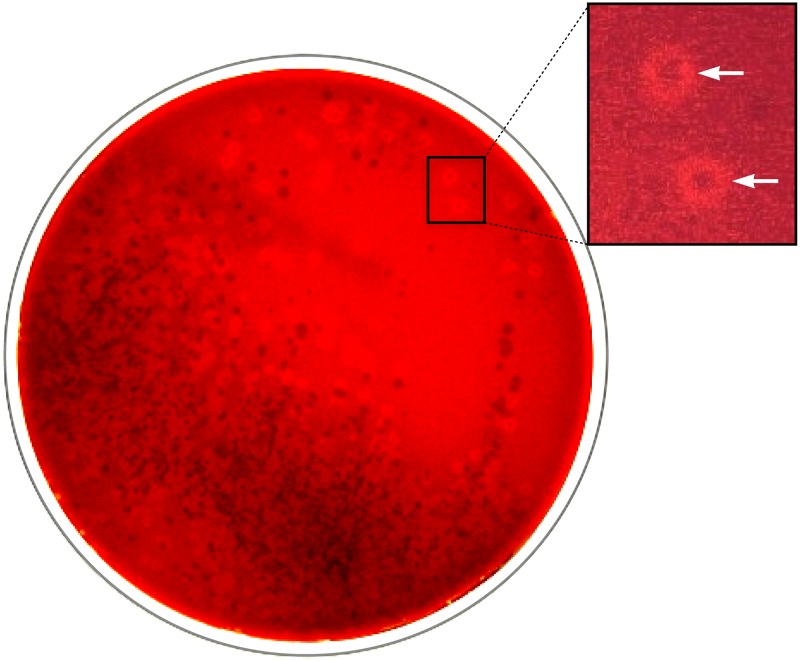
The recognition of K. kingae and K. negevensis in cultures of upper respiratory tract specimens is difficult and requires special media and incubation conditions because the rich oropharyngeal microbiota tends to hide the presence of these elusive species. On the basis of the fact that K. kingae strains are beta-hemolytic and universally resistant to glycopeptide antimicrobials, a selective and indicative culture medium consisting of Trypticase soy agar with 5% added sheep hemoglobin and 2 μg/ml of vancomycin (BAV medium) has been developed (1). A variant of the original BAV formulation based on Columbia agar has also been successfully employed in France for detecting asymptomatic K. kingae carriage (20). To date, no comparison of the performance of the two preparations has been performed. Plates inoculated with oropharyngeal secretions are incubated under aerobic conditions in a 5% CO2-enriched atmosphere at 35°C for 48 h and examined daily (1). The rationale behind this methodology is that the glycopeptide antibiotic inhibits the growth of the competing Gram-positive buccal and pharyngeal flora, whereas the aerobic conditions suppress the development of anaerobic species; the added CO2 enhances the growth of capnophilic K. kingae and K. negevensis, and the hemoglobin content facilitates the visualization of hemolytic colonies of both organisms (1, 2) (Fig. 2). The use of chocolate-based media for primary isolation is not recommended, since it does enable the recognition of beta-hemolysis and probably contributed to failure to identify respiratory carriers in the investigation of an outbreak of K. kingae disease in a North Carolina day care center (1). In a field study in which the performance of the BAV medium and that of Trypticase soy agar with 5% added hemoglobin (here, blood-agar) for the primary isolation of K. kingae were compared, the selective medium detected 43 of 44 (97.7%) carriers, whereas the blood-agar plate identified only 10 (22.7%) (P < 0.001 by the chi-square test) (1).
FIG 2.
Oropharyngeal specimen cultured on BAV medium. Inset shows rings of beta-hemolysis surrounding colonies of K. kingae (arrows).
IDENTIFICATION OF K. KINGAE AND K. NEGEVENSIS
Phenotypic methods.
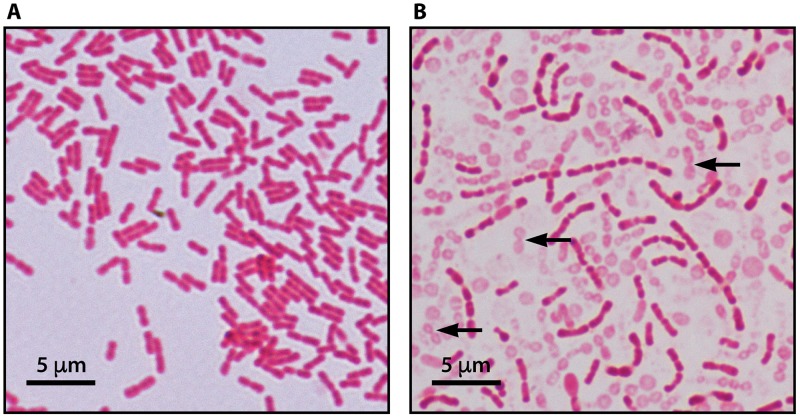
Kingella kingae is a facultative anaerobic bacterium that appears as pairs or short chains of Gram-negative coccobacilli with tapered ends (1) (Fig. 3A). The organism is beta-hemolytic, and this property is conferred by a wide-spectrum repeat-in-toxin (RTX) cytotoxin, which is also elaborated by K. negevensis (1, 2, 14, 25, 26). Kingella kingae can be subcultured on blood-agar, chocolate-agar, and Thayer-Martin media but fails to grow on Kligler and MacConkey plates (1). Growth is characterized by marked pitting of the agar surface, which is made more conspicuous after harvesting the colonies. Kingella kingae is a nonmotile and non-spore-forming organism, exhibits negative catalase, urease, and indole tests and, with rare exceptions, shows oxidase activity (1). Kingella kingae produces acid from glucose and maltose (the latter reaction is rarely negative) but not from other sugars, hydrolyzes indoxyl phosphate and l-prolyl-β-naphthylamide, and exhibits positive alkaline and acid phosphatase reactions (1). Beta-hemolytic colonies growing on BAV medium and exhibiting a typical Gram stain, a positive oxidase reaction, and negative catalase activity are presumptively identified as K. kingae and should be further tested for confirmation. A number of commercial systems can be used for the identification and include the quadFERM+ kit (Analytab Products, Plainview, NY), the API NH kit, (bioMérieux, Marcy-l'Etoile, France), the Vitek 2 instrument (bioMérieux, Marcy-l'Etoile, France), and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) technology. However, K. kingae is misidentified by the Remel RapID NH system (Thermo Scientific, Lenexa, KS) as Gardnerella vaginalis and occasionally as Haemophilus ducreyi or Haemophilus parainfluenzae (1).
FIG 3.
(A) Typical Gram stain of Kingella kingae depicting Gram-negative coccobacilli with tapered ends arranged in pairs and short chains. (B) Gram stain of Kingella negevensis exhibiting long chains of Gram-negative coccobacilli and “ghost-like” cells representing autolysis after a 36-h incubation.
Kingella negevensis forms long chains of plump Gram-negative coccobacilli and exhibits early autolysis (Fig. 3B). The organism is beta-hemolytic, grows as pinpoint colonies on blood-agar, chocolate-agar, and BAV plates, and does not display growth on Thayer-Martin, Kligler, or MacConkey culture media (2). Corroding of the agar surface is also observed beneath K. negevensis colonies. Enhanced growth, without modifying the long-chain configuration, is obtained by seeding the bacterium on GC-based medium but not on chocolate-agar medium (1, 2). Kingella negevensis shows aerobic and facultative anaerobic growth, with an optimal growth temperature of 35°C, with or without 5% added CO2, but optimal development is observed under aerobic/capnophilic conditions (2). The species shows positive oxidase and negative catalase tests, produces acid from glucose but not from other sugars (including maltose), and exhibits alkaline phosphatase, acid phosphatase, and leucine arylamidase enzymatic activity (2). In summary, the typical Gram-stain morphology, differences in growth/no growth on Thayer-Martin medium, and acid production from maltose make a clear and easy distinction between K. negevensis and K. kingae. The phenotypic features that enable the identification of K. kingae and K. negevensis and their differentiation from other members of the genus are shown in Table 1.
TABLE 1.
Phenotypic characteristics that differentiate the five Kingella species
| Characteristic | K. kingae | K. negevensis | K. denitrificans | K. oralis | K. potus |
|---|---|---|---|---|---|
| Gram stain | Coccobacilli in pairs and short chains | Coccobacilli in long chains, early autolysis | Coccobacilli in pairs and short chains | Rods or coccobacilli, sometimes in pairs and short chains | Rods |
| Hemolysis | β | β | − | − | − |
| Pitting of the agar | + | + | + | + | − |
| Pigment | − | − | − | − | + |
| Oxidase | + | + | + | + | + |
| Catalase | − | − | − | − | − |
| Acid production form glucose | + | + | + | + | − |
| Acid production from maltose | + | − | − | − | − |
| H2S production | − | − | − | + | − |
| DNase | − | − | − | − | + |
| NO2− reduction | − | − | + | − | − |
| NO3− reduction | − | − | + | − | − |
| Phosphatase activity | + | + | − | + | − |
Currently, K. negevensis is not identified correctly by the commercial MALDI-TOF technology instruments, since the organism has not been incorporated yet into the databases. When examined by the Brüker Daltonik instrument, K. negevensis exhibits protein spectrum scores lower than 1.9, precluding its identification, and the Vitek MS system misidentifies the organism as K. kingae (2, 25).
Identification by molecular assays.
Genomic comparison of the 16S rRNA gene shows that K. kingae and K. negevensis share <97% identity; therefore, correct identification of the isolates and differentiation between these two closely related species can be made by sequencing the gene (2). However, because of the close taxonomic proximity of K. kingae and K. negevensis, partial sequencing of the 16S rRNA gene does not necessarily distinguish between the two species. It is recommended to amplify and sequence the whole gene or at least the more informative and hypervariable V1, V2, V6, and V9 regions (2). Due to the fact that K. kingae and K. negevensis elaborate identical RTX toxins (2, 14, 25), molecular assays that target the rtxA and rtxB genes do not distinguish between the two organisms (26). To overcome the problem, a K. negevensis-specific real-time PCR assay that amplifies the groEL gene has been developed (28). The test accurately discriminated between 20 different strains of K. negevensis, 42 K. kingae strains, and other members of the Kingella genus and Neisseriaceae family (28). Although recent studies have confirmed that amplification of the K. kingae groEL gene is a reliable strategy for the detection of the organism in clinical specimens (5, 28), the primers and probes of groEL-based quantitative PCR (qPCR) assays designed by Ilharreborde et al. (4) and Levy et al. (29) display sequence mismatches that may result in suboptimal sensitivity and a failure to identify K. kingae isolates. Because invasive K. kingae infections are characterized by a low bacterial load, ranging from 11 to 300 CFU per ml of joint exudate (1), these concentrations may approach or exceed the lower limit of detection of NAATs. Therefore, any loss of sensitivity may result in a false-negative result of the molecular assay. To overcome this potential problem, an alternative NAAT targeting the species' housekeeping malate dehydrogenase (mdh) gene has been recently developed (30). Because the mdh gene is absent from other kingellae genomes—including K. negevensis—while its sequence is highly conserved among K. kingae strains, this novel test accurately differentiated between K. kingae and other members of the genus. The assay demonstrated improved specificity and sensitivity and was successfully applied to diagnose invasive K. kingae infections, as well as asymptomatic respiratory carriage, in young children (30). The primers and probes employed in real-time PCR assays for the identification of K. kingae and K. negevensis are summarized in Table 2.
TABLE 2.
Targeted genes, sequences of primers and probes, and species detection by Kingella-specific NAATs
| Targeted gene | Sequence (5′→3′) |
Result |
Reference | ||
|---|---|---|---|---|---|
| Primer (forward, reverse) | Probe | K. kingae | K. negevensis | ||
| rtxA | GCGCACAAGCAGGTGTACAA, ACCTGCTGCTACTGTACCTGTTTTAG | TTGAACAAAGCTGGACACG | + | + | 22 |
| TGCCAAAGTAAAACCAGCTGAA, AACTTACCTAATTTTGGCAAAGCAA | TGACAACAAACCGCTAATCATTTCTAAGGCC | + | + | 23 | |
| rtxB | CAACATAAGCCGCCAGTTGA, ACAATTAAAGCAATGGCAGTTGAG | ATCCCAACGGCGCGTCATTTGT | + | + | 23 |
| groEL | GCAAGAAGTCGGCAAAGAG, GTCAAACAACAACACAAATGGG | CGCGATCGCGACAAGTAGCCACGGTCAAGATCGCG | + | − | 4 |
| CACGTTCTGCATTGAAATCTG, GTTCACTACTACAGACGCTTC | CGCTGACCAAGAAGCTGGCGTG | − | + | 26 | |
| CCGATTTGAAACGCGGTATT, TTTGCCAACTTGCTCGTCAG | AGTGGCGGCTTTGGTTGGG | + | − | 29 | |
| mdh | TGTTCCGCATTGCTTCTG, TCATGCCGTCCAACAATG | CATCATCACGCCCTGAACGGCTT | + | − | 30 |
STORAGE AND RECOVERY OF ISOLATES
Kingella kingae is a highly fastidious organism, and the maintenance of viable strains requires subculturing the bacterium every 48 to 72 h. Kingella negevensis is an even more frail organism, and early autolysis is already evident in cultures aged <24 h (Fig. 3B). Unless subcultures are performed daily, K. negevensis strains may be irreversibly lost. Colonies from fresh cultures are harvested and inoculated in vials containing Mueller-Hinton broth with 15% added glycerol and kept frozen at −70°C. Our strain collection currently comprises hundreds of frozen K. kingae isolates from healthy pharyngeal carriers and patients with invasive diseases, many of them isolated in the 1990s and 2000s, as well as K. negevensis organisms isolated more than 10 years ago. With very few exceptions, the frozen strains remain viable today.
CONCLUSIONS
Over the last 3 decades, the use of improved culture methods has resulted in an increasing appreciation of the role of K. kingae as a pediatric pathogen and the recognition of the organism as the most common etiology of skeletal system infections in early childhood. The development of the selective BAV medium has revealed that the oropharynx is K. kingae's initial colonization site from which it penetrates into the bloodstream and disseminates to bone and joint tissues and the endocardium. Culture of oropharyngeal specimens has also disclosed chains of person-to-person transmission of the organism among family members and attendees to day care center facilities. In-depth research of K. kingae isolates has enabled the identification of a wide-spectrum cytotoxin, pili, and capsular polysaccharides involved in the colonization of the respiratory epithelium and invasion of the blood and skeletal tissues and the determination of its antibiotic susceptibility pattern. More recently, culture of the oropharynges of young children made possible the isolation and characterization of the novel K. negevensis species. Although the adoption of sensitive NAATs has greatly improved the bacteriological diagnosis of invasive infections caused by kingellae and the management of septic arthritis, osteomyelitis, and discitis in young children, culture isolation remains a cornerstone of future developments in the identification of virulence factors of K. kingae and K. negevensis and the study of the pathogenesis of pediatric diseases caused by these emerging pathogens and should not be abandoned.
REFERENCES
- 1.Yagupsky P. 2015. Kingella kingae: carriage, transmission, and disease. Clin Microbiol Rev 28:54–79. doi: 10.1128/CMR.00028-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Houmami N, Bakour S, Bzdrenga J, Rathored J, Seligmann H, Robert C, Armstrong N, Schrenzel J, Raoult D, Yagupsky P, Fournier PE. 2017. Isolation and characterization of Kingella negevensis sp. nov., a novel Kingella species detected in a healthy paediatric population. Int J Syst Evol Microbiol 67:2370–2376. doi: 10.1099/ijsem.0.001957. [DOI] [PubMed] [Google Scholar]
- 3.Moumile K, Merckx J, Glorion C, Berche P, Ferroni A. 2003. Osteoarticular infections caused by Kingella kingae in children; contribution of polymerase chain reaction to the microbiologic diagnosis. Pediatr Infect Dis J 22:837–839. doi: 10.1097/01.inf.0000083848.93457.e7. [DOI] [PubMed] [Google Scholar]
- 4.Ilharreborde B, Bidet P, Lorrot M, Even J, Mariani-Kurkdjian P, Ligouri S, Vitoux C, Lefevre Y, Doit C, Fitoussi F, Pennecot G, Bingen E, Mazda K, Bonacorsi S. 2009. New real-time PCR-based method for Kingella kingae DNA detection: application to samples collected from 89 children with acute arthritis. J Clin Microbiol 47:1837–1841. doi: 10.1128/JCM.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chometon S, Benito Y, Chaker M, Boisset S, Ploton C, Bérard J, Vandenesch F, Freydière AM. 2007. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J 26:377–381. doi: 10.1097/01.inf.0000259954.88139.f4. [DOI] [PubMed] [Google Scholar]
- 6.Juchler C, Spyropoulou V, Wagner N, Merlini L, Dhouib A, Manzano S, Tabard-Fougère A, Samara E, Ceroni D. 2018. The contemporary bacteriologic epidemiology of osteoarticular infections in children in Switzerland. J Pediatr 194:190.e1–196.e1. doi: 10.1016/j.jpeds.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Ceroni D, Dubois-Ferrière V, Anderson R, Combescure C, Lamah L, Cherkaoui A, Schrenzel J. 2012. Small risk of osteoarticular infections in children with asymptomatic carriage of Kingella kingae. Pediatr Infect Dis J 31:983–985. doi: 10.1097/INF.0b013e31825d3419. [DOI] [PubMed] [Google Scholar]
- 8.Yagupsky P, Dagan R, Prajgrod F, Merires M. 1995. Respiratory carriage of Kingella kingae among healthy children. Pediatr Infect Dis J 14:673–678. doi: 10.1097/00006454-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Yagupsky P, Weiss-Salz I, Fluss R, Freedman L, Peled N, Trefler R, Porat N, Dagan R. 2009. Dissemination of Kingella kingae in the community and long-term persistence of invasive clones. Pediatr Infect Dis J 28:707–710. doi: 10.1097/INF.0b013e31819f1f36. [DOI] [PubMed] [Google Scholar]
- 10.Yagupsky P, Porat N, Pinco E. 2009. Pharyngeal colonization by Kingella kingae in children with invasive disease. Pediatr Infect Dis J 28:155–157. doi: 10.1097/INF.0b013e318184dbb8. [DOI] [PubMed] [Google Scholar]
- 11.Slonim A, Walker ES, Mishori E, Porat N, Dagan R, Yagupsky P. 1998. Person-to-person transmission of Kingella kingae among day care center attendees. J Infect Dis 178:1843–1846. doi: 10.1086/314488. [DOI] [PubMed] [Google Scholar]
- 12.Basmaci R, Bidet P, Yagupsky P, Muñoz-Almagro C, Balashova NV, Doit C, Bonacorsi S. 2014. Major intercontinentally distributed sequence types of Kingella kingae and development of a rapid molecular typing tool. J Clin Microbiol 52:3890–3897. doi: 10.1128/JCM.01609-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amit U, Porat N, Basmaci R, Bidet P, Bonacorsi S, Dagan R, Yagupsky P. 2012. Genotyping of invasive Kingella kingae isolates reveals predominant clones and association with specific clinical syndromes. Clin Infect Dis 55:1074–1079. doi: 10.1093/cid/cis622. [DOI] [PubMed] [Google Scholar]
- 14.Kehl-Fie TE, St Geme JW III. 2007. Identification and characterization of an RTX toxin in the emerging pathogen Kingella kingae. J Bacteriol 189:430–436. doi: 10.1128/JB.01319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang D, Nudell Y, Lau J, Zakharian E, Balashova NV. 2014. RTX toxin plays a key role in Kingella kingae virulence in an infant animal model. Infect Immun 82:2318–2328. doi: 10.1128/IAI.01636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muñoz VL, Porsch EA, St Geme JW III. 2018. Kingella kingae surface polysaccharides promote resistance to human serum and virulence in a juvenile rat model. Infect Immun 86:e00100-. doi: 10.1128/IAI.00100-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starr KF, Porsch EA, Seed PC, Heiss C, Naran R, Forsberg LS, Amit U, Yagupsky P, Azadi P, St Geme JW III. 2016. Kingella kingae expresses four structurally distinct polysaccharide capsules that differ in their correlation with invasive disease. PLoS Pathog 12:e1005944. doi: 10.1371/journal.ppat.1005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porsch EA, Starr KE, Yagupsky P, St Geme JW III. 2017. The type a and type b polysaccharide capsules predominate in an international collection of invasive Kingella kingae isolates. mSphere 2:e00060-. doi: 10.1128/mSphere.00060-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagupsky P, Ben-Ami Y, Trefler R, Porat N. 2016. Outbreaks of invasive Kingella kingae infections in closed communities. J Pediatr 169:135–139. doi: 10.1016/j.jpeds.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Bidet P, Collin E, Basmaci R, Courroux C, Prisse V, Dufour V, Bingen E, Grimprel E, Bonacorsi S. 2013. Investigation of an outbreak of osteoarticular infections caused by Kingella kingae in a childcare center using molecular techniques. Pediatr Infect Dis J 32:558–560. doi: 10.1097/INF.0b013e3182867f5e. [DOI] [PubMed] [Google Scholar]
- 21.El Houmami N, Bzdrenga J, Pons JC, Minodier P, Durand GA, Oubraham A, Ceroni D, Yagupsky P, Raoult D, Bidet P, Fournier PE. 2017. A modified multilocus sequence typing protocol to genotype Kingella kingae from oropharyngeal swabs without bacterial isolation. BMC Microbiol 17:200. doi: 10.1186/s12866-017-1104-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Basmaci R, Ilharreborde B, Bidet P, Doit C, Lorrot M, Mazda K, Bingen E, Bonacorsi S. 2012. Isolation of Kingella kingae in the oropharynx during K. kingae arthritis on children. Clin Microbiol Infect 18:E134–E136. doi: 10.1111/j.1469-0691.2012.03799.x. [DOI] [PubMed] [Google Scholar]
- 23.Ceroni D, Belaieff W, Kanavaki A, Della Llana RA, Lascombes P, Dubois-Ferrière V, Dayer R. 2013. Possible association of Kingella kingae with infantile spondylodiscitis. Pediatr Infect Dis J 32:1296–1298. doi: 10.1097/INF.0b013e3182a6df50. [DOI] [PubMed] [Google Scholar]
- 24.Ceroni D, Dubois-Ferrière V, Cherkaoui A, Gesuele R, Combescure C, Lamah L, Manzano S, Hibbs J, Schrenzel J. 2013. Detection of Kingella kingae osteoarticular infections in children by oropharyngeal swab PCR. Pediatrics 131:e230–e235. doi: 10.1542/peds.2012-0810. [DOI] [PubMed] [Google Scholar]
- 25.Opota O, Laurent S, Pillonel T, Léger M, Traschel S, Prod'hom G, Jaton K, Greub G. 2017. Genomics of the new species Kingella negevensis: diagnostic issues and identification of a locus encoding a RTX toxin. Microbes Infect 19:546–552. doi: 10.1016/j.micinf.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 26.El Houmami N, Bzdrenga J, Durand G, Minodier P, Seligmann H, Prudent E, Bakour S, Bonacorsi S, Raoult D, Yagupsky P, Fournier PE. 2017. Molecular tests that target the RTX locus do not distinguish between Kingella kingae and the recently described Kingella negevensis species. J Clin Microbiol 55:3113–3122. doi: 10.1128/JCM.00736-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satzkea C, Turnerd P, Virolainen-Julkunenf A, Adriang PV, Antonioh M, Harei KM, Henao-Restrepo AM, Leach AJ, Klugman KP, Portera BD, Sá-Leão R, Scott JA, Nohynek H, O'Brien KL, WHO Pneumococcal Carriage Working Group. 2013. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 32:165–179. doi: 10.1016/j.vaccine.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 28.El Houmami N, Fournier PE, Ceroni D. 2017. Targeting the Kingella kingae groEL gene is a reliable method for the molecular diagnosis of K. kingae infection and carriage. J Paediatr Child Health 53:1030–1031. doi: 10.1111/jpc.13672. [DOI] [PubMed] [Google Scholar]
- 29.Levy PY, Fournier PE, Fenollar F, Raoult D. 2013. Systematic PCR detection in culture-negative osteoarticular infections. Am J Med 126:1143.e25–1143.e33. doi: 10.1016/j.amjmed.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 30.El Houmami N, Durand GA, Bzdrenga J, Darmon A, Minodier P, Saligmann H, Raoult D, Fournier PE. 6 June 2018. A new highly sensitive and specific real-time PCR assay targeting the malate dehydrogenase gene of Kingella kingae and application to 201 pediatric clinical specimens. J Clin Microbiol doi: 10.1128/JCM.00505-18. [DOI] [PMC free article] [PubMed] [Google Scholar]