Abstract
Oxidative stress can cause injury in retinal endothelial cells. Carboxymethyl cellulose modified with collagen peptide (CMCC) is of a distinct antioxidant capacity and potentially a good drug carrier. In this study, the protective effects of CMCC against H2O2‐induced injury of primary retinal endothelial cells were investigated. In vitro, we demonstrated that CMCC significantly promoted viability of H2O2‐treated cells, efficiently restrained cellular reactive oxygen species (ROS) production and cell apoptosis. Then, the CMCC was employed as both drug and anti‐inflammatory drug carrier for treatment of retinal ischaemia/reperfusion (I/R) in rats. Animals were treated with CMCC or interleukin‐10‐loaded CMCC (IL‐10@CMCC), respectively. In comparisons, the IL‐10@CMCC treatment exhibited superior therapeutic effects, including better restoration of retinal structural thickness and less retinal apoptosis. Also, chemiluminescence demonstrated that transplantation of IL‐10@CMCC markedly reduced the retinal oxidative stress level compared with CMCC alone and potently recovered the activities of typical antioxidant enzymes, SOD and CAT. Therefore, it could be concluded that CMCC provides a promising platform to enhance the drug‐based therapy for I/R‐related retinal injury.
Keywords: antioxidant carrier, drug delivery, IL‐10, retinal ischaemia/reperfusion injury
1. INTRODUCTION
Retinal ischaemia/reperfusion (I/R) injury was one of important factors responsible for the pathogenesis of multiple ocular diseases, including diabetic retinopathy, acute glaucoma and retinopathy of prematurity.1, 2 Moreover, retinal I/R injury developed vision loss and even blindness, owing to eternal damage to the retina, particularly retinal neurons.3, 4, 5 Ischaemia blocked blood flow to retina, causing the transient deficiency of oxygen and other physiological nutrients, such as adenosine triphosphate.6 The following reperfusion further aggravated the tissue damage through producing reactive oxygen species (ROS) and pro‐inflammatory mediators, which rendered oxidative stress and inflammation.7, 8 Therefore, the development of antioxidative and anti‐inflammatory treatments was gradually accepted as the main therapeutic strategies for retinal I/R injury.9
Carboxymethyl cellulose consisted of water‐soluble anionic polysaccharide and semisynthetic derivative of cellulose.10 It possessed a broad range of practical applications, such as pharmaceuticals,11 drug delivery12 and wound dressing,13 due to their high water content, biodegradability and biocompatibility. Interestingly, carboxymethyl cellulose, as functional medical materials, has been found to be able to reduce the high level of ROS.14, 15 However, this antioxidative capability of carboxymethyl cellulose against ROS was rather limited.16 An improving approach was modifying the cellulose with a stronger antioxidative group. Collagen peptide was known for its abundant physiological functions, such as chemotaxis, platelet aggregation, suppressing osteoclast differentiation and, in particular, protecting against oxidative free radical.17, 18, 19 Thus, the functionalization of carboxymethyl cellulose with collagen was expected to effectively improve its antioxidative capacity.
IL‐10, a potent anti‐inflammatory cytokine, is known for its potent capacity that hampered inflammation and modulated pathogenesis of inflammatory syndromes.20 The IL‐10 has been found to play a critical protective role in multiple inflammatory diseases, such as inflammatory bowel disease, experimental allergic encephalomyelitis and atherosclerosis.21, 22, 23
In this study, we prepared carboxymethyl cellulose grafted collagen peptides and investigated its antioxidative property in vitro against H2O2‐simulated oxidative stress environment. Then, IL‐10‐loaded antioxidative gels were delivered into the eyes of rats after retinal I/R injury for practical attempts. In addition, we preliminarily studied the underlying mechanism of enhanced restoration of retina from apoptosis with IL‐10‐loaded antioxidative gels by investigating the level of antioxidant enzyme and the mRNA expression of inflammatory cytokines.
2. MATERIALS AND METHODS
2.1. Materials
The commercial collagen peptide (M w = 800) was purchased from Huashun Biological Technology Co. Ltd., China. The other chemicals at least analytical purity, including N‐Hydroxy sulfosuccinimide (NHS), 2‐(N‐morpholino) ethanesulfonic acid (MES) and 1‐ethyl‐(dimethylaminopropyl) carbodiimide (EDC), were purchased from Sinopharm Chemical Reagent Co., Ltd., China. Interleukin‐10 (IL‐10) and sodium carboxymethyl cellulose (M w = 7.8 × 103) were purchased from Sigma‐Aldrich. The culture medium was renewed every 48 hours when the cells were grown up to 80%‐90% confluence.
2.2. Preparation of carboxymethyl cellulose grafted collagen peptide
The synthetic approach was based on the previous method.24 Typically, first, carboxymethyl cellulose was first dissolved with magnetic stirring, followed by NHS‐EDC coupling reaction (EDC:CMC, 1:2). Then, after reacting for 1 hour, collagen peptide with the mass ratio of 5:3 to CMC was added into the mixture and the grafting reaction was undertaken in MES buffer (pH 6) for 20 hours at 55°C with a constant vigorous stirring. The final products, carboxymethyl cellulose grafted collagen peptide (CMCC), were lyophilized and stored before dialysis for 3 days.
2.3. Isolation and culture, viability and identification of retina endothelial cells
Three‐week‐old male Sprague‐Dawley (SD) rats were applied to the isolation of primary retina endothelial cells (rRECs), according to previous protocol.25 Typically, rats eyes were first enucleated and hemisected. The retinas were separated with a dissecting microscope, immersed in HBSS in the presence of penicillin/streptomycin (Sigma) and rinsed. A total of twenty‐four retinas were undertaken digestion with 0.5% collagenase type I (Worthington, NJ) in serum‐free DMEM for 45 minutes at 37°C. Then, endothelial cell‐specified medium containing 1% ECM (5% foetal bovine serum, 100 mg/mL streptomycin and 100 U/mL penicillin ScienCell Research Laboratories, CA) was supplemented. The obtained cells undertook filtering through 70‐ and 40‐μm nylon mesh (Becton, USA), successively, and were redispersed in ECM followed by incubating with goat antimouse magnetic beads pre‐coated via mouse anti‐rat PECAM‐1. After thoroughly washing with HBSS for 6 times and resuspending in ECM, the beads contained solution was then seeded on 6‐well plates pre‐treated with human fibronectin (Thermo Fisher Scientific, USA).
The viability of the resultant cells underwent propidium iodide (PI) evaluation for viability by flow cytometry, according to previously reported method.26 Briefly, after incubation with PI (100 ng/mL) in phosphate‐buffered saline (PBS) for 1 minute, cells were centrifuged to remove free PI and then resuspended in PBS containing 2% FBS for analysis using a BD LSR II flow cytometer (BD Biosciences, USA). Viable cells (PI cells) were calculated by flow cytometry at a constant flow rate. rRECs were confirmed by a positive immunofluorescence staining for endothelial cells markers: factor VIII‐related antigen. Briefly, cells, grown in cell slides of the 24‐well plates, were fixed with 4% paraformaldehyde for 10 minutes. After rinsing with PBS, they were blocked with 5% normal goat serum in PBS for 30 minutes and incubated with rabbit anti‐factor VIII‐related antigen polyclonal antibody (Santa Cruz, CA, USA) at 4°C overnight. Cells then were incubated with rhodamine‐conjugated anti‐rabbit secondary antibody at 37°C for 30 minutes. Afterwards, cells were washed with PBS 3 times and were staining with 4'6‐diamidino‐2‐phenylindole (DAPI, Sigma) for 5 minutes. The resulting samples were examined using a Olympus FV1000 confocal laser scanning microscope.
2.4. Cell viability assessment of rRECs
Cell viability was evaluated via methylthiazolyldiphenyl‐tetrazolium bromide (MTT) method (Sigma, USA) assay. The rRECs were cultivated in the medium containing different concentrations of H2O2 or μg/mL CMC. After 12‐hours incubation, the treated cells were rinsed and supplemented with 100 μL of DMEM containing MTT (20 μL in PBS, 5 mg/mL) for 4 hours. Subsequently, after removal of supernatant, 150 μL of DMSO was added and the absorbance of the solution in each well was recorded using a multimode microplate reader. Eight replicates were prepared for each treatment group.
2.5. Determination of intracellular reactive oxygen species
The intracellular ROSs were analysed using dihydroethidium (DHE) according to the previous protocol. Typically, rRECs were added with 1 mM DHE, incubating at 37°C for 25 minutes. The suspension was gently washed with PBS buffers for 3 times. The stained cells were then harvested and underwent FACScan flow cytometer (BD Biosciences) analysis.
2.6. Terminal deoxynucleotidyl transferase UTP nick end labelling (TUNEL) assay
TUNEL assay kits were purchased from Beyotime Biotechnology, China, and were performed as described in the manufacturer's protocols. Typically, the air‐dried rRECs were fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X‐100 containing 0.1% sodium citrate. Then, these cells were darkly incubated with TUNEL reagent. After washing, the cells were casted onto glass slides and immersed with a DAPI‐containing antifade mounting medium. TUNEL staining (green) images were attained through the Flouview‐FV300 Laser Scanning Confocal system (Olympus, Japan).
2.7. Western blotting assay
The proteins of lysed rRECs and retina were analysed using BCATM Protein Assay Kit (Pierce, USA), according to the manufacturer's protocol. Briefly, protein sample was first separated with SDS‐PAGE approach. The discrete protein strips were then electrophoretically transferred to nitrocellulose membranes, followed by incubating with primary antibodies against cleaved caspase 3 (Csp3), Bax, Bcl‐2, TNF‐α, IL‐1β, iNOS, ICAM‐1 and MCP‐1 overnight, respectively, and then the corresponding secondary antibodies marked with HRP for 1 hour. β‐Action and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) served as internal reference for these cytokines. The corresponding antibodies were purchased from Cell Signal Technology, USA.
2.8. IL‐10 loading and releasing assay in vitro
The IL‐10 loading CMCC (IL‐10@CMCC) was obtained by soaking CMCC in saturated IL‐10 solution overnight at 4°C. The releasing assay of IL‐10@CMCC was performed using ELISA to detect the released IL‐10. Briefly, aliquots of the IL‐10@CMCC were separately dispersed and standing in 5 mL 1 × PBS (pH7.4). Subsequently, the supernatants were collected and analysed at different days. Each sample was repeated 3 times.
2.9. Retinal ischaemia/reperfusion injury
Sprague‐Dawley rats with similar weights were anesthetized using sodium pentobarbital and then received surgery, performed as described previously.27 Briefly, the anterior chambers of rat eyes were cannulated utilizing a 27‐gauge infusion needle connected with a normal saline reservoir. The intraocular pressure was then tuned up to 110 mm Hg for 1 hours. The retinal ischaemia/reperfusion was verified through examining the fundus whitening and the retinal blood flow restoration.
2.10. Injection of IL‐10@CMCC into vitreous
The injection procedures followed a previous protocol with modification.28 Briefly, after anaesthesia with ketamine (50 mg/kg) and phenergan (25 mg/kg), both pupils of rat eyes were dilated using 1% atropine and 1% compound tropicamide eye drops (Zhongshan Ophthalmic Center, China). 1% Tetracaine eye drops (Zhejiang Jiuxu Pharmaceutical Co., Ltd., China) was used for topical anaesthesia followed by the puncture of anterior chamber. Aqueous humour was aspirated to avoid the increase in intraocular tension, while eyeballs were immobilized with toothed forceps. Under surgical microscope, No. 4.5 needle (outlet: 0.46 mm, inlet: 0.25 mm) was inserted from pars plana and directed towards the midvitreous cavity. Subsequently, CMCC or IL‐10@CMCC in PBS was injected. The conjunctival sac was then casted with chlortetracycline eye ointment. After injection, rats underwent antibiotic eye drop for a week.
2.11. Measurement of retinal thickness
The retinal thickness of rats was measured as previously described.29 In brief, the rats were first anaesthetized at day 21, followed by the enucleation of their eyes. The eyecups then successively underwent the 2‐hours fixation in 4% paraformaldehyde, the 12‐hours immersion in 30% sucrose solution, and the final embedment in OCT media (Sakura Finetek, USA), after removing cornea, lens and vitreous. 0.5 mm of retinal sections were cut off along the vertical meridian of eye and across the optic nerve head. The cut‐off sections were stained with haematoxylin and eosin (H&E) and imaged via the light microscope. The retinal thickness measurements were taken at every 250 μm in a range of 800‐1200 μm centralized at the optic nerve head.
2.12. Immunofluorescence staining of Csp3
Typically, the cut‐off retinal sections received fixing with 4% paraformaldehyde for 10 minutes, permeabilizing with 0.1% Triton X‐100 for 15 minutes and blocking with 5% normal goat serum for 60 minutes. rRECs were then incubated with Csp3 antibody (1/200 diluted) overnight and secondary antibody modified with Alexa Fluor 568 for 1 hour (1/500 diluted, Molecular Probes, USA). DAPI staining was used as reference. Images were also attained via the Flouview‐FV300 Laser Scanning Confocal system.
2.13. Inflammation level assessment via quantitative polymerase chain reaction (PCR)
The extraction of total RNA from the retina was performed using TRIzol reagent (Invitrogen‐Life, USA). Then, 1 μg of total RNA was annealed with 300 ng of oligo (dT) (Promega, USA) for 5 minutes at 65°C, followed by reversely transcribing to cDNA utilizing 80U Moloney murine leukaemia virus reverse transcriptase (Gibco‐life, USA) at 37°C for 1 hour. The obtained cDNA was applied to real‐time qPCR using iTaq Universal SYBR Green Supermix (Bio‐Rad) using a CFX96 Touch Real‐Time PCR Detection System (Bio‐Rad). The following primers were used for amplification reaction: TNF‐α: forward primer 5’‐GCA TGA TCC GAG ATG TGG AA‐3’, reverse primer 5’‐ACG AGC GGG AAT GAG AAG AG‐3’; IL‐1β: forward primer 5’‐GGA GAA GCT GTG GAC GCT A‐3’, reverse primer 5’‐GCT GATGTA CCA GTT GGG GA‐3’; iNOS: forward primer 5’‐ TAT CTG CAG ACA CAT ACT TTA CGC‐3’, reverse primer 5’‐ TCC TGG AAC CAC TCG TAC TTG‐3’; ICAM‐1: forward primer 5’‐TCC TAA AAT GAC CTG CAG ACG‐3’, reverse primer 5’‐AGT TTT ATG GCC TCC TCC TGA‐3’; MCP‐1: forward primer 5’‐GCT CAT AGC AGC CAC CTT CAT TC‐3’, reverse primer 5’‐GTC TTC GGA GTT TGG GTT TGC‐3’.
2.14. Measurement of reactive oxygen species in retina
The chemilumigenic probes, luminol (5‐amino‐2,3‐dihydro‐1,4‐phthalazinedione, Sigma) and lucigenin (bis‐N‐methylacridiniumnitrate, Sigma), were used to assess the ROS level in rat retina.30 Briefly, retinas were isolated at days 3, 7 and 14 after euthanasia and then balanced with 1 × PBS. Samples were then placed into vials containing PBS‐HEPES buffer (pH 7.2). The level of ROS was analysed by supplementing lucigenin and luminol with a final concentration of 0.2 mM. The luminescent intensity was recorded at every 1 minutes through luminescence reader (BioTek, USA).
2.15. Measurements of the activities of antioxidant enzymes in retina
Colorimetric methods were aplicated to evaluate superoxide dismutase (SOD) and catalase (CAT) activities using kits (Cayman Chemical, USA) in the retina at day 3, 7 and 14. Assays were conducted according to the manufacturer's protocols.
2.16. Measurements of glutathione levels in retina
Glutathione (GSH) ELISA kits (Cayman Chemical, USA) were used to determine the concentration of GSH. The absorbance of the dithionitrobenzoic acid (DTNB) method at 412 nm at days 3, 7 and 14 was recorded and then calculated to the expression of GSH level as μmol/mg protein.
2.17. Statistical analysis
Digital data were expressed as mean ± SD. Statistical significance was denoted as P value < .05. The analysis within groups employed one‐way ANOVAs followed by Tukey's post hoc test for multiple pairwise tests.
3. RESULTS
3.1. rRECs viability and identification
As can be seen in Figure 1A, the morphology of the isolated rRECs was fusiform. The PI evaluation showed that viable cells (PI‐) reached 98.8%, indicating desirable isolation efficiency (Figure 1B). Furthermore, the obtained cells were immunofluorescence stained with the specific biomarker of rRECs (factor VIII) and DAPI for cell nucleus. As shown in Figure 1C, the almost complete overlapping of the 2 markers indicated the high purity of rRECs.
Figure 1.
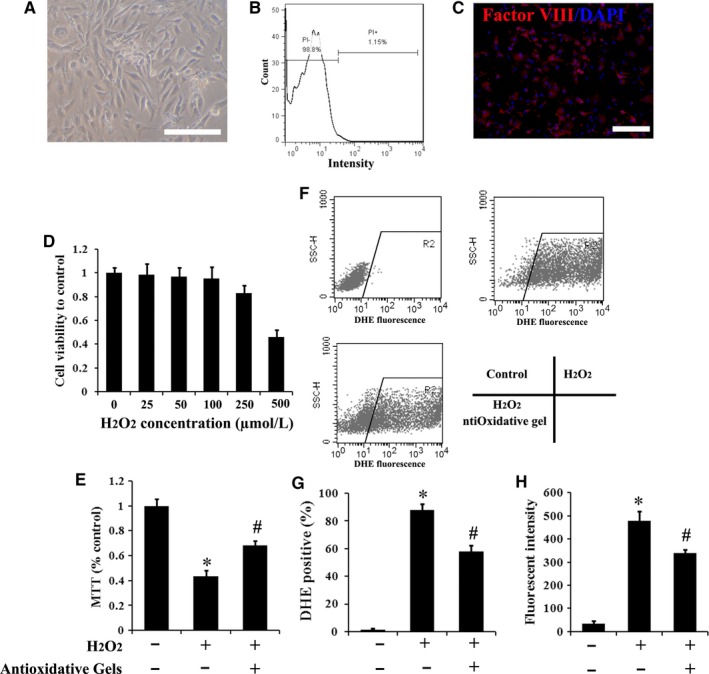
Characterization of rRECs and the effects of antioxidative gels, CMCC, on their intracellular ROS levels and apoptosis. A, Morphological image of isolated rRECs was attained by the inverted microscopy. Bar scale = 100 μm. B, Flow cytometry of rREC stained by PI. C, Immunofluorescence staining with antibodies against factor VIII‐related antigen showed positive staining in rRECs. Bar scale = 100 μm. D, The cell viability was dose‐dependently related to H2O2. E, The H2O2‐mediated decrease in cell viability was attenuated by add CMCC. F, Intracellular ROS levels in rRECs were analysed by flow cytometry. G and H, The quantitative detection of DHE staining‐positive rRECs and fluorescence intensity of DHE. (n = 5). * P < .05 compared with control; # P < .05 compared with H2O2 group
3.2. CMCC protect rRECs against H2O2‐induced oxidative stress
The introduction of H2O2 into rRECs culture medium was used to establish in vitro oxidative stress model and assess whether CMCC was able to strengthen the viability of rRECs under the condition. As shown in Figure 1D, the viability of rRECs decreased when the H2O2 concentration elevated up to 250 μmol/L. When the concentration of H2O2 reached 500 μmol/L, only around half of the cells can survive and this level of H2O2 was thus chosen in the following experiments. The CMCC was known for its antioxidative capability. As predicted, the 500 μmol/L H2O2‐induced decrease in rRECs viability can be considerably offset by the addition of this antioxidative gels (Figure 1E). Furthermore, as shown in Figure 1F‐H, normal rRECs possessed a relatively low ROS level. Hence, few DHC‐positive cells and weak fluorescent intensity were detected. However, DHE response dramatically increased after introducing H2O2. This augment generated by H2O2 could be significantly attenuated (P < .05) by adding antioxidative gels.
The typical TUNEL staining images (Figure 2) were in agreement with the flow cytometry measurements. TUNEL‐positive cells were much less in the control group than that in the H2O2‐treated one, while the increased number of TUNEL‐positive cells by oxidative stress was reduced via the introduction of CMCC. The following quantification results of TUNEL by flow cytometry (Figure 3A) clearly indicated that apoptosis in the control group was significantly lower than H2O2‐treated group and this change could be considerably ameliorated by supplementing antioxidative gels.
Figure 2.
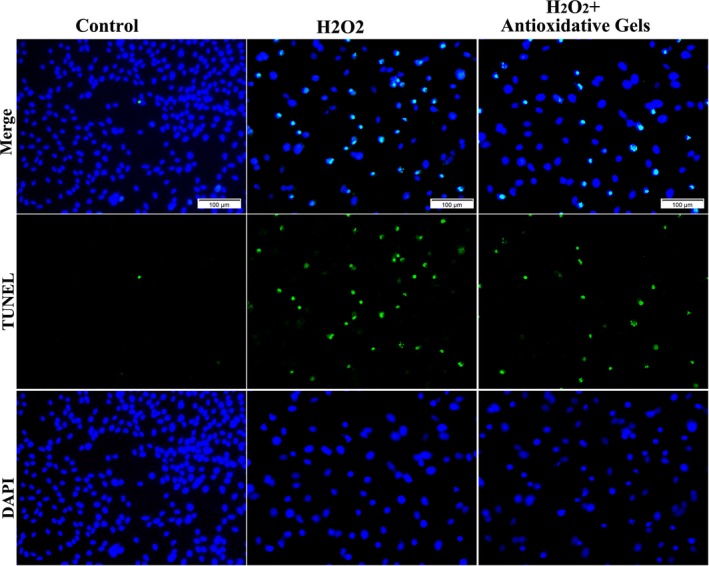
Representative TUNEL images of rRECs. The rRECs were pre‐treated with or without H2O2 and antioxidative gels. The blue was nucleus staining with DAPI and the green was TUNEL staining, representing apoptotic rRECs. Three groups. Bar scale = 100 μm
Figure 3.
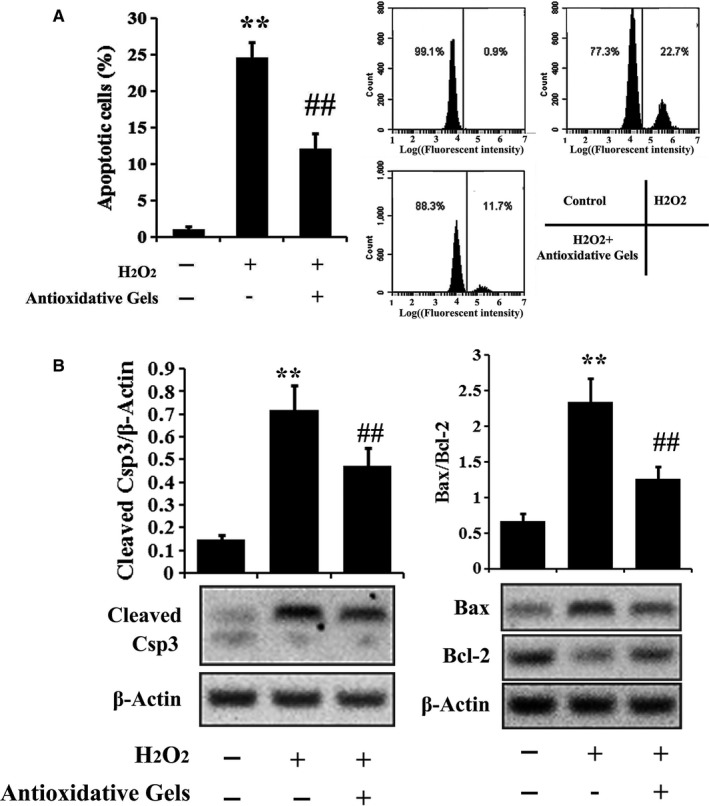
Assessment of apoptosis‐related proteins in rRECs. A, Apoptosis of rRECs treated with or without H2O2 or following antioxidative gels was analysed by flow cytometry. B, The levels of apoptotic proteins in H2O2‐treated rRECs were analysed by Western blotting (n = 5). ** P < .01 compared with control; ## P < .01 compared with H2O2 group
3.3. Assessment of apoptotic proteins
The investigation of apoptotic proteins expression in rRECs further validated the underlying mechanism. As shown in Figure 3B, H2O2‐induced oxidative stress significantly up‐regulated pro‐apoptotic protein, Csp3 and the ratio of Bax to Bcl‐2. This modulation in rRECs was then significantly suppressed when the antioxidative gels, CCPCC, were introduced.
3.4. In vitro assay of IL‐10 releasing
The released IL‐10 in supernatant was measured at days 0, 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10, respectively (Figure 4). It can be seen that about 25% amount of drugs were released at the first day. With time, the releasing speed was correspondingly decreased. At day 10, only 2% increase was detected, compared with the releasing amount at day 9, indicating the relative retention of loaded IL‐10 in antioxidative gels.
Figure 4.
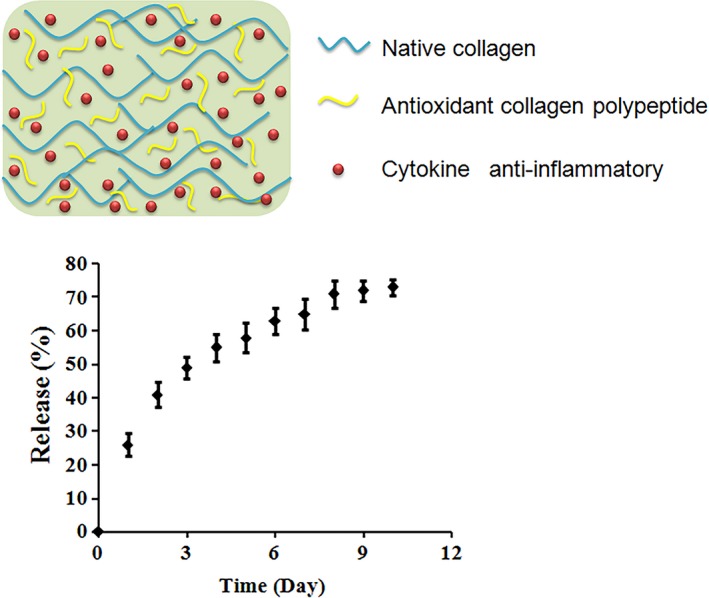
IL‐10 releasing measurement in vitro. Upper panel) The schematic image of IL‐10@CMCC structure. Bottom panel) the releasing progress was recorded at 1‐day interval and up to 10 d
3.5. Effects of IL‐10@CMCC on retinal histology after I/R injury
As shown in Figure 5A, retinal I/R injury obviously caused the decrease in retinal thickness, compared with normal group at day 21. Marked increase in retinal thickness was found in the I/R + Gel group and this restoration was enhanced in the I/R + Gel/IL‐10 group, compared with the I/R group. The data of bar plotting clearly supported the observation through H&E staining. In addition, the thicknesses of inner plexiform layer (IPL), inner nuclear layer (INL) and outer nuclear layer (ONL) were generally reduced by the treatment of I/R (Table 1). The introduction of antioxidative gels significantly attenuated the thinness. The beneficial effect of gels could be further amplified by the loading of IL‐10.
Figure 5.
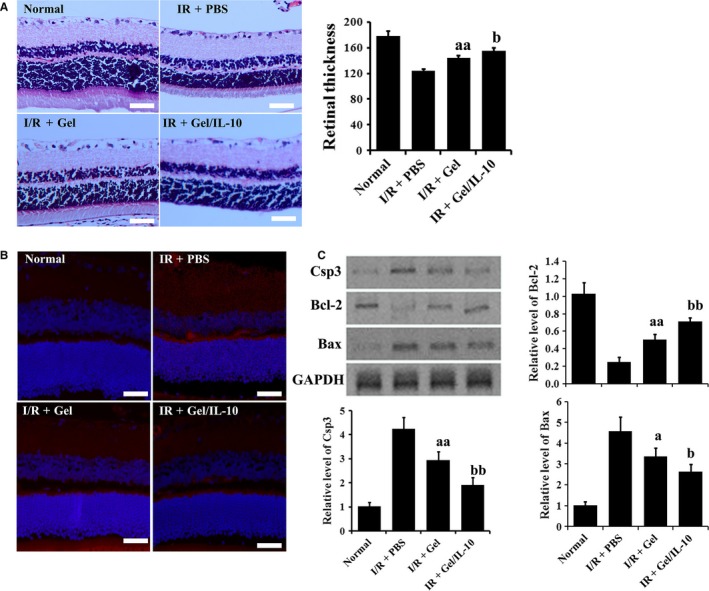
Effects of IL‐10@CMCC on retinal histology at 21 d post‐I/R injury. A, Representative retinal section images of control, I/R + PBS, I/R + Gel or I/R + Gel/IL‐10 group were attained by H&E staining and the corresponding quantitative total retinal thickness (from the inner limiting membrane to the pigment epithelium). B, Representative immunofluorescence images specified for Csp3 in the 4 groups. C, Western blotting analysis of apoptotic proteins of rRECs, for example Csp3, Bcl‐2 and Bax, in the 4 groups. n = 5, bar scale = 50 μm, a P < .05 compared with I/R + PBS group; aa P < .01 compared with I/R + PBS group; b P < .05 compared with I/R + Gel group; bb P < .01 compared with I/R + Gel group
Table 1.
The thickness of the inner plexiform layer (IPL), the inner nuclear layer (INL) and the outer nuclear layers (ONL) in 4 groups
| Normal | I/R + PBS | I/R + Gel | IR + Gel/IL‐10 | |
|---|---|---|---|---|
| IPL (μm) | 68.2 ± 3.7 | 42.4 ± 3.0 | 51.4 ± 2.3a | 57 ± 3.4b |
| INL (μm) | 40.4 ± 2.4 | 26.7 ± 2.0 | 33.0 ± 1.7a | 36.6 ± 2.0 |
| ONL (μm) | 57.7 ± 1.3 | 41 ± 2.6 | 48.8 ± 1.1a | 52.4 ± 1.9b |
n = 5.
P < .05 compared with I/R + PBS group.
P < .05 compared with I/R + Gel group.
The immunofluorescence staining of Csp3 revealed the apoptosis in retina (Figure 5B). The red dots were notably emerged in I/R group, compared with the normal one. However, the number and distribution of red stains were decreased in I/R + Gel group. Moreover, a further decrease was found in the I/R + Gel/IL‐10 group. Furthermore, as shown in Figure 5C, Western blotting of apoptosis‐related proteins clearly showed that the introduction of gels significantly elevated the expression of anti‐apoptotic protein, for example Bcl‐2 and lowered that of apoptotic proteins, for example Csp3 and Bax. Such modulation was further enhanced by loading IL‐10, indicating the protective effect of antioxidative gels and IL‐10 against the retinal apoptosis induced by I/R.
3.6. Effects of IL‐10@CMCC on the expression of inflammatory cytokine
At day 3, as shown in Figure 6A‐E, the mRNA expression levels of TNF‐α, IL‐1β, iNOS, ICAM‐1 and MCP‐1 were much higher in I/R‐injured group than the normal rats. In I/R + Gel, the expression of TNF‐α, IL‐1β, iNOS, ICAM‐1 and MCP‐1 significantly reduced, compared with that in I/R group (P < .05). Moreover, the mRNA levels of TNF‐α, IL‐1β, iNOS, ICAM‐1 and MCP‐1 were significantly less in the IL‐10 involved group than in the only CMCC containing group (P < .05). The further Western blotting results (Figure 6F) provided a supportive evidence for the effects of IL‐10@CMCC on the expression of the inflammatory cytokines.
Figure 6.
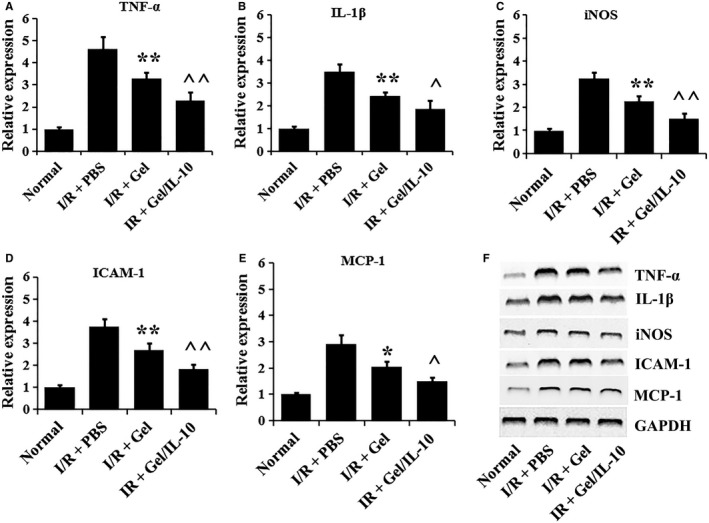
Effects of IL‐10@CMCC on the expression of inflammatory mediators in retinal cells after I/R injury. At day 3, the mRNA expression of inflammatory mediators, for example A, TNF‐α, B, IL‐1β, C, iNOS, D, ICAM‐1 and E, MCP‐1 in retinas of control, I/R + PBS, I/R + Gel or I/R + Gel + IL‐10 group, was investigated via qPCR based on 2^‐Ct (n = 5). F) Western blotting images of the corresponding mediators in the 4 groups. * P < .05 compared with I/R + PBS group; ** P < .01 compared with I/R + PBS group; ^ P < .05 compared with I/R + Gel group; ^^ P < .01 compared with I/R + Gel group
3.7. Effects of IL‐10@CMCC on the level of ROS and the activity of anti‐oxidases
The changes in oxidative states in retinas were finally assessed by evaluating the level of ROS and the activity of antioxidant enzymes. Lucigenin‐ and luminol‐enhanced chemiluminescence (CL) methods revealed the in vivo ROS level at days 3, 7 and 14 respectively. As shown in Figure 7, I/R injury could significantly trigger the CL in retina. The administration of antioxidative gels and IL‐10 remarkably decreased the luminol‐ and lucigenin‐enhanced CL signal over 2 weeks, compared with I/R group. Conversely, the activity of SOD and CAT as well as the level of GSH in retina was found significantly reduced in I/R group, compared with those in normal group, as shown in Figure 7C,D. Such significant decrease in the activity of SOD and CAT enzymes and GSH level induced by I/R injury was considerably inhibited by the supplementation with antioxidative gels, CMCC, particularly loaded with IL‐10 at days 3, 7 and 14.
Figure 7.
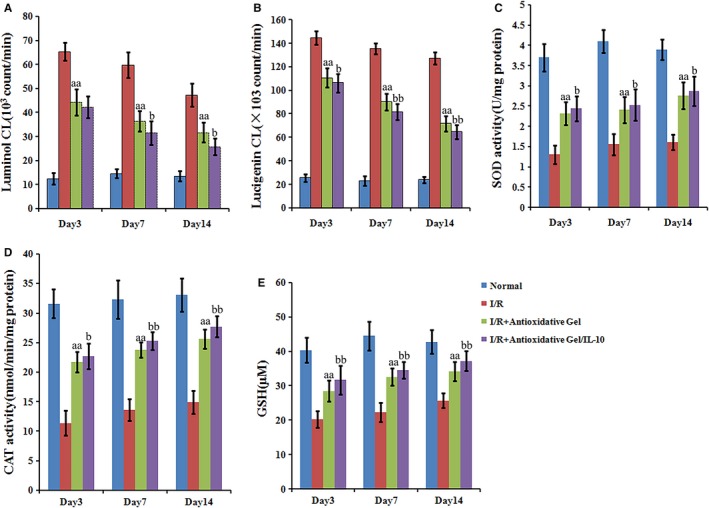
Effects of IL‐10@CMCC on retinal ROS and anti‐oxidase activity post‐I/R injury. A, Luminol‐ and (B) lucigenin‐enhanced chemiluminescence as well as the activity of (C) SOD and (D) CAT enzymes as well as E) GSH levels in retinas of the control, I/R + PBS, I/R + CMCC or I/R + IL‐10@CMCC group were investigated at days 3, 7 and 14, respectively (n = 5). aa P < .01 compared with I/R + PBS group; b P < .05 compared with I/R + Gel group; bb P < .01 compared with I/R + Gel group
4. DISCUSSION
Oxidative stress and inflammation were reported to play key roles in retinal damage caused by I/R injury.31, 32 Hence, drugs or treatments capable of attenuating ROS level or/and inflammation in retina are considered as efficacious and main therapies for retinal I/R injury.31 H2O2 was regarded as one of the most important species in ROS, due to its stability and key status in ROS‐related metabolism.33, 34 Thus, in vitro H2O2 tests were gradually accepted as featured tools for the study of ROS or oxidative stress.35, 36 Here, H2O2 was employed to simulate in vitro oxidative stress microenvironment and rRECs were selected as model cells due to its physiological function of retinal vascularization, which is essential for structural and functional recovery of the retina.37
Our study was aimed at the establishment of a desirable drug slow‐release system for the retinal treatment of I/R injury. The antioxidative gel carriers, CMCC, were found to significantly decrease intracellular ROS level and thus apoptosis in rRECs. The following analysis of the apoptotic cytokines offered us evidence in molecular level for the CMCC‐based protection on rRECs. The accumulation of intracellular ROS could inhibit the Akt‐signal pathway,38 followed by down‐regulating survival signals, including Bcl‐2 and activating pro‐apoptotic factors, such as Bax and Csp3.39 Additionally, the high concentration of ROS was conducive to triggering the opening of the mitochondria permeability transition gates, thus leading to the release of apoptosis‐activating cytokines.40 Moreover, the expression of pro‐apoptotic protein of mitochondria membrane, Bax, was enhanced and anti‐apoptotic protein, Bcl‐2, was, correspondingly, blocked. The continuing apoptotic response would ultimately activate the late‐stage apoptotic protein, Csp3.41, 42
The therapeutic efficiency of this antioxidative gel cargo could be further enhanced by loading drugs. IL‐10 was selected for its anti‐inflammation drug potentials.43 Previous studies have already utilized IL‐10 to mitigate inflammatory response to I/R injury.44, 45 For instance, the up‐regulation of IL‐10 through pre‐conditioning with CpG‐oligonucleotides could mitigate the myocardial I/R injury.46 In this study, we demonstrated that IL‐10 load enhanced the protective effect of CMCC on retinal cells against I/R injury. The restoration of retinal morphology and the decrease in apoptosis confirmed the therapeutic effect of IL‐10. In addition, the IL‐10@CMCC delivery system synergistically mitigated retinal oxidative stress damage and suppressed the expression of inflammatory cytokines (TNF‐α, IL‐1β, iNOS, ICAM‐1 and MCP‐1). The pathogenesis of retinal I/R injury was found to up‐regulate the expression of multiple inflammatory mediators, including TNF‐α, IL‐1β, iNOS, ICAM‐1 and MCP‐1, which was in accordance with previous reports.3 TNF‐α played a pivotal role in regulating neuron death caused by retinal I/R injury.47, 48 IL‐1β was involved in the degeneration of inner retinal compositions owe to I/R injury.49 The iNOS‐triggered delayed neuronal cell death after ischaemia mediated the production of nitric oxide (NO),50 which would finally develop into inflammatory oxidants.51 ICAM‐1 directly activated inflammatory responses to the blood vessel wall through stimulating endothelial cell and generating atherosclerotic plaque.52 MCP‐1 contributed to the combination between monocytes/macrophages and target tissues.53 In this study, we clearly demonstrated that the drug‐carrying platform, antioxidant gels loading with IL‐10, could inhibit the expression of TNF‐α, IL‐1β, iNOS, ICAM‐1 and MCP‐1. Our results complied with those of previous studies,54, 55 demonstrating that the gels and IL‐10 synergistically displayed anti‐inflammatory activities through the expression inhibition of various inflammatory mediators.
Afterwards, another important factor to induce apoptosis after I/R, in vivo oxidative stress, was investigated. Previous studies have confirmed that I/R injury produced a myriads of ROS, including H2O2, OH‐, hypochlorite, peroxynitrite and superoxide radicals, resulting in cellular damage and apoptosis.3, 31 Our data of in vivo ROS analysis were consistent with that of the previous report,27 indicating that I/R injury induced apoptosis in retina was accompanied by significant augment of ROS level. In addition, the decreases in oxidant scavenges, typically SOD, CAT and GSH, were found after I/R injury,27 which further weakened the capacity of the degradation of intracellular ROS. In our study, the IL‐10@CMCC hybrids were capable of ameliorating the augment of ROS level. After injecting these IL‐10‐loaded hydrogels, the expressive abnormity of ROS and oxidant scavenges was significantly offset, facilitating to recover retinal cells from apoptosis and ultimately retinal thickness as well as morphology. Albeit plausible results achieved in retinal treatment post‐I/R injury, there is still a long way to improve therapeutic effects of drug‐delivery system on the retina I/R injury. We hence need more endeavour to explore and optimize the in vivo performance of this slow‐releasing system in the future.
5. CONCLUSION
In summary, we here utilized H2O2 to simulate in vitro oxidative stress microenvironment and thus tested the performance of CMCC, the drug carrier, establishing the antioxidative and thus anti‐apoptotic features of this drug cargo. The next in vivo study demonstrated that I/R injury induced oxidative stress and inflammation could be potently mitigated by the treatment of these drug slow‐releasing systems. The reduction in apoptotic retinal cells and thus the raise of retinal thickness fully demonstrated the therapeutic effects of IL‐10@CMCC on retinal I/R injury.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
ACKNOWLEDGEMENTS
This work was supported by the China Postdoctoral Science Foundation (Grant No. 20100471803), Health and Family Planning Commission Research Project of Heilongjiang Province (Grant No. 2014‐299) and Heilongjiang Postdoctoral Science Foundation (Grant No. LBH‐Q15101).
Mu H, Wang Y, Wei H, et al. Collagen peptide modified carboxymethyl cellulose as both antioxidant drug and carrier for drug delivery against retinal ischaemia/reperfusion injury. J Cell Mol Med. 2018;22:5008–5019. 10.1111/jcmm.13768
REFERENCES
- 1. Stefansson E, Machemer R, de Juan E Jr, McCuen BW 2nd, Peterson J. Retinal oxygenation and laser treatment in patients with diabetic retinopathy. Am J Ophthalmol 1992;113:36‐38. [DOI] [PubMed] [Google Scholar]
- 2. Levin LA. Models of neural injury. J Glaucoma. 2001;10:S19‐S21. [DOI] [PubMed] [Google Scholar]
- 3. Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91‐147. [DOI] [PubMed] [Google Scholar]
- 4. Szabo ME, Haines D, Garay E, et al. Antioxidant properties of calcium dobesilate in ischemic/reperfused diabetic rat retina. Eur J Pharmacol. 2001;428:277‐286. [DOI] [PubMed] [Google Scholar]
- 5. Kusari J, Padillo E, Zhou SX, et al. Effect of brimonidine on retinal and choroidal neovascularization in a mouse model of retinopathy of prematurity and laser‐treated rats. Invest Ophthalmol Vis Sci. 2011;52:5424‐5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yokota H, Narayanan SP, Zhang W, et al. Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Invest Ophthalmol Vis Sci. 2011;52:8123‐8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei Y, Gong J, Yoshida T, et al. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia‐reperfusion injury. Free Radic Biol Med. 2011;51:216‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laskowski I, Pratschke J, Wilhelm MJ, Gasser M, Tilney NL. Molecular and cellular events associated with ischemia/reperfusion injury. Ann Transplant. 2000;5:29‐35. [PubMed] [Google Scholar]
- 9. Pan H, He M, Liu R, Brecha NC, Yu AC, Pu M. Sulforaphane protects rodent retinas against ischemia‐reperfusion injury through the activation of the Nrf2/HO‐1 antioxidant pathway. PLoS ONE. 2014;9:e114186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yadav M, Rhee KY, Jung IH, Park SJ. Eco‐friendly synthesis, characterization and properties of a sodium carboxymethyl cellulose/graphene oxide nanocomposite film. Cellulose. 2013;20:687‐698. [Google Scholar]
- 11. Bajpai AK, Giri A. Water sorption behaviour of highly swelling (carboxy methylcellulose‐g‐polyacrylamide) hydrogels and release of potassium nitrate as agrochemical. Carbohyd Polym. 2003;53:271‐279. [Google Scholar]
- 12. Agarwal R, Alam MS, Gupta B. Polyvinyl alcohol‐polyethylene oxide‐carboxymethyl cellulose membranes for drug delivery. J Appl Polym Sci. 2013;129:3728‐3736. [Google Scholar]
- 13. Garrett Q, Xu SJ, Simmons PA, Vehige J, Flanagan JL, Willcox MD. Expression and localization of carnitine/organic cation transporter OCTN1 and OCTN2 in ocular epithelium. Invest Ophthalmol Vis Sci. 2008;49:4844‐4849. [DOI] [PubMed] [Google Scholar]
- 14. Yager DR, Nwomeh BC. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999;7:433‐441. [DOI] [PubMed] [Google Scholar]
- 15. Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen. 2002;10:16‐25. [DOI] [PubMed] [Google Scholar]
- 16. Moseley R, Walker M, Waddington RJ, Chen WYJ. Comparison of the antioxidant properties of wound dressing materials‐carboxymethylcellulose, hyaluronan benzyl ester and hyaluronan, towards polymorphonuclear leukocyte‐derived reactive oxygen species. Biomaterials. 2003;24:1549‐1557. [DOI] [PubMed] [Google Scholar]
- 17. Guillerminet F, Beaupied H, Fabien‐Soule V, et al. Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: an in vitro and in vivo study. Bone. 2010;46:827‐834. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Zhang CL, Zhang Q, Li P. Composite electrospun nanomembranes of fish scale collagen peptides/chito‐oligosaccharides: antibacterial properties and potential for wound dressing. Int J Nanomed. 2011;6:667‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watanabe‐Kamiyama M, Shimizu M, Kamiyama S, et al. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J Agric Food Chem. 2010;58:835‐841. [DOI] [PubMed] [Google Scholar]
- 20. Rajasingh J, Bord E, Luedemann C, et al. IL‐10‐induced TNF‐alpha mRNA destabilization is mediated via IL‐10 suppression of p38 MAP kinase activation and inhibition of HuR expression. Faseb J. 2006;20:2112‐2114. [DOI] [PubMed] [Google Scholar]
- 21. Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL‐10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71‐109. [DOI] [PubMed] [Google Scholar]
- 22. Li Z, Ma JY, Kerr I, et al. Selective inhibition of p38alpha MAPK improves cardiac function and reduces myocardial apoptosis in rat model of myocardial injury. Am J Physiol Heart Circ Physiol. 2006;291:H1972‐H1977. [DOI] [PubMed] [Google Scholar]
- 23. Wang MJ, Tsai BM, Turrentine MW, Mahomed Y, Brown JW, Meldrum DR. p38 mitogen activated protein kinase mediates both death signaling and functional depression in the heart. Ann Thorac Surg. 2005;80:2235‐2241. [DOI] [PubMed] [Google Scholar]
- 24. Fan LH, Peng M, Zhou XY, et al. Modification of carboxymethyl cellulose grafted with collagen peptide and its antioxidant activity. Carbohyd Polym. 2014;112:32‐38. [DOI] [PubMed] [Google Scholar]
- 25. Shi K, Wang XL, Zhu J, Cao GQ, Zhang K, Su ZG. Salidroside protects retinal endothelial cells against hydrogen peroxide‐induced injury via modulating oxidative status and apoptosis. Biosci Biotechnol Biochem. 2015;79:1406‐1413. [DOI] [PubMed] [Google Scholar]
- 26. Chang JH, Wang YY, Shao LJ, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fang IM, Yang CM, Yang CH, Chiou SH, Chen MS. Transplantation of induced pluripotent stem cells without C‐Myc attenuates retinal ischemia and reperfusion injury in rats. Exp Eye Res. 2013;113:49‐59. [DOI] [PubMed] [Google Scholar]
- 28. Wang FX, He SZ, Chen B. Retinoic acid‐loaded alginate microspheres as a slow release drug delivery carrier for intravitreal treatment. Biomed Pharmacother. 2018;97:722‐728. [DOI] [PubMed] [Google Scholar]
- 29. Liu L, Jiang YD, Steinle JJ. Compound 49b Restores Retinal Thickness and Reduces Degenerate Capillaries in the Rat Retina following Ischemia/Reperfusion. PLoS ONE. 2016;11:e0159532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fang IM, Yang CH, Yang CM, Chen MS. Chitosan oligosaccharides attenuates oxidative‐stress related retinal degeneration in rats. PLoS ONE. 2013;8:e77323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fang IM, Yang CM, Yang CH. Chitosan oligosaccharides prevented retinal ischemia and reperfusion injury via reduced oxidative stress and inflammation in rats. Exp Eye Res. 2015;130:38‐50. [DOI] [PubMed] [Google Scholar]
- 32. Szabo ME, Droy‐Lefaix MT, Doly M, Carre C, Braquet P. Ischemia and reperfusion‐induced histologic changes in the rat retina. Demonstration of a free radical‐mediated mechanism. Invest Ophthalmol Vis Sci. 1991;32:1471‐1478. [PubMed] [Google Scholar]
- 33. Miao Z‐H, Wang H, Yang H, Li Z‐L, Zhen L, Xu C‐Y. Intrinsically Mn2 + ‐chelated polydopamine nanoparticles for simultaneous magnetic resonance imaging and photothermal ablation of cancer cells. ACS Appl Mater Interfaces. 2015;7:16946‐16952. [DOI] [PubMed] [Google Scholar]
- 34. Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem. 2014;289:8735‐8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cia D, Vergnaud‐Gauduchon J, Jacquemot N, Doly M. Epigallocatechin gallate (EGCG) prevents H2O2‐induced oxidative stress in primary rat retinal pigment epithelial cells. Curr Eye Res. 2014;39:944‐952. [DOI] [PubMed] [Google Scholar]
- 36. Ma T, Chen T, Li P, et al. Heme oxygenase‐1 (HO‐1) protects human lens epithelial cells (SRA01/04) against hydrogen peroxide (H2O2)‐induced oxidative stress and apoptosis. Exp Eye Res. 2016;146:318‐329. [DOI] [PubMed] [Google Scholar]
- 37. Su XJ, Sorenson C, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis. 2003;9:171‐178. [PubMed] [Google Scholar]
- 38. Panieri E, Gogvadze V, Norberg E, Venkatesh R, Orrenius S, Zhivotovsky B. Reactive oxygen species generated in different compartments induce cell death, survival, or senescence. Free Radic Biol Med. 2013;57:176‐187. [DOI] [PubMed] [Google Scholar]
- 39. Marte BM, Downward J. PKB/Akt: connecting phosphoinositide 3‐kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355‐358. [DOI] [PubMed] [Google Scholar]
- 40. Sun L, Jin Y, Dong L, Sumi R, Jahan R, Li Z. The neuroprotective effects of Coccomyxa gloeobotrydiformis on the ischemic stroke in a rat model. Int J Biol Sci. 2013;9:811‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Q, Huang WD, Lv XY, Yang YM. Ghrelin protects H9c2 cells from hydrogen peroxide‐induced apoptosis through NF‐kappaB and mitochondria‐mediated signaling. Eur J Pharmacol. 2011;654:142‐149. [DOI] [PubMed] [Google Scholar]
- 42. Han J, Tan P, Li Z, et al. Fuzi attenuates diabetic neuropathy in rats and protects schwann cells from apoptosis induced by high glucose. PLoS ONE. 2014;9:e86539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oberholzer A, Oberholzer C, Moldawer LL. Interleukin‐10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti‐inflammatory drug. Crit Care Med. 2002;30:S58‐S63. [PubMed] [Google Scholar]
- 44. Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM. The dual role of IL‐10. Trends Immunol.2003; 24: 36‐43. [DOI] [PubMed] [Google Scholar]
- 45. Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL‐10 secretion. J Clin Investig. 2010;120:559‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Markowski P, Boehm O, Goelz L, et al. Pre‐conditioning with synthetic CpG‐oligonucleotides attenuates myocardial ischemia/reperfusion injury via IL‐10 up‐regulation. Basic Res Cardiol. 2013;108:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barone FC, Arvin B, White RF, et al. Tumor necrosis factor‐alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233‐1244. [DOI] [PubMed] [Google Scholar]
- 48. Fontaine V, Mohand‐Said S, Hanoteau N, Fuchs C, Pfizenmaier K, Eisel U. Neurodegenerative and neuroprotective effects of tumor Necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci 2002;22:RC216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoneda S, Tanihara H, Kido N, et al. Interleukin‐1beta mediates ischemic injury in the rat retina. Exp Eye Res. 2001;73:661‐667. [DOI] [PubMed] [Google Scholar]
- 50. Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28:1283‐1288. [DOI] [PubMed] [Google Scholar]
- 51. Eiserich JP, Hristova M, Cross CE, et al. Formation of nitric oxide‐derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393‐397. [DOI] [PubMed] [Google Scholar]
- 52. Lawson C, Wolf S. ICAM‐1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22‐32. [DOI] [PubMed] [Google Scholar]
- 53. Jo N, Wu GS, Rao NA. Upregulation of chemokine expression in the retinal vasculature in ischemia‐reperfusion injury. Invest Ophthalmol Vis Sci. 2003;44:4054‐4060. [DOI] [PubMed] [Google Scholar]
- 54. Khodagholi F, Eftekharzadeh B, Maghsoudi N, Rezaei PF. Chitosan prevents oxidative stress‐induced amyloid beta formation and cytotoxicity in NT2 neurons: involvement of transcription factors Nrf2 and NF‐kappaB. Mol Cell Biochem. 2010;337:39‐51. [DOI] [PubMed] [Google Scholar]
- 55. Wei P, Ma P, Xu QS, et al. Chitosan oligosaccharides suppress production of nitric oxide in lipopolysaccharide‐induced N9 murine microglial cells in vitro. Glycoconj J. 2012;29:285‐295. [DOI] [PubMed] [Google Scholar]


