Abstract
Given sex‐related differences in brain disorders, it is of interest to study if there is a sex difference in the permeability of the blood‐cerebrospinal fluid barrier (BCSFB) and the blood‐brain barrier (BBB). The CSF/serum albumin ratio (QA lb) is a standardized biomarker that evaluates the function of these barriers. In previous studies, contradictory results have been reported with respect to sex difference using this quotient, possibly because of small population sizes and heterogeneity with respect to ages. QA lb measurements in more than 20 000 patients between 1 and 90 years visiting our hospitals revealed a significant sex difference in all age groups also when excluding patients with pathologically high CSF albumin > 400 mg/L. Similar pattern was found in 335 healthy volunteers in similar age intervals. Although also other factors are likely important, our observation is consistent with lower integrity of the brain barriers in males. If the difference in QA lb is caused mainly by a difference in barrier function, this may require different drug doses and strategies for efficient central nervous system (CNS) delivery in males and females, as well as it may indicate differences in brain metabolism. Moreover, our study emphasizes that different reference values should be used both for different ages and sexes.
Keywords: brain‐barriers integrity, CSF/plasma albumin ratio, sex differences
1. INTRODUCTION
The BBB and the BCSFB selectively regulate the transfer of molecules between the blood, brain parenchyma and CSF. Both blood‐CNS barriers are affected during brain ageing possibly preceding neuronal degeneration.1
The QAlb is a standardized biomarker reflecting the function of these barriers.2 As albumin is almost exclusively produced in the liver, increased QAlb indicates brain damage.3 However, reduction in CSF drainage or production, and low turnover rate may also account for increased CSF albumin levels.2, 5, 6, 7
Given the sex‐related difference in prevalence and incidence of brain disorders8 as well as in drug absorption, bioavailability and its response in brain,9 we have explored an aspect rarely investigated on: whether or not there is a sex difference in the permeability of the brain barriers.
In the past, we measured QAlb in controls and patients with a broad spectrum of neurological diseases.10 When re‐analysing our data by grouping the subjects into sex category, we found a significant sex‐related difference in all the populations studied, including the control subjects (Leoni et al, unpublished). Moreover, male patients with lower lumbar pain without positive findings in myelography were reported to have higher CSF albumin than corresponding female patients.11 A similar sex difference was found in a population of AD patients.12
Contrary to the above studies, no significant sex difference was reported in the QAlb of 93 subjects searching advice at a neurological clinic without showing any pathological findings.13 A later study on 105 healthy volunteers also failed to find a sex difference.6 A weakness in all the studies referred to above is the relative small population size. Age heterogeneity might mask sex differences in terms of QAlb. Indeed, most of the studies showing sex difference present a narrow age distribution.
Based on QAlb measurements on a great number of patients (>20 000) in our hospitals during 8 years and healthy volunteers (n = 335) with a range of age between 1 and 90 years, evidence is presented here for a sex difference in QAlb.
2. METHODS
Results for CSF and plasma albumin of 27 263 measurements anonymous patients were obtained in routine diagnostic procedures from the clinical chemistry laboratory at the Karolinska University Hospital Huddinge and Solna during 2008‐2016. Quantification of CSF albumin was performed using immunochemical assays from either Siemens or Roche Diagnostics, and plasma albumin was quantified using either the Siemens immunochemical assay or a bromocresol purple assay from Roche Diagnostics or Beckman Coulter. For multiple measurements on a sample, the mean value was calculated and used in subsequent calculation. In some cases, more than one measurement on the same individual may be present, because of the anonymity of the samples. We estimate that the number of unique patients to be over approximately 20 000.
Data from the previous study on 105 healthy volunteers6 were expanded with additional 230 healthy volunteers and grouped into sex categories. The studies were approved by the local ethical committee.
For statistical computing and graphics, we used GraphPad Prism Software, version 5.0. QAlb did not show a normal Gaussian distribution. Non‐parametric group comparisons of means were performed using Kruskal‐Wallis test followed by Dunn's post hoc test. Two‐tailed P‐values < 0.05 were considered as statistically significant.
3. RESULTS
Figure 1 shows the medians of QAlb values obtained from 27 263 measurements on at least 20 000 anonymous patients grouped by age range. No marked sex differences were observed in childhood or at puberty or menopause. We found greater age‐dependent changes than those related to sex. Differences in QAlb were because of changes in CSF albumin levels rather than plasma albumin levels. The overall sex difference of the latter levels was only 1.5%.
Figure 1.
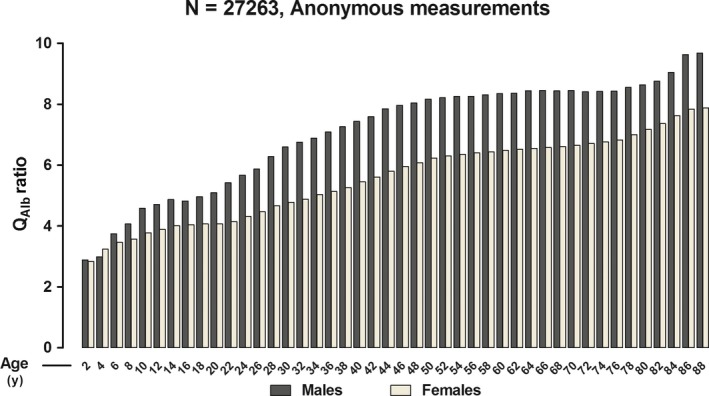
Anonymous Measurements of the Cerebrospinal Fluid/Plasma Albumin Ratio (QA lb Ratio) in Males and Females between 1 and 90 y. Data (N = 27 263) are displayed as median and are grouped in 2‐y age interval
Figure 2A shows the same data grouped in 5 different age intervals. Figure 2B shows the median values after removing the patients with pathologically high CSF albumin levels (>400 mg/L). The sex difference was statistically significant in all age groups. Figure 2C shows in same age intervals, the median QAlb values from the previous investigation on 105 healthy volunteers6 expanded with additional 230 healthy subjects. The sex difference was statistically significant in the age interval 61‐80 but not in the other age categories, probably because of the lower number of subjects. However, QAlb in the different age groups was found to be very similar to that of the anonymous patients after truncation (Figure 2B).
Figure 2.

Measurements of QA lb Ratio from Anonymous Patients before (A, N = 27 263) and after (B, N = 22 596) Removing CSF Albumin Levels (>400 mg/L); and QA lb Ratio from Healthy Controls (C, N = 335). Data are expressed as the median of males and females grouped in 20‐y interval. Kruskal‐Wallis test followed by Dunn's multiple post hoc test (Two‐tailed *P < 0.05, **P < 0.01, ***P < 0.001)
4. DISCUSSION
In the present study QAlb was measured in more than 20 000 anonymous subjects. This quotient is often included in the investigation of patients searching for headache or suspected neurological or neurodegenerative diseases. The number of measurements was about 25% higher for females.
Here, we uncover a sex difference in QAlb evident from around 6 years of age up to 90 consistent with a greater integrity of the BCSFB and/or BBB in females. If the observed sex‐related difference in QAlb is caused only by a difference in barrier function, this may require different drug strategies for efficient CNS delivery in males and females.
Many studies have previously defined sex‐based effects on CNS disease and progression, and here we add an additional sex‐dependent factor that might be relevant. BBB dysfunction has been suggested to be causative for many CNS diseases; however, sex differences have not been discussed in this connection.
The fact that the sex difference was not markedly changed at puberty or at menopause in our study population does not support the contention that hormonal factors are of major importance. The possibility must be considered that sex chromosome genes are more important for the differences observed than hormonal levels.8
A limitation of the study is the unavailability of data related to bodyweight. Part of the sex differences may be explained by the higher body mass in males. Seyfert, et al7 demonstrated that body mass index (BMI) influences on QAlb. Moreover, high BMI at middle age can predict high QAlb levels at an advanced age. In this context, we cannot exclude other determinants that may influence to increased CSF albumin levels such as reduced CSF flow‐rate, especially in elderly patients.
If the sex difference is because of a higher proportion of male patients with diseases associated with BBB disturbance, this difference should be reduced after removal of the patients with the highest ratios. After truncation, there was a decrease in the sex difference. A general problem when evaluating levels of different factors in CSF is the generation of suitable reference values. A common strategy is to analyse CSF from patients who have been searched the neurological clinic for subjective symptoms with no objective findings. Healthy volunteers represent the most optimal reference population, but it is difficult to get such subjects motivated to donate CSF. Here we were able to recruit 335 healthy subjects for our study. The age distribution was not optimal with most of the subjects in the age category 61‐89 years, which provided significant sex difference. The pattern was very similar in all age intervals to that obtained in the population of anonymous patients.
Regardless of the relative importance of the different factors that may affect QAlb our study emphasizes that different reference values should be used both as for different ages as for the two sexes.
POTENTIAL CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
CPF, KB, MH, ACM and IB conceived and designed the study. MH, KB and IB contribute to acquisition of data. CPF, KB, MH, VL and IB contribute to analysis of data and/or to drafting the text and/or preparing the figures.
ACKNOWLEDGEMENTS
We would like to acknowledge Karolinska Hospitals of Solna and Huddinge, Stockholm, Sweden for providing the anonymous measurements. We thank all colleagues and staff at the Departments of Anaesthesiology, Orthopaedics and Surgery, Mölndal Hospital and Sahlgren Hospital, Göteborg, Sweden, who helped to obtain a control sample for the CSF studies. This work was supported by the following Swedish foundations: Stiftelsen för Gamla Tjänarinnor, Gun och Bertil Stohnes Stiftelse, Stockholm County Council, The Margaretha af Ugglas Foundation, Karolinska Institutet fund for geriatric research, the regional agreement on medical training and clinical research (ALF) between the Stockholm County Council and Karolinska Institutet.
Parrado‐Fernández C, Blennow K, Hansson M, Leoni V, Cedazo‐Minguez A, Björkhem I. Evidence for sex difference in the CSF/plasma albumin ratio in ~20 000 patients and 335 healthy volunteers. J Cell Mol Med. 2018;22:5151–5154. 10.1111/jcmm.13767
Contributor Information
Cristina Parrado‐Fernández, Email: Cristina.parrado@ki.se.
Ingemar Björkhem, Email: Ingemar.Bjorkhem@ki.se.
REFERENCES
- 1. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tibbling G, Link H, Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Din Lab Invest. 1977;37:385‐390. [DOI] [PubMed] [Google Scholar]
- 3. Farrall AJ, Wardlaw JM. Blood‐brain barrier: ageing and microvascular disease–systematic review and meta‐analysis. Neurobiol Aging. 2009;30:337‐352. [DOI] [PubMed] [Google Scholar]
- 4. Hegen H, Auer M, Zeileis A, Deisenhammer F. Upper reference limits for cerebrospinal fluid total protein and albumin quotient based on a large cohort of control patients: Implications for increased clinical specificity. Clin Chem Lab Med. 2016;54:285‐292. [DOI] [PubMed] [Google Scholar]
- 5. Reiber H. Flow rate of cerebrospinal fluid (CSF) ‐ A concept common to normal blood‐CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. 1994;122:189‐203. [DOI] [PubMed] [Google Scholar]
- 6. Blennow K, Fredman P, Wallin A, et al. Protein analysis in cerebrospinal fluid: II. Reference values derived from healthy individuals 18‐88 years of age. Eur Neurol. 1993;33:129‐133. [DOI] [PubMed] [Google Scholar]
- 7. Seyfert S, Kunzmann V, Schwertfeger N, Koch HC, Faulstich A. Determinants of lumbar CSF protein concentration. J Neurol. 2002;249:1021‐1026. [DOI] [PubMed] [Google Scholar]
- 8. Loke H, Harley V, Lee J. Biological factors underlying sex differences in neurological disorders. Int J Biochem Cell Biol. 2015;65:139‐150. [DOI] [PubMed] [Google Scholar]
- 9. Soldin OP, Chung SH, Mattison DR. Sex differences in drug disposition. J Biomed Biotechnol. 2011;2011:187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leoni V, Masterman T, Patel P, Meaney S, Diczfalusy U, Björkhem I. Side chain oxidized oxysterols in cerebrospinal fluid and the integrity of blood‐brain and blood‐cerebrospinal fluid barriers. J Lipid Res. 2003;44:793‐799. [DOI] [PubMed] [Google Scholar]
- 11. Ahonen A, Myllylä VV, Hokkanen E. Measurement of reference values for certain proteins in cerebrospinal fluid. Acta Neurol Scand. 1978;57:358‐365. [DOI] [PubMed] [Google Scholar]
- 12. Algotsson A, Winblad B. The integrity of the blood‐brain barrier in Alzheimer's disease. Acta Neurol Scand. 2007;115:403‐408. [DOI] [PubMed] [Google Scholar]
- 13. Link H, Tibbling G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand J Clin Lab Invest. 1977;37:397‐401. [DOI] [PubMed] [Google Scholar]


