Abstract
Glioma has been regarded as the most common, highly proliferative and invasive brain tumour. Advances in research of miRNAs in glioma are toward further understanding of the pathogenesis of glioma. MiR‐19, a member of miR‐17~92 cluster, was reported to play an oncogenic role in tumourigenesis. Here we review the identified data about the effect of miR‐19 on proliferation, apoptosis, migration and invasion of glioma cells, the target genes regulated by miR‐19, and correlation of miR‐19 with the sensitivity of glioma cells to chemotherapy and radiotherapy. It is concluded that miR‐19 plays an important role in the pathogenesis of glioma and can be a potential target for gene therapy of glioma.
Keywords: chemotherapy, glioma, miR‐19, radiotherapy, target gene
1. INTRODUCTION
MicroRNAs, a class of endogenous non‐coding RNA with 18‐25 nucleotides, repress the expression of corresponding genes by binding to the 3′‐UTR region of target genes at post‐transcriptional level.1, 2 MiRNAs have been identified to participate in a variety of cell biological processes such as proliferation, apoptosis, migration and invasion.3, 4 During the development of cancer, miRNAs are dysregulated and play oncogenic or tumour suppressive role by enhancing or suppressing proliferation, invasion of tumour cells.5, 6 Thus, miRNA deregulation is one of the key mechanisms in glioma pathogenesis. The relevant miRs can be used as new targets of glioma therapy and provide clues for diagnosis.
This review will discuss the role of miR‐19 in glioma cell proliferation, apoptosis and migration and its effect on chemotherapy and radiotherapy of glioma.
MiR‐19 is a member of miR‐17‐92 cluster, this cluster participates not only in the development of heart and lung,7, 8 but also in ageing and cancer.9 The target genes of miR‐17‐92 cluster have been experimentally identified so far including: STAT3, Mapk1410 and Rb2/p130.11 MiR‐17‐92 cluster plays an important role in tumourigenesis of thyroid cancer, leukaemia and lymphoma.12, 13, 14 The expression of miR‐17‐92 cluster is up‐regulated in glioma tissues. MiR‐17‐92 cluster inhibition decreases cell proliferation and induces apoptosis in glioblastoma spheroids culture by up‐regulating the expression of CDKN1A (cyclin‐dependent kinase inhibitor 1A), E2F1 and PTEN.15 MiR‐17‐92 cluster is regarded as the first miRNA cluster with oncogenic potential,15 the cluster includes 6 single mature miRNAs, miR‐19 has been supposed to be the key oncogenic miRNA among the six members of miR‐17‐92 cluster. MiR‐19 is located on chromosome 13 in c13orf25 16 and its expression is up‐regulated in bladder cancer, breast cancer, pancreatic cancer, gastric cancer and laryngeal squamous cell carcinoma.17, 18, 19, 20, 21 MiR‐19 promotes tumourigenesis by regulating target genes and related signalling pathways. In human B‐cell lymphomas, miR‐19 promotes tumour cell survival by inhibiting PTEN directly and activating AKT/mTOR pathway.22 Expression of miR‐19 is elevated in SHH medulloblastoma, a subgroup of medulloblastoma characeterized as constitutive activation of the Sonic Hedgehog pathway, anti‐miR‐19 treatment restrains proliferation of tumour cells and prolongs survival of tumour‐bearing mice23 (Figure 1).
Figure 1.
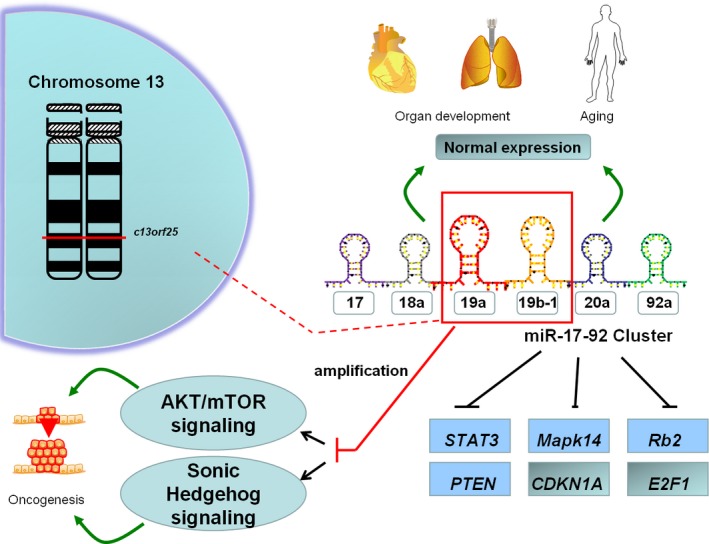
Sketch of miR‐17‐92 cluster and miR‐19
2. MIR‐19 IN GLIOMA
The expression of miR‐19 is up‐regulated in glioma. In 75 archival paraffin‐embedded glioma specimens with different grades of malignancy and five normal control, miR‐19 is significantly up‐regulated in glioma tissues and positively correlates with the tumour grade.24 Increased expression level of miR‐19 is also detected in glioma cell lines.25 MiR‐19 is confirmed to participate in the process of glioma recurrence. A research report has demonstrated that miR‐19 is progression‐associated up‐regulation in patients who has been operated as WHO grade II originally and spontaneously progress to WHO grade IV secondary glioblastoma.26 This indicates that miR‐19 plays an important role in glioma progression. MiR‐19 is also regarded as prognostic biomarker of glioma, high expression of miR‐19 in patient's serum is associated with poor survival.27 MiR‐19 exerts its effect on biological characters of tumour cells through regulation on its target genes. It has been identified to regulate hundred of target genes in TargetScan and Pictar database, among them there are a few target genes have been experimentally confirmed such as PTEN,24 which play a significant role in glioma pathogenesis and progression. The effect of miR‐19 on glioma cell proliferation, apoptosis and migration and the impact of miR‐19 on chemotherapy and radiation therapy of glioma will be discussed separately as follows.
2.1. MiR‐19 and apoptosis
Studies demonstrate that miR‐19 inhibits apoptosis of glioma cells. Anti‐miR‐19 (antisense oligonucleotide of miR‐19) is introduced to knock down miR‐19 expression, apoptosis is induced in glioma cells.28 We have confirmed PTEN as the target gene of miR‐19 experimentally.24 PTEN plays a significant role as a tumour suppressor gene that induces glioma cell apoptosis and as a negative regulator of PI3K/AKT pathway, whereas PI3K/AKT inhibits apoptosis through repressing JNK and p38, or promoting FoxM1 expression in glioma.29, 30 Wang also reported that miR‐19 was suppressed by resveratrol in glioma and induced apoptosis, PTEN up‐regulation and PI3K/AKT repression.31 It has been reported that p53 is up‐regulated when miR‐19 is inhibited in glioma cells.31 p53 is a key proapoptosis gene. p53 induces p53‐dependent apoptosis through enhanced expression of transcription targets including STAG1, PUMA and PERP.32, 33, 34 MiR‐19 expression has been identified up‐regulated in glioma as oncomiR,24 apoptosis inducing proteins such as PTEN (directly suppressed by miR‐19) and p53 (negative regulated by miR‐19) are suppressed, so miR‐19 reduces apoptosis to promote tumour cell survival. It has been reported that miR‐19 also inhibit apoptosis in SH‐SY5Y human neuroblastoma cells, transfection of miR‐19 inhibitor leads to induction of apoptosis and increases expression of apoptosis‐related proteins including PTEN, p53, Bax and caspase‐3, decreases the expression of Bcl‐2.35
2.2. MiR‐19 and cell proliferation
Inhibition of miR‐19 by anti‐miR‐19 results in diminished proliferation of glioma cell in vitro.28 MiR‐19 depresses growth of glioma cells by negative regulation of PTEN, which can inhibit glioma cell proliferation by suppressing PI3k/AKT pathway.36 It also has been reported that miR‐19 promotes glioma progression by directly suppressing PPARα (the peroxisome proliferator‐activated receptor α, PPARα).37 PPAR belongs to nuclear receptor family which includes three subtypes, ie, PPARα, PPARγ and PPARδ. PPARα is well known for its role in regulating lipid and glucose metabolism, cell proliferation, tumourigenesis and inflammation. PPARα can inhibit glioma cell proliferation and induce cell cycle arrest by promoting nuclear translocation of FoxO1, then increases the expression of a FoxO1‐dependent cell cycle‐related protein, p27kip in glioma cells.38 The antitumour role of PPARα also can be mediated by direct and indirect antiangiogenic effect on tumour cells.39 MiR‐19 directly down‐regulates the expression of PPARα by binding to 3′‐UTR region of PPARα mRNA in gliomas. Inhibitory effect of PPARα on glioma cell proliferation is blocked by targeting regulation of miR‐19, progression of tumour is enhanced.37 p53 has also been identified to be the target gene of miR‐19 in Hela cell.40 In glioma, expression of p53 is correlated negatively with that of miR‐19.31 p53 can impose cell cycle arrest and inhibit cell growth by decreasing the expression of Cyclin E1 and CDK2.41, 42 MiR‐19 promotes glioma cell proliferation that might be through inhibition on p53. MiR‐19 also has been reported to promote tumour cell proliferation in other tumours such as pancreatic cancer, castration‐resistant prostate cancer43, 44 and suppress tumour growth in lung cancer45 as well.
2.3. MiR‐19 and cell migration
It has been demonstrated that knocking down miR‐19 suppresses migration of glioma cell,28 whereas overexpression of miR‐19 promotes glioma cell migration and invasion. Long nocoding RNA: MEG3 suppresses glioma cell migration by playing a role as competing endogenous RNA (ceRNA) of miR‐19.46 As to the target genes of miR‐19 relevant to glioma cell invasion, it has been reported that RUNX3 suppresses glioma cell invasion by depressing the transcription activity of β‐catenin/TCF4 and expression of downstream factors of β‐catenin/TCF4 such as c‐MYC and AKT1,47 so miR‐19 possibly promotes glioma cell migration by direct negative regulation on RUNX3. RhoB, another target gene of miR‐19, is a member of Rho GTPase family. It participates in diverse cellular process including actin organization, differentiation to block cell migration activity. Introduction of exogenous RhoB suppresses glioma cell mobility and invasiveness by reducing activation of PKC iota and PKB/AKT.48 MiR‐19 is reported to promote glioma cell invasion and migration by directly suppressing RhoB by miR‐19.25 In addition, leucine‐rich repeats and immunoglobulin‐like domains 1 (LRIG1): as one of identified target genes of miR‐19, are confirmed to participate in the regulation on glioma invasion.49 Overexpression of LRIG1 suppresses U251 malignant glioma cell migration and invasion by reducing MMP2/9 level.50 Besides, LRIG1 inhibits phosphorylation of MAPK, EGFR and AKT signalling molecules and affects biological behaviours including migration and invasion by inactivation of EGFR/AKT signalling pathway.51, 52 Thus, the other alternative pathway that MiR‐19 promoting glioma migration might by directly inhibiting LRIG1 expression and indirect activation of EGFR/AKT signalling pathway. Since miR‐19 might negatively regulate EGFR via LRIG1, the impact of EGFR on invasion and angiogenesis of glioma cell may be connected with miR‐19. However, the connection between miR‐19 and EGFR should be studied further. In summary, miR‐19 promotes glioma cell migration mainly through the negative regulation on RUNX3, RhoB and LRIG1. Mir‐19 also exerts different effects on cell migration and invasion in diverse tumour cells. It has been demonstrated that miR‐19 enhances invasiveness of colorectal tumour cells by regulating target gene TG2 (Transglutaminase‐2)53 and promotes cell metastasis in hepatocellular carcinoma.54 However, miR‐19 exerts inhibitory effect on the ability of breast cancer cell migration and invasion,55 and it also inhibits migration and invasion of colon cancer cell by suppressing MMP9 and tissue factor (TF).56
3. MIR‐19 AND TREATMENT OF GLIOMA
3.1. MiR‐19 may be a novel target for gene therapy of glioma
Gliomas are the most challenging malignant brain tumours. Current standard treatment includes surgical resection, followed by radiotherapy and chemotherapy, co‐administration of temozolomide (TMZ). However, the prognosis of the patients with high‐grade glioma is still poor and the medium survival of GBM patients is only 15 months. Hence, researches on new therapeutic option are becoming hotspots in glioma study. Molecular target therapy against specific genes is an expectation for glioma treatment. Molecular target drugs like EGFR inhibitors and VEGFR antibodies have been applied in clinical practice. More and more studies on searching new target genes, including microRNAs, for molecular target therapy are carried out.
Overexpression of miR‐19 has been demonstrated to promote proliferation and invasion of glioma cells,25 knocking down miR‐19 by RNAi blocks tumour growth, induces cell apoptosis,28 all these results indicating that miR‐19 plays an important role in glioma progression. It suggests that miR‐19 can be identified as a potential target gene for glioma therapy. Since multiple proteins including RUNX3,47 RhoB,25 LRIG149 and PTEN24 have been proved to be the target genes of miR‐19, when miR‐19 is knocked down by miR‐19 inhibitor, the expression of its target genes will be regulated accordingly, so it is predictable that multiple genes therapeutic effect can be achieved if novel anti‐oncomiR‐19 measures are adopted.
3.2. MiR‐19 and radiotherapy and chemotherapy sensitivity of glioma
In order to improve the prognosis of patients, malignant glioma patients are given radiotherapy and chemotherapy simultaneously after surgical resection. However, resistance to radiotherapy and chemotherapy turn out to be the important source of glioma recurrence.
MiR‐19 has been reported to exert effects on drug resistance in diverse tumour chemotherapy. In breast cancer, miR‐19 expression is up‐regulated in MDR (multidrug resistance) cell lines compared with corresponding parent cell line, knocking down miR‐19 restores sensitivity of MDR cells to chemotherapeutic agents by up‐regulating the expression of PTEN and decreasing the expression of MDR‐related transporters: BCRP (breast cancer resistance protein), MDR (multidrug resistance protein), MDR‐1 (P‐glycoprotein) and MRP‐1 (multidrug resistance‐associated protein 1).57 In gastric cancer, miR‐19 decreases the sensitivity of tumour cell to chemotherapy by targeting PTEN and promoting expression of AKT, pAKT and MDR‐1.58 Our literature reviews indicate that there is no report about the relationship of miR‐19 and sensitivity of glioma cells to chemotherapy up to now. While some studies provide clue for us to predict the effect of miR‐19 which might exert on reaction of glioma cell to chemotherapeutic agents. In multidrug resistance glioablastoma cell lines, MDR protein 1 (multidrug resistance protein 1) serves as drug resistance to decrease chemotherapy sensitivity of cells, MDR protein 1 up‐regulation is PI3K/AKT pathway activation dependent.59 PTEN—the negative regulator of PI3K/AKT pathway: also improves the sensitivity of tumour cell to drug in glioma cells.60 MiR‐19 might trigger the activation of PI3K/AKT pathway and up‐regulate the expression of drug resistance gene‐MDR protein 1 by directly inhibiting PTEN, so mir‐19 probably participates in promoting drug resistance of gloma cells by targeting PTEN.
Leung has shown that miR‐19 is significantly up‐regulated in breast cancer cell MDA‐MB‐361 cells after exposed to radiation, indicating that miR‐19 appears to be radiation‐associated miRNA in breast cancer.61 Knocking down miR‐19 in SiHa cervical cancer cells, the radiotherapy sensitivity of SiHa cells is increased and cell proliferation is inhibited.62 Above studies demonstrate that miR‐19 is associated with radiosensitivity of tumour cells. However, research on the relationship between miR‐19 and radiotherapy in glioma is limited. Chaudhry has reported that miR‐19 is up‐regulated in both irradiated glioma cell line M059J which is deficient in DNA‐PK (DNA‐dependent protein kinase) and glioma cell line M059K with normal DNA‐PK activity, indicating that miR‐19 paritcipates in the reaction of different types of glioma cell lines to ionizing radiation.63 Some of the target genes of miR‐19 are related to the radiosensitivity of glioma cells. As previously reported, PTEN overexpression leads to an increase in sensitivity to ionizing radiation in glioma cells,60 LRIG1 enhances the sensitivity of radiotherapy in glioma cells by suppressing EGFR/AKT pathway.64 However, RhoB induces radioresistance in glioma cells.65 It has been identified that CtIP is the target gene of miR‐19 and CtIP plays a role in the DNA end resection and homologous recombination in response to DNA damage.66 Then we can infer that miR‐19 aberrant expression down‐regulating CtIP suppresses the repair of the most hazardous type of lesion, DNA double‐strand breaks induced by ionizing radiation, and may be helpful to enhance the effect of radiotherapy. Certainly, this assumption will be contradictory to the anti‐miR‐19 therapy for its oncogenic potential in glioma and the relationship between miR‐19 and radiation response of glioma needs to be investigated further.
4. CONCLUSION AND PERSPECTIVE
To date, studies on miR‐19 have been demonstrated to reduce apoptosis, promote proliferation and migration of glioma cells. Besides, it is associated with sensitivity of chemotherapy and radiotherapy of glioma cells. Intensive studies of the mechanism by which miR‐19 regulates proliferation and invasion of glioma cells will be helpful for providing a noval therapeutic target for glioma treatment. Standard care of glioma patients with combining surgery, chemotherapy and radiation therapy does not improve the survival rate of patients. Targeting oncomiRs seems to be the new trends in the development of miRNA therapeutic strategies. Thus, miR‐19 can be a new target in glioma treatment, or might regulate the effect of chemotherapy and radiotherapy by adjusting the sensitivity of glioma cells, which will provide a new clue for glioma therapy (Figure 2). Further study on miR‐19 will demonstrate its more roles in the pathogenesis of glioma, biomarker of prognosis and glioma treatment.
Figure 2.
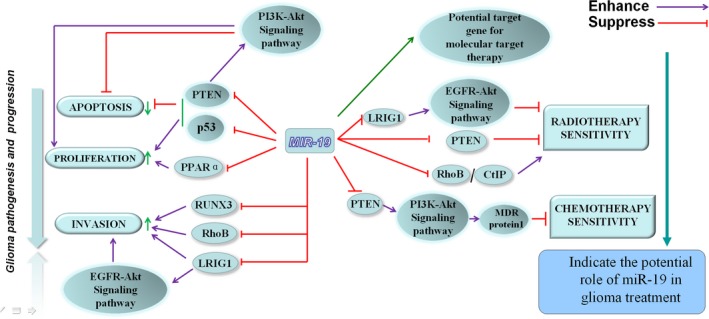
The role of miR‐19 in proliferation, migration, apoptosis and treatment of glioma
CONFLICT OF INTEREST
The authors declare that we have no conflict of interest.
ACKNOWLEDGEMENT
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81101915).
Wang W, Zhang A, Hao Y, Wang G, Jia Z. The emerging role of miR‐19 in glioma. J Cell Mol Med. 2018;22:4611–4616. 10.1111/jcmm.13788
W. Wang and A. Zhang contribute equally to this work.
REFERENCES
- 1. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281‐297. [DOI] [PubMed] [Google Scholar]
- 2. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post‐transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9(2):102‐114. [DOI] [PubMed] [Google Scholar]
- 3. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15‐20. [DOI] [PubMed] [Google Scholar]
- 4. Hayder H, O'Brien J, Nadeem U, Peng C. MicroRNAs: crucial regulators of placental development. Reproduction. 2018;155(6):R259‐R271. [DOI] [PubMed] [Google Scholar]
- 5. Frixa T, Donzelli S, Blandino G. Oncogenic MicroRNAs: key players in malignant transformation. Cancers (Basel). 2015;7(4):2466‐2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285(2):116‐126. [DOI] [PubMed] [Google Scholar]
- 7. Danielson LS, Park DS, Rotllan N, et al. Cardiovascular dysregulation of miR ‐17 ‐92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. FASEB J. 2013;27(4):1460‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khuu C, Utheim TP, Sehic A. The three paralogous microRNA clusters in development and disease, miR‐17‐92, miR‐106a‐363, and miR‐106b‐25. Scientifica (Cairo). 2016;2016:1379643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grillari J, Hackl M, Grillari‐Voglauer R. miR‐17‐92 cluster: ups and downs in cancer and aging. Biogerontology. 2010;11(4):501‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carraro G, El‐Hashash A, Guidolin D, et al. miR‐17 family of microRNAs controls FGF10‐mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E‐Cadherin distribution. Dev Biol. 2009;333(2):238‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Q, Li YC, Wang J, et al. miR‐17‐92 cluster accelerates adipocyte differentiation by negatively regulating tumor‐suppressor Rb2/p130. Proc Natl Acad Sci USA. 2008;105(8):2889‐2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takakura S, Mitsutake N, Nakashima M, et al. Oncogenic role of miR‐17‐92 cluster in anaplastic thyroid cancer cells. Cancer Sci. 2008;99(6):1147‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brockway S, Zeleznik‐Le NJ. WEE1 is a validated target of the microRNA miR‐17‐92 cluster in leukemia. Cancer Genet. 2015;208(5):279‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robaina MC, Faccion RS, Mazzoccoli L, et al. miR‐17‐92 cluster components analysis in Burkitt lymphoma: overexpression of miR‐17 is associated with poor prognosis. Ann Hematol. 2016;95(6):881‐891. [DOI] [PubMed] [Google Scholar]
- 15. Ernst A, Campos B, Meier J, et al. De‐repression of CTGF via the miR‐17‐92 cluster upon differentiation of human glioblastoma spheroid cultures. Oncogene. 2010;29(23):3411‐3422. [DOI] [PubMed] [Google Scholar]
- 16. He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feng Y, Liu J, Kang Y, et al. MiR‐19a acts as an oncogenic microRNA and is up‐regulated in bladder cancer. J Exp Clin Cancer Res. 2014;33:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Q, Liu M, Ma F, et al. Circulating miR‐19a and miR‐205 in serum may predict the sensitivity of luminal A subtype of breast cancer patients to neoadjuvant chemotherapy with epirubicin plus paclitaxel. PLoS ONE. 2014;9(8):e104870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan Y, Yin H, Zhang H, et al. Sp1‐driven up‐regulation of miR‐19a decreases RHOB and promotes pancreatic cancer. Oncotarget. 2015;6(19):17391‐17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Q, Yang Z, An Y, et al. MiR‐19a/b modulate the metastasis of gastric cancer cells by targeting the tumour suppressor MXD1. Cell Death Dis. 2014;5:e1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu TY, Zhang TH, Qu LM, et al. MiR‐19a is correlated with prognosis and apoptosis of laryngeal squamous cell carcinoma by regulating TIMP‐2 expression. Int J Clin Exp Pathol. 2013;7(1):56‐63. [PMC free article] [PubMed] [Google Scholar]
- 22. Olive V, Bennett MJ, Walker JC, et al. miR‐19 is a key oncogenic component of mir‐17‐92. Genes Dev. 2009;23(24):2839‐2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murphy BL, Obad S, Bihannic L, et al. Silencing of the miR‐17~92 cluster family inhibits medulloblastoma progression. Cancer Res. 2013;73(23):7068‐7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jia Z, Wang K, Zhang A, et al. MiR‐19a and miR‐19b overexpression in gliomas. Pathol Oncol Res. 2013;19(4):847‐853. [DOI] [PubMed] [Google Scholar]
- 25. Chen Q, Guo W, Zhang Y, Wu Y, Xiang J. MiR‐19a promotes cell proliferation and invasion by targeting RhoB in human glioma cells. Neurosci Lett. 2016;628:161‐166. [DOI] [PubMed] [Google Scholar]
- 26. Malzkorn B, Wolter M, Liesenberg F, et al. Identification and functional characterization of microRNAs involved in the malignant progression of gliomas. Brain Pathol. 2010;20(3):539‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhi F, Shao N, Wang R, et al. Identification of 9 serum microRNAs as potential noninvasive biomarkers of human astrocytoma. Neuro Oncol. 2015;17(3):383‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang K, Jia Z, Zhang A, Wang G, Hao J, Pu P. Inhibitory effects of knocking down microRNA‐19a and microRNA‐19b on glioma cell growth in vitro. Chin J Neuromed. 2011;4(10):365‐368. [Google Scholar]
- 29. Ou YW, Zhao ZT, Wu CY, Xu BN, Song YM, Zhan QM. Mig‐2 attenuates cisplatin‐induced apoptosis of human glioma cells in vitro through AKT/JNK and AKT/p38 signaling pathways. Acta Pharmacol Sin. 2014;35(9):1199‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang M, Liu Y, Gao Y, Li S. Silibinin‐induced glioma cell apoptosis by PI3K‐mediated but Akt‐independent downregulation of FoxM1 expression. Eur J Pharmacol. 2015;765:346‐354. [DOI] [PubMed] [Google Scholar]
- 31. Wang G, Dai F, Yu K, et al. Resveratrol inhibits glioma cell growth via targeting oncogenic microRNAs and multiple signaling pathways. Int J Oncol. 2015;46(4):1739‐1747. [DOI] [PubMed] [Google Scholar]
- 32. Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683‐694. [DOI] [PubMed] [Google Scholar]
- 33. Anazawa Y, Arakawa H, Nakagawa H, Nakamura Y. Identification of STAG1 as a key mediator of a p53‐dependent apoptotic pathway. Oncogene. 2004;23:7621‐7627. [DOI] [PubMed] [Google Scholar]
- 34. Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW. PERP, an apoptosis‐associated target of p53, is a novel member of the PMP‐22/gas3 family. Genes Dev. 2000;14:704‐718. [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu M, Li B, Ma X, et al. Folic acid protected neural cells against aluminum‐maltolate‐induced apoptosis by preventing miR‐19 downregulation. Neurochem Res. 2016;41(8):2110‐2118. [DOI] [PubMed] [Google Scholar]
- 36. Liu C, Wu H, Li Y, et al. SALL4 suppresses PTEN expression to promote glioma cell proliferation via PI3K/AKT signaling pathway. J Neurooncol. 2017;135(2):263‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi Y, Tao T, Liu N, et al. PPARα, a predictor of patient survival in glioma, inhibits cell growth through the E2F1/miR‐19a feedback loop. Oncotarget. 2016;7(51):84623‐84633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han DF, Zhang JX, Wei WJ, et al. Fenofibrate induces G0/G1 phase arrest by modulating the PPARα/FoxO1/p27 kip pathway in human glioblastoma cells. Tumour Biol. 2015;36(5):3823‐3829. [DOI] [PubMed] [Google Scholar]
- 39. Panigrahy D, Kaipainen A, Huang S, et al. PPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci USA. 2008;105(3):985‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fan Y, Yin S, Hao Y, et al. miR‐19b promotes tumor growth and metastasis via targeting TP53. RNA. 2014;20(6):765‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou X, Wu W, Zeng A, et al. MicroRNA‐141‐3p promotes glioma cell growth and temozolomide resistance by directly targeting p53. Oncotarget. 2017;8(41):71080‐71094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9(10):749‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lu K, Liu C, Tao T, et al. MicroRNA‐19a regulates proliferation and apoptosis of castration‐resistant prostate cancer cells by targeting BTG1. FEBS Lett. 2015;589(13):1485‐1490. [DOI] [PubMed] [Google Scholar]
- 44. Wang X, Wang L, Mo Q, Jia A, Dong Y, Wang G. A positive feedback loop of p53/miR‐19/TP53INP1 modulates pancreatic cancer cell proliferation and apoptosis. Oncol Rep. 2016;35(1):518‐523. [DOI] [PubMed] [Google Scholar]
- 45. Li J, Yang S, Yan W, et al. MicroRNA‐19 triggers epithelial‐mesenchymal transitionof lung cancer cells accompanied by growth inhibition. Lab Invest. 2015;95(9):1056‐1070. [DOI] [PubMed] [Google Scholar]
- 46. Qin N, Tong GF, Sun LW, Xu XL. Long noncoding RNA MEG3 suppresses glioma cell proliferation, migration, and invasion by acting as a competing endogenous RNA of miR‐19a. Oncol Res. 2017;25(9):1471‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sun J, Jia Z, Li B, et al. MiR‐19 regulates the proliferation and invasion of glioma by RUNX3 via β‐catenin/Tcf‐4 signaling. Oncotarget. 2017;8(67):110785‐110796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baldwin RM, Parolin DA, Lorimer IA. Regulation of glioblastoma cell invasion by PKC iota and RhoB. Oncogene. 2008;27(25):3587‐3595. [DOI] [PubMed] [Google Scholar]
- 49. Wu S, Chen Q, Wu T, Li Y, Li J, Liu J. Effect of microRNA‐19a down‐regulation on invasiveness of glioma cells and the potential mechanism. Chin J Exp Surg. 2015;32(2):336‐339. [Google Scholar]
- 50. Mao F, Wang B, Xiao Q, et al. A role for LRIG1 in the regulation of malignant glioma aggressiveness. Int J Oncol. 2013;42(3):1081‐1087. [DOI] [PubMed] [Google Scholar]
- 51. Ye F, Gao Q, Xu T, et al. Upregulation of LRIG1 suppresses malignant glioma cell growth by attenuating EGFR activity. J Neurooncol. 2009;94:183‐194. [DOI] [PubMed] [Google Scholar]
- 52. Xie R, Yang H, Xiao Q, et al. Downregulation of LRIG1 expression by RNA interference promotes the aggressive properties of glioma cells via EGFR/Akt/c‐Myc activation. Oncol Rep. 2013;29(1):177‐184. [DOI] [PubMed] [Google Scholar]
- 53. Cellura D, Pickard K, Quaratino S, et al. miR‐19‐mediated inhibition of transglutaminase‐2 leads to enhanced invasion and metastasis in colorectal cancer. Mol Cancer Res. 2015;13(7):1095‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yin Z, Huang J, Ma T, et al. Macrophages activating chemokine (C‐X‐C motif) ligand 8/miR‐17 cluster modulate hepatocellular carcinoma cell growth and metastasis. Am J Transl Res. 2017;9(5):2403‐2411. [PMC free article] [PubMed] [Google Scholar]
- 55. Shi X, Tang X, Su L. Over‐expression of long non‐coding RNA PTENP1 inhibits cell proliferation and migration via suppression of miR‐19b in breast cancer cells. Oncol Res. 2017. 10.3727/096504017X15123838050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yu G, Li H, Wang X, et al. MicroRNA‐19a targets tissue factor to inhibit colon cancer cells migration and invasion. Mol Cell Biochem. 2013;380(1–2):239‐247. [DOI] [PubMed] [Google Scholar]
- 57. Liang Z, Li Y, Huang K, Wagar N, Shim H. Regulation of miR‐19 to breast cancer chemoresistance through targeting PTEN. Pharm Res. 2011;28:3091‐3100. [DOI] [PubMed] [Google Scholar]
- 58. Wang F, Li T, Zhang B, et al. MicroRNA‐19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem Biophys Res Commun. 2013;434(3):688‐694. [DOI] [PubMed] [Google Scholar]
- 59. Xi G, Hayes E, Lewis R, et al. CD133 and DNA‐PK regulate MDR1 via the PI3K‐ or Akt‐NF‐κB pathway in multidrug‐resistant glioblastoma cells in vitro. Oncogene. 2016;35(2):241‐250. [DOI] [PubMed] [Google Scholar]
- 60. Inaba N, Kimura M, Fujioka K, et al. The effect of PTEN on proliferation and drug‐, and radiosensitivity in malignant glioma cells. Anticancer Res. 2011;31(5):1653‐1658. [PubMed] [Google Scholar]
- 61. Leung CM, Chen TW, Li SC, et al. MicroRNA expression profiles in human breast cancer cells after multifraction and single‐dose radiation treatment. Oncol Rep. 2014;31(5):2147‐2156. [DOI] [PubMed] [Google Scholar]
- 62. Wang Y, Wang Y, Zhong W, Gulina K. Correlation between miR‐19a inhibition and radiosensitivity in SiHa cervical cancer cells. J BUON. 2017;22(6):1505‐1508. [PubMed] [Google Scholar]
- 63. Chaudhry MA, Sachdeva H, Omaruddin RA. Radiation‐induced micro‐RNA modulation in glioblastoma cells differing in DNA‐repair pathways. DNA Cell Biol. 2010;29(9):553‐561. [DOI] [PubMed] [Google Scholar]
- 64. Yang JA, Liu BH, Shao LM, et al. LRIG1 enhances the radiosensitivity of radioresistant human glioblastoma U251 cells via attenuation of the EGFR/Akt signaling pathway. Int J Clin Exp Pathol. 2015;8(4):3580‐3590. [PMC free article] [PubMed] [Google Scholar]
- 65. Delmas C, Heliez C, Cohen‐Jonathan E, et al. Farnesyltransferase inhibitor, R115777, reverses the resistance of human glioma cell lines to ionizing radiation. Int J Cancer. 2002;100(1):43‐48. [DOI] [PubMed] [Google Scholar]
- 66. Hühn D, Kousholt AN, Sørensen CS, Sartori AA. miR‐19, a component of the oncogenic miR‐17~92 cluster, targets the DNA‐end resection factor CtIP. Oncogene. 2015;34(30):3977‐3984. [DOI] [PubMed] [Google Scholar]


